Abstract
Using phage display, we generated a panel of optimized neutralizing antibodies against the human and mouse receptors for interleukin 21 (IL-21), a cytokine that is implicated in the pathogenesis of many types of autoimmune disease. Two antibodies, Ab-01 and Ab-02, which differed by only four amino acids in VL CDR3, showed potent inhibition of human and mouse IL-21R in cell-based assays and were evaluated for their pharmacological and pharmacodynamic properties. Ab-01, but not Ab-02, significantly reduced a biomarker of disease (anti-dsDNA antibodies) and IgG deposits in the kidney in the MRL-Faslpr mouse model of lupus, suggesting that anti-IL-21R antibodies may prove useful in the treatment of lupus. Ab-01 also had a consistently higher exposure (AUC0-∞) than Ab-02 following a single dose in rodents or cynomolgus monkeys (2-3-fold or 4-7-fold, respectively). Our data demonstrate that small differences in CDR3 sequences of optimized antibodies can lead to profound differences in in vitro and in vivo properties, including differences in pharmacological activity and pharmacokinetic profiles. The lack of persistent activity of Ab-02 in the MRL-Faslpr mouse lupus model may have been a consequence of faster elimination, reduced potency in blocking the effects of mouse IL-21R, and more potent/earlier onset of the anti-product response relative to Ab-01.
Introduction
Biologic therapies for the treatment of autoimmune disease have the potential to target specific pathogenic cytokines or receptors with less immunosuppression than conventional small-molecule drugs. Several biologics have been developed that successfully target cytokine pathways and have significant beneficial effects on clinical disease. In particular, monoclonal antibodies (mAbs) targeting the cytokine TNFα, infliximab (Remicade; Centocor), a chimeric human/murine mAb, and adalimumab (Humira, Abbott), a human mAb derived by phage display technology, are approved to treat several autoimmune diseases.Citation1 Several mAbs antagonizing cell surface receptors are also approved for therapeutic use, such as daclizumab (Zenapax, Hoffman-LaRoche), which targets the IL-2 receptor, CD25, and antagonizes IL-2 bioactivity.Citation2 Phage display and affinity maturation technologies allow the selection of fully human antibodies with high ligand specificity and binding affinity. Many human antibodies developed by phage display technology are currently in clinical trials.Citation3
Interleukin 21 (IL-21), a pleiotropic member of the gamma-common chain family of cytokines, is increasingly implicated in the pathogenesis of many types of autoimmune disease. IL-21 is expressed by activated CD4+ T cellsCitation4 and NKT cells and is most highly expressed the CD4+TFH subset in the lymphoid follicles,Citation5,Citation6 and extrafollicular T cells in the MRL-Faslpr autoimmune mouseCitation7 suggesting an important role for this cytokine pathway in development of antibody responses. IL-21 is also an important growth factor for the initiation and expansion of the TH17 subset, commonly associated with chronic inflammation.Citation8–Citation10
IL-21 signals through a heterodimeric receptor, binding to the high-affinity IL-21-specific alpha chain (referred to as IL-21R), which leads to the recruitment of the gamma common chain and subsequent signaling through the JAK-STAT pathway. Many lymphoid cell types express the IL-21R, including B, T, NK and cells of the myeloid lineage.Citation8–Citation10 The IL-21R can be upregulated on non-lymphoid tissues as well, suggesting a significant role for this cytokine in orchestrating many aspects of the inflammatory response. Increased expression of IL-21 and IL-21R have been associated with human rheumatoid arthritis,Citation11–Citation13 lupusCitation14 and Crohn disease.Citation15,Citation16 Blockade of the IL-21 pathway with a fusion of the IL-21R extracellular domain to the Fc portion of murine IgG (mIL-21R-Fc) neutralizes IL-21 bioactivity in vitro and reduces disease in murine models of lupus,Citation17,Citation18 arthritisCitation19 and inflammatory bowel disease.Citation20 A complementary approach to blocking the IL-21/IL-21R pathway is to target IL-21R instead of the cytokine. In this report, we describe in vitro and in vivo properties (including affinity to human, monkey, and mouse IL-21R; potency in cell-based assays; pharmacokinetics in mice, rats and monkeys; and pharmacology in a mouse lupus model) of affinity-matured antibodies against IL-21R. Our data suggest that anti-IL-21R antibodies may provide an effective treatment for lupus.
Results
Isolation and in vitro characterization of optimized anti-IL-21R antibodies.
A panel of antibodies that bind human IL-21R (hIL-21R) and block its interaction with IL-21 was isolated by phage display. The most potent inhibitor in this set, antibody 18A5, inhibited the IL-21-dependent proliferation of hIL-21R-transfected BaF3 cells or TF1 cells with IC50 of 1.7 and 14 nM, respectively, similar to that of hIL-21R-Fc (, , and Suppl. Fig. 1). 18A5 was also able to inhibit the hIL-21-dependent proliferation of primary human B and T cells with IC50 of 1.4 and 1.9 nM, respectively ( and Suppl. Fig. 1). 18A5 also had detectable but relatively weak inhibitory activity of IL-21-dependent proliferation of BaF3 cells transfected with mouse IL-21R (mIL-21R; , ), suggesting that it would require optimization for use in mouse pharmacology models.
Variants of 18A5 with increased affinity for both human and mouse IL-21R were identified in a subsequent phage display campaign. Four phage libraries displayed variants of 18A5 in which blocks of six adjacent amino acids in the CDR3 loops of the VH or VL domains were randomized to all 20 amino acids (). These libraries were selected in alternating rounds against biotinylated human and murine IL-21R extracellular domains, while the target concentration was reduced from 10 nM to 0.2 pM over the course of five or six cycles. Among 8,280 clones examined, 287 scFv in crude periplasmic extracts competed better for binding of 18A5 IgG to hIL-21R than did the parental 18A5 scFv, and this group contained 7 and 153 unique variants in the VH and VL CDR3 loops, respectively. Neutralization of human and mouse IL-21R by 108 unique scFv variants with highest binding activity was confirmed in BaF3 proliferation assays (data not shown).
Only a few alterations of VH CDR3 appeared among the improved variants, with the starting sequence GGGISRPEY being changed to a more hydrophobic consensus CDR3 sequence of F-L/M/F-GFGRPEY (). The small number of isolates from the libraries with randomization in the C terminal portions of either the VL CDR3 (1 of 171 isolates) or the VH CDR3 (2 of 121 isolates), showed comparatively low neutralization potency and were not pursued further (data not shown). By contrast, five of the six positions at the germline-encoded N terminus of the VL CDR3 (parental sequence NSRDSSGNPHVL) were highly tolerant of sequence changes (). The arginine at position 3 was retained in all isolated sequences, and the aspartate at position 4 was replaced by non-charged amino acids, largely serine and alanine. Indeed, few acidic residues were found in the optimized VL CDR3s, and a majority of the most potent scFv had additional basic residues, most commonly a lysine at position 6. A consensus (hydrophobic/small polar)-(S/A/T)-R-(S/A)-X-basic was apparent in the most potent N-terminally-altered VL CDR3s ().
From the scFv that showed the highest potency against both human and mouse IL-21R in BaF3 assays, 15 mutant VL CDR3 sequences and four mutant VH CDR3 sequences were cloned into the corresponding light or heavy chains of 18A5 IgG lambda and human IgG1 expression vectors, respectively. The 18A5 sequences had been modified so that all framework sequences matched those of the closest human VH and VL germline genes, DP-67/VHJ4-4B+ and DPL16/VL3.1. The IgG1 constant region sequence contained mutations that reduced potential effector activity.Citation21 None of the antibodies tested had detectable activity in ADCC or C1q binding assays (data not shown). Testing of all 80 combinations of mutant and parental heavy and light chains expressed transiently in cos-7 cells showed that, with the exception of several combinations involving the VH3 heavy chain, pairing of the chains in the combinations in which they were selected as scFv produced the highest neutralization potency in human and murine cell-based assays (data not shown). In IgG1 format, all of the optimized antibodies had modestly improved potency (compared to the 18A5 parent) against hIL-21R in BaF3 and TF1 assays (2- to 8-fold) and dramatically improved potency against mIL-21R in BaF3 assays. Analysis of the most potent clones following Protein A purification showed an improvement of 520- to 4,400-fold over 18A5 in the murine IL-21R BaF3 assay (, ). None of the optimized antibodies showed binding to the closest human homologs of IL-21R (IL-4Rα, IL-2Rβ) in surface plasmon resonance studies, while all bound to rat and cynomolgus monkey IL-21R expressed transiently on the surface of CHO cells (data not shown).
The seven most potent hIL-21R-neutralizing antibodies based on assays in engineered cells (numbered Ab-01 through Ab-07) were also found to block IL-21-dependent proliferation of CD3-activated human primary CD4+ T cells with potency greater than that of 18A5. Similar results were obtained for primary human B cells activated with anti-CD40, with the seven antibodies showing 2–8 fold improvements in IC50s, relative to 18A5, consistent with their increased affinity to the hIL-21R. All seven antibodies blocked the IL-21-dependent proliferation of mouse CD8+ T cells. Ab-01 was consistently the most potent in neutralizing IL-21-dependent proliferation of mouse cells (and comparable to mIL-21R-Fc), while it was consistently 2- to 3-fold less potent than the other antibodies in neutralizing hIL-21R.
These seven antibodies were also analyzed by surface plasmon resonance for the kinetics of their binding to monomeric forms of the human and mouse IL-21R extracellular domains. When captured at low density on anti-human IgG, the optimized antibodies showed affinities to hIL-21R ranging from 2 nM (Ab-01) to 0.2 nM (Ab-04), compared with 4 nM for the parental 18A5 antibody ( and Suppl. Fig. 2). Affinities to mIL-21R ranged from 16 nM (Ab-01 and Ab-02) to values close to the 18A5 affinity of 62 nM. Most of the affinity improvements resulted from a reduced dissociation rate. The affinities of Ab-01 and Ab-02 for cynomolgus monkey IL-21R were similar to their affinities for hIL-21R (data not shown).
Interestingly, the gains in potency of neutralization in cell-based assays did not correlate with the magnitude in change in affinity. This was most dramatic in the case of murine IL-21R neutralization, where Ab-01 had a 4,400-fold improvement in potency against mIL-21R-BaF3 cells compared with 18A5 (an IC50 shift from 177 nM to 0.04 nM), but only a 4.3-fold improvement in affinity. In primary human T cells, a 10-fold increase in affinity was associated with a 47-fold increase in potency (Ab-05). In engineered cell lines with the human IL-21R, gains in potency more closely mirrored gains in affinity.
Two of the optimized antibodies were selected for evaluation in vivo. Ab-01 was selected because it had the highest potency in mouse cell based assays () and was thus most appropriate for the proof-of-concept studies in mouse pharmacology models. Ab-02 was chosen because it had among the highest potency of human IL-21R inhibition while still retaining strong mouse IL-21R inhibition (). The affinities of both antibodies to mouse IL-21R were about 16 nM, while Ab-02 bound human IL-21R with a 5-fold higher affinity than did Ab-01 (0.4 nM vs. 2 nM).
PK profiles of anti-IL-21R antibodies Ab-01 and Ab-02 after single administration to mice and rats.
After IV administration to healthy CD-1 mice, Ab-01 was eliminated slowly, with elimination half-life (t1/2; ∼6.8 days) and clearance (CL; ∼1.4 mL/hr/kg) that were within the range reported for other human IgG1 in mice. When healthy CD-1 mice were dosed IV with Ab-02, exposure (AUC0−∞) was ∼2-fold lower and CL was ∼2 fold faster (∼2.2 mL/hr/kg) compared to Ab-01 (), while t1/2 was similar to that of Ab-01 (∼8.8 days). After IP administration to healthy DBA mice, AUC0−∞ of Ab-02 was also ∼2-fold lower compared to Ab-01 (), while t1/2 was ∼5.8 and 3.3 days for Ab-01 and Ab-02, respectively. After IP administration to lupus-prone MRL-Faslpr mice, Ab-01 and Ab-02 were eliminated faster (t1/2 ∼2 and 0.9 days, respectively), resulting in the lower dose-normalized AUC0−∞, compared to the respective values in healthy DBA mice (). The difference in AUC0−∞ between the Ab-01 and Ab-02 was more pronounced among MRL-Faslpr mice than among the other mouse strains tested (∼3 fold difference, and ).
A sharp decline in mean Ab-01 and Ab-02 concentrations at the terminal phase was detected in both the healthy and in MRL-Faslpr mice ∼2–3 weeks and 5–10 days after a single dose, respectively ( and data not shown). Anti-product antibodies were detected during the period of rapid clearance in animals treated with Ab-01 (Suppl. Table 1). MRL-Faslpr mice have polyclonal B-cell expansionCitation22,Citation23 and appeared to have faster onset and/or higher frequency of anti-Ab-01 antibodies (detected in 100% of tested samples), compared to healthy mice (detected in ∼37–75% of tested samples) (Suppl. Table 1).
Following a single 10 mg/kg IV dose to Sprague-Dawley rats, Ab-01 and Ab-02 were eliminated slowly (CL ∼1.6 mL/hr/kg for Ab-01 and ∼2.1 mL/hr/kg for Ab-02; and ); albeit significantly faster (p < 0.05) than that for an isotype control IgG (∼0.4 mL/hr/kg, and data not shown). Mean serum concentrations of Ab-01, Ab-02, and the control IgG started to diverge as early as 24 hr post-dose, with the control IgG displaying the highest serum concentrations and Ab-02 the lowest ones. At the one week time-point, the difference between the mean IgG control and Ab-01 concentrations was ∼2.5-fold, while that between the IgG and Ab-02 was more than 10-fold. The AUC0−∞ for Ab-02 was ∼2-fold lower than that of Ab-01. All rats dosed with Ab-01 or Ab-02 had a sharp decline in test article serum concentrations in the terminal phase, such that there was no detectable Ab-01 and Ab-02 in serum after day 15–17 post-dose. The sharp decline in Ab-01 serum concentrations correlated with the presence of anti-Ab-01 antibodies detected at day 15 and all subsequent time points in all six animals (Suppl. Table 1). Samples were not tested for the presence of anti-Ab-02 antibodies. It should be noted that differences in concentration-time profiles in rats between the Ab-01, Ab-02 and the control IgG are not likely to be explained entirely by anti-product responses, since mean serum concentrations started to diverge much earlier than anti-product responses could have developed.
PK profiles of anti-IL-21R antibodies Ab-01 and Ab-02 after single administration to cynomolgus monkeys.
After a single 10 or 100 mg/kg IV dose to monkeys, Ab-01 was eliminated slowly (CL ∼1 mL/hr/kg); albeit faster (p < 0.05) than that for an isotype control IgG (CL ∼0.25 mL/hr/kg) (). Bioavailability of Ab-01 after a 10 mg/kg SC dose was approximately 43%. All six monkeys that were administered 10 mg/kg of Ab-01 (n = 3 for IV and n = 3 for SC) had a rapid decline in Ab-01 levels. These monkeys also had detectable anti-Ab-01 antibodies starting at 3 weeks post-dose ( and Suppl. Table 1), and which persisted at all subsequent time points (up to 27 weeks). Therefore, elimination half-life (t1/2) was calculated with and without time-points at which a rapid decline in Ab-01 concentration was observed and was approximately 3 days or 8 days, respectively (). The t1/2 for an isotype control IgG, which did not show a rapid decline, was approximately 14 days.
The difference between the pharmacokinetic profiles of Ab-01 and Ab-02 was more pronounced in monkeys than in rodents. Following 10 or 100 mg/kg IV administration to monkeys, Ab-02 was eliminated significantly faster (p < 0.05) than Ab-01 (). For example, at 168 hours (one week) after a single 10 mg/kg IV dose, mean Ab-02 levels were ∼0.53 µg/mL, while those for Ab-01 were ∼13 µg/mL. Mean Ab-02 levels were below the limit of detection in all Ab-02-dosed monkeys after 2 and 4 weeks following single 10 and 100 mg/kg IV doses, respectively. Formation of anti-Ab-02 antibodies was not examined in this study. However, in a separate study, all three monkeys dosed with 10 mg/kg IV of Ab-02 were positive in a cell-based neutralizing antibody assay (data not shown). The estimated CL of Ab-02 was ∼5–7 fold faster and Ab-02 exposure (AUC0−∞) was 4–7 fold lower, compared to Ab-01 and t1/2 of Ab-02 was ∼2–4 days ().
In vivo pharmacology of anti-IL-21R antibodies Ab-01 and Ab-02 in MRL-Faslpr mice.
Blockade of the IL-21-pathway with murine IL-21R.Fc has previously been shown to reduce auto-antibody titers to dsDNA in the MRL-Faslpr model of lupus.Citation18 To examine the in vivo pharmacology of the anti-IL-21R antibodies, we treated MRL-Faslpr mice 3x/wk with saline, 10 mg/kg IP dose of Ab-01, Ab-02 or isotype control antibodies for 10 weeks, measured serum anti-dsDNA IgG antibody titers bi-weekly, and sacrificed the mice 3 days after the last dose. At the end of the study, kidney sections were examined for IgG deposits by immunohistochemistry. Urine was collected bi-weekly and tested for protein using Albustix test strips, but neither control animals nor treated animals developed significant proteinuria during the study (data not shown).
Twelve-week old male MRL-Faslpr mice had high titers of anti-dsDNA IgG serum antibody, and anti-dsDNA antibody titers were not reduced by treatment with an isotype control antibody ( and Suppl. Fig. 3). Administration of Ab-01 significantly reduced anti-dsDNA antibody titers in MRL-Faslpr mice within two weeks of treatment (wk 0 median of 120,751 Ab units versus wk 2 median of 22,104 Ab units). By week 4 of treatment with Ab-01, median anti-dsDNA antibody titers were approximately 100-fold lower in MRL-Faslpr mice compared to baseline (wk 0 median of 104,200 Ab units versus wk 4 median of 1,000 units = lower limit of detection) and 8 of 10 mice had undetectable anti-dsDNA IgG serum antibodies. Ab-01-induced reduction of median anti-dsDNA antibody titers (compared to the baseline, saline control or an isotype control) persisted throughout the treatment period. Specifically, anti-dsDNA antibodies were reduced to undetectable levels in 8 of 10 mice at week 4, in 5 of 10 mice at week 6, and in 7 of 10 mice at week 8, in contrast to saline-and isotype-control-antibody-treated animals, in which all mice had detectable anti-dsDNA antibody titers. However, treatment with Ab-02 only slightly reduced median anti-dsDNA IgG serum antibody titers in MRL-Faslpr mice (compared to median baseline values) over the course of the first four weeks of treatment, but these changes were not statistically significant compared to IgG control groups and were not observed at the later time points. In addition, Ab-01 but not Ab-02 significantly reduced IgG deposits in the kidney at week 10 time point, when compared with either saline or an isotype control groups ().
Anti-product antibody titers in mice treated with anti-Ab-02 were 10-100-fold higher than those measured in animals treated with Ab-01 (Suppl. Fig. 4). However, it should be noted that circulating test articles could have interfered with the detection of anti-product antibodies in mouse serum.
In a similar study, MRL-Faslpr mice were administered Ab-01 and Ab-02 by IP injection (3x/week for ∼10 weeks) at 10 and 20 mg/kg, respectively, and trough serum concentrations, as well as anti-dsDNA titers, were measured bi-weekly until the terminal time point (week 10). In accordance with the results of the first study, treatment with Ab-01 but not Ab-02 led to persistent reduction in anti-dsDNA titers (data not shown). Despite the higher dosage used for Ab-02, trough median serum Ab-02 concentrations were lower than those of Ab-01 at all time points. Trough median Ab-01 serum concentrations were ∼138–200 µg/mL at 2–6 weeks and ∼50–70 µg/mL at 8–10 weeks, while trough median Ab-02 serum concentrations were ∼94 and 13 µg/mL at 2 and 4 weeks, respectively, and below the detection limit at subsequent time points (Suppl. Fig. 5). Thus, differences in pharmacological potencies between the two anti-IL-21R antibodies correlated with the differences in trough serum concentrations.
Discussion
In this report we demonstrated that small differences in CDR3 sequence of optimized antibodies can lead to profound differences in behavior of the molecules both in vitro and in vivo, including differences in pharmacological activity and pharmacokinetic profiles. A panel of variants of the anti-IL-21R antibody 18A5, which blocks IL-21 cytokine binding to the receptor, was identified in an affinity optimization campaign. Phage display libraries with blocks of six adjacent randomized codons in the CDR3 loops of the 18A5 heavy or light chains were selected for scFv that could bind to both human and mouse IL-21R at low (0.2–2 pM) concentrations. Within the resulting set of optimized clones, nearly all were derived from libraries in which the N terminal portion of one of the CDR3s was randomized. Among these, only a few unique variants of the VH CDR3 were isolated, while the VL CDR3 sequence showed high tolerance for sequence substitution in five of six randomized positions. Combinations of independently-optimized VH and VL sequences were nearly always less potent than the combinations present in the selected scFv, suggesting that residues from both the VH and VL CDR3s work in concert to form the antigen binding surface.
Optimized CDR3 sequences tended to be more basic than the parental sequence, retaining the arginine residues at VH CDR3 position 6 and VL CDR3 position 3, nearly always losing the aspartic acid at VH CDR3 position 4, and frequently acquiring arginine or lysine residues at positions 5 and 6 of the VL CDR3. This trend toward basic character could reflect interaction with the proposed IL-21 binding interface of IL-21R, which is believed to have a high negative charge.Citation24,Citation25 Different sequence classes appeared to provide optimal binding to mouse and human receptors, e.g., in binding assays in which scFvs competed with the 18A5 scFv for binding to mIL-21R (data not shown). Clones with isoleucine or methionine at the first position in the VL CDR3 were among the strongest binders to mIL-21R, but bound more weakly than most to hIL-21R. Given the low sequence identity between the two receptors (62%), it seems likely that at a fine-structure level, an optimal fit to each species’ IL-21R may require a different spectrum of amino acids at key positions in the antigen binding site.
Improved affinity, largely a function of slower dissociation rates, was generally associated with a proportional improvement in potency in cell-based assays for hIL-21R, while for mIL-21R, antibodies with small differences in affinity showed large differences in potency in cell-based assays. For example, Ab-01 bound to mIL-21R with a 4.3-fold higher affinity than that of the parental antibody 18A5, while it neutralized the IL-21 dependent proliferation of mIL-21R-BaF3 cells with a 4,400-fold greater potency. Likewise, 18A5, Ab-03 and Ab-04 bound mIL-21R with similar affinity (KD values of 72, 69 and 65 nM, respectively), but 18A5 failed to neutralize mouse primary T cell proliferation, while the two optimized antibodies had sub-nanomolar IC50s. Both differences in binding kinetics (3.9-fold slower association rate and 4.2-fold slower dissociation rate of Ab-03 compared to that of Ab-04) and subtle differences in the molecular interactions at the antigen binding site are likely to contribute to the differences in potency.
Two of the optimized antibodies were selected for evaluation in vivo, including PK studies in rodents and monkeys and pharmacology studies in a mouse lupus model. Ab-01 and Ab-02 are identical except for four amino acids in VL CDR3 (MSRSIWGNPHVL vs. VARSNKGNPHVL), and both bind mIL-21R with a KD of ∼16 nM. Ab-01 was chosen because it had the highest potency in mouse cell based assays and was thus most appropriate for the proof-of-concept studies in mouse pharmacology models. Ab-02 was chosen because it was among the most potent inhibitors of both human and mouse IL-21R.
Despite their similar structures and in vitro properties, Ab-01 and Ab-02 had significantly different pharmacokinetics and pharmacological activities. Ab-01 significantly reduced autoantibody levels in the MRL-Faslpr mouse model of lupus. MRL-Faslpr mice dosed with Ab-01 had reduced anti-dsDNA antibody titers within 2 weeks of administration, and over the next 6 weeks of treatment, the anti-dsDNA antibody levels in 5–8 out of 10 mice were below the level of detection. In addition, Ab-01 significantly reduced IgG deposits in the kidneys in the MRL-Faslpr mice at the end of the study (week 10). These data indicate that anti-IL-21R antibodies may prove useful in the treatment of lupus, corroborating the observation that IL-21R deficiency prevents development of lupus-like symptoms in MRL-Faslpr mice.Citation18 In contrast, Ab-02 did not reduce anti-dsDNA antibodies or IgG deposits in the kidney in MRL-Faslpr mice.
Ab-01 and Ab-02 had consistently different PK profiles in all species tested. In single-dose PK studies in rodents (CD-1, DBA and MRL-Faslpr mice and S-D rats), Ab-02 exposure was 2–3 fold lower than that of Ab-01 administered at the same dosage/route. The differences in pharmacokinetics of Ab-01 and Ab-02 in cynomolgus monkeys were more striking than those in mice. Following 10 or 100 mg/kg IV administration to cynomolgus monkeys, Ab-02 exposure was 4–7 fold lower than that of Ab-01. The mechanism of fast elimination of Ab-02 in rodents and monkeys is not known, but is unlikely to be explained entirely by increased binding to IL-21R, since it appeared non-saturable at the relatively high dose of 100 mg/kg. In addition, another anti-IL-21R antibody with human IL-21R affinity equivalent to that of Ab-02 showed a PK profile similar to that of Ab-01 (data not shown), suggesting that the fast elimination of Ab-02 is not a necessary consequence of high affinity. The development of an anti-product immune response is likely to be responsible for the sharp drop in circulating Ab-01 and Ab-02 concentration observed in the terminal phase in rodents and monkeys. However, the more rapid elimination of Ab-02 in monkeys cannot be explained entirely by differences in anti-product responses, since the concentration profiles of Ab-01 and Ab-02 started to diverge as early as 6 hours post-dose and differed by more than 20-fold at the one week time point, prior to the appearance of anti-product antibodies. There were no significant differences in the glycosylation profiles, thermal stability or whole blood stability at 37°C between Ab-01 and Ab-02 (data not shown). Further studies are needed to delineate the mechanism of fast clearance of Ab-02 in monkeys and rodents.
The absence of persistent efficacy of Ab-02 in the MRL-Faslpr mouse lupus model may be the result of both rapid elimination and insufficient potency in blocking the effects of mouse IL-21R. Because IL-21 has a critical role in the primary antibody responses,Citation26 it is plausible that an antibody that neutralizes IL-21R effectively upon repeated dosing also reduces or delays the development of mouse-anti-human antibodies (MAHA) against itself, which in turn would lead to slower elimination of human anti-IL-21R antibodies compared to that after a single dose. In contrast, multiple injections with an antibody that only weakly blocks IL-21R, may instead trigger a potent MAHA response and lead to rapid human antibody elimination as well as to neutralization of any remaining anti-IL-21R activity. This effect may be enhanced in a mouse strain such as MRL-Faslpr, which is prone to autoimmune disease and polyclonal B-cell expansion and which also has fast elimination of normal IgG, possibly due to the disease-induced impairment of the function of FcRn.Citation27 Consistent with this hypothesis, the anti-product response in MRL-Faslpr mice treated with Ab-01 was significantly lower than that observed with Ab-02 (Suppl. Fig. 4). Furthermore, in a separate study, median Ab-01 trough serum concentrations in MRL-Faslpr mice dosed 3 times per week at 10 mg/kg were up to 10-fold higher than those simulated using the concentration profile observed following a single 10 mg/kg dose of Ab-01 (Suppl. Fig. 5), consistent with the hypothesis that effective blockade of the IL-21R reduced anti-Ab-01 formation and anti-Ab-01-mediated clearance of Ab-01. In contrast, Ab-02 serum concentrations were undetectable after week 4 of a similar dosing regimen at 20 mg/kg, suggesting that even the high initial Ab-02 serum concentration was insufficient to prevent anti-Ab-02-mediated clearance of Ab-02. The Ab-02 CDR3 sequence per se is unlikely to be more immunogenic than that of Ab-01, since the majority of the anti-product response against both antibodies was against the common portion of the molecule (Guay et al., unpublished observations). Also, it should be noted that circulating test articles could have interfered with the detection of anti-product antibodies in mouse samples.
In summary, using phage display, we generated a panel of optimized anti-IL-21R neutralizing antibodies. Two antibodies, Ab-01 and Ab-02, which differed by only four amino acids in VL CDR3, both showed potent inhibition of human and mouse IL-21R in cell-based assays. Ab-01, but not Ab-02, significantly reduced a biomarker of disease (anti-dsDNA antibodies) and IgG deposits in the kidney in the MRL-Faslpr mouse model of lupus. Ab-01 also had a consistently higher exposure (AUC0−∞) than Ab-02 following a single dose to rodents or cynomolgus monkeys. Our data suggest that IL-21R-neutralizing antibodies may provide an effective treatment for lupus, which would fill a major unmet medical need. The differences in in vivo behavior of Ab-01 and Ab-02 suggest further that an effective therapeutic would require high neutralization potency and optimization of PK properties.
Materials and Methods
Antibody isolation by phage display
The scFv parental clone 18A5 (accession number GP230882) was obtained from the CS human scFv library (Cambridge Antibody Technology)Citation28 by standard phage display methods,Citation29,Citation30 using BaF3 cells expressing human IL-21R as a target in rounds 1 and 3 and biotinylated IL-21R-Fc fusion protein as a target in round 2. It was identified on the basis of its ability to compete with MuF, an anti-IL-21R antibody previously identified in the same screen, for binding to biotinylated hIL-21R-Fc in ELISA. Subsequently, four phage display libraries were made based upon the parental 18A5 scFv, in which blocks of six adjacent codons in either the VH CDR3 (GGGISR in library VH-A and ISRPEY in library VH-B) or the VL CDR3 (NSRDSS in library VL-A and GNPHVL in library VL-B) were replaced with random sequences encoded by NNS codons, where N is any of the four nucleotides A, T, G or C, and S is either of the nucleotides G or C. Phage able to bind in solution to biotinylated human and mouse IL-21R extracellular domain His-FLAG fusion proteins (IL-21R-H/F) were selected using standard methods,Citation30 starting from 10 nM target and descending over 5–6 selection rounds to 0.2 pM, alternating between human and mouse IL-21R-H/F. VH-randomized libraries were kept separate from VL-randomized libraries, but the two VH libraries were combined with each other, and the two VL libraries were combined with each other, after 2–3 selection rounds.
ScFv able to compete with the parental 18A5 antibody for binding to human or murine IL-21R were identified by a homogeneous time-resolved fluorescence (HTRF) assay. Parental 18A5 antibody was conjugated to cryptate, a derivative of europium (Cisbio, Bedford, MA). Periplasmic extracts of scFv were prepared in TES buffer (30 mM Tris-HCl (pH 8.0)/0.6 mM EDTA/12% sucrose), diluted to 0.25% in PBS/0.4 M potassium fluoride/0.1% BSA, mixed with cryptate-18A5 and XL665-streptavidin (Cisbio), and brought to a final concentration of either 1.2 nM biotin-hIL-21R-H/F or 10 nM biotin-mIL-21R-H/F. Following a 2-hr incubation, binding of the 18A5 antibody to IL-21R was measured by time-resolved FRET (340 nm excitation, 615 nm and 665 nm emission).
scFv sequence determination.
PCR amplification of scFvs was carried out on diluted bacterial cultures using VENT® DNA Polymerase (New England Biolabs, Ispwich, MA) in HN buffer (Epicentre Biotechnologies, Madison, WI) according to the manufacturer’s instructions. 0.2 µM each of the PUC forward (5′ CGC CAG GGT TTT CCC AGT CAC GAC 3′) and PUC Reverse (5′ AGC GGA TAA CAA TTT CAC ACA GG 3′) primers were used under the following cycling conditions: 94°C (2 min), 30 cycles of 94°C (1 min), 55°C (2 min), and 72°C (1 min), followed by a final 5-min extension at 72°C. PCR products were verified by agarose gel electrophoresis and cleaned with ExoI/SAP (shrimp alkaline phosphatase) prior to dye-termination sequencing with the M13rev primer.
BaF3/TF1 proliferation assay.
Individual scFv clones were purified from periplasmic extracts of 20 ml cultures using PHYTIP® Ni-NTA columns (Phynexus, Inc., San Jose, CA).Citation31 BaF3 cells, a murine pre-B cell line, and TF1 cells, a human erythroid cell line, were retrovirally transduced with IL-21R and green fluorescent protein (GFP). Cells were grown in RPMI 1640 with 10% FBS, 2 mM L-glutamine, 100 U/ml penicillin, 100 µg/ml streptomycin, 0.00036% β-mercaptoethanol, and supplementary growth factors (50 ng/ml hIL-21 for hIL-21R-BaF3; 10 U/ml mIL-3 for mIL-21R-BaF3; 50 ng/ml GM-CSF for hIL-21R-TF1). Cells were washed and incubated for 6 hr in assay medium lacking supplemental growth factors, added at 5,000 cells per well to a 96-well flat-bottomed white tissue culture plate (Thermo Scientific, Waltham, MA), and incubated for 30 min. with diluted scFv or IgG. Human or murine IL-21 was added to each well (100–400 pg/ml), and the cells were incubated for an additional 48 hr. Proliferation was measured with CELLTITER-GLO® (Promega, Madison, WI).
Primary B and T cell proliferation assays.
Buffy coat cells from healthy human donors were obtained from Massachusetts General Hospital (Boston, MA). B cells were isolated with a ROSETTESEP™ B cell enrichment kit (Stem Cell Technologies, Vancouver, BC) according to the manufacturer’s instructions. The resulting population (60–80% CD19+ B cells) was cultured in RPMI containing 10% FBS, 50 µg/ml penicillin, 50 µg/ml streptomycin, and 2 mM L-glutamine at 1 × 105/well in 96-well flat-bottom plates (37°C, 5% CO2). Cells were pretreated with antibodies for 30 min and then stimulated with 0.5 µg/ml anti-CD40 mAb (BD Biosciences, San Jose, CA) and 10 ng/ml IL-21 cytokine for 3 days. Cultures were pulsed with 0.5 µCi/well 3H-thymidine (Perkin Elmer (NEN)) and harvested 5 hr later onto glass fiber filter mats. 3H-thymidine incorporation was determined by liquid scintillation counting.
Human CD4+ T cells were isolated from buffy coats with a ROSETTESEP™ CD4+ T cell enrichment cocktail (Stem Cell Technologies) according to the manufacturer’s instructions. Cells (80–90% CD4+/CD3+ T cells) were activated for 3 days with anti-CD3/anti-CD28-coated microspheres in RPMI containing 10% FBS, 100 U/ml penicillin, 100 µg/ml streptomycin, 2 mM L-glutamine, and HEPES (37°C, 5% CO2). Microspheres were then removed, and the cells were washed once prior to resting overnight at ∼1 × 106 cells/ml in culture medium and once before addition to assay plates. Antibodies were diluted in culture medium in flat-bottomed 96-well plates, followed by sequential addition of human IL-21 (20 ng/ml final concentration) and 105 cells/well. After 72 hr, cells were pulsed with 1 µCi/well 3H-thymidine (Perkin Elmer (NEN)) for 6 hr and harvested onto glass fiber filter mats for liquid scintillation counting.
Murine CD8+ T cells were isolated from the spleens and popliteal, axillary, brachial, and inguinal lymph nodes from 12-week-old female BALB/C mice. A single-cell suspension of the spleen cells was depleted of red blood cells using 0.16 M NH4Cl in 0.017 M Tris (pH 7.4). Spleen and lymph node cells were pooled and enriched for CD8+ cells using a murine T cell CD8 Subset Column Kit (R&D Systems). 3 × 104 murine CD8+ cells (in DMEM containing 10% fetal calf serum, 0.05 mM β-mercaptoethanol, 2 mM L-glutamine, 0.1 mM nonessential amino acids, 1 mM sodium pyruvate, 100 U/ml penicillin, 100 µg/ml streptomycin and 50 µg/ml gentamicin) were plated in 96-well anti-mCD3 activation plates (BD Biosciences). 50 ng/ml mIL-21 and the test antibodies were added to the wells, and cells were grown for 3 days in a 37°C/10% CO2 incubator. Cells were labeled for 5 hr with 0.5 µCi methyl-3H-thymidine/well (GE Healthcare, Piscataway, NJ) and harvested for liquid scintillation counting.
Determination of in vitro kinetics.
The kinetics of binding of anti-IL-21R antibodies to human, murine and cynomolgus monkey IL-21R-H/F at 25°C was tested on a BIAcore® surface plasmon resonance instrument. Anti-huIgG antibodies (CALTAG™ Laboratories/Invitrogen) were immobilized (2950–3405 resonance units) onto a CM5 chip using standard amine coupling, and remaining activated groups were blocked with ethanolamine-HCl. Antibodies were diluted to 0.1–0.2 µg/ml in HBS/EP buffer/0.2% BSA and loaded onto the chip. Following a brief washing period, IL-21R solutions were injected over the chip at a flow rate of 50 µl/min for 3 min and then allowed to dissociate (0–100 nM hIL-21R-H/F with 15 min dissociation or 10–500 nM mIL-21R-H/F with 5 min dissociation), followed by regeneration of the chip surface with glycine (pH 1.5). Blank and buffer effects were subtracted for each sensorgram using double referencing.
PK study design.
Mice (male DBA and CD-1; female MRL-Faslpr) and rats (male Sprague-Dawley) were obtained from Jackson Laboratories (Bar Harbor, ME), Taconic Farms (Germantown, NY), or Charles River (Wilmington, MA). Anti-IL-21R or control antibodies were administered via a single intravenous (IV), intraperitoneal (IP) or subcutaneous (SC) dose at indicated dose levels (). For IV administration, antibodies were administered as a single bolus dose into the tail vein, jugular vein via catheter, or saphenous vein for mice, rats and monkeys, respectively. The dose was based on the most recent body weights prior to dosing. The dosing volume was 4 mL/kg for mice, 1 mL/kg for rats, and 1–2 mL/kg for monkeys in formulation buffer containing either 10 mM L-histidine, 5% sucrose, pH 6.0 or PBS, pH 7.2.
For the mouse studies, non-serial sampling design was used (n = 4–9 per time point), with 1–3 blood draws per mouse (either by retro-orbital bleeding or by cardiac puncture). Serial sampling design was used for rat (n = 6 per group for Ab-01 and Ab-02 and n = 4 for huIgG control antibody) and monkey studies (n = 3 per group for Ab-01 and Ab-02 and n = 4 for huIgG control antibody). In mouse and rat studies, serum for test article concentration analysis was collected at 5 min and up to 4 weeks post-dose. In the monkey studies, serum for test article concentration analysis was collected at 5 min and then up to 6 weeks post-dose for all studies, except the 100 mg/kg studies, in which an 8 week collection period was used. In the Ab-01 studies, additional serum samples for anti-Ab-01 analysis were collected at pre-dose and up to 4 weeks in mouse and rat studies and up to 27 weeks in the 10 mg/kg monkey study.
The Wyeth Institutional Animal Care and Use Committee approved all aspects of PK and pharmacology studies.
ELISA for determination of Ab-01, Ab-02 and huIgG control serum concentrations.
Anti-IL-21R antibody in serum samples was captured onto a microtiter plate pre-coated with hIL-21R-H/F (CD-1 mice, S-D rats and cynomolgus monkeys) or with a mouse monoclonal antibody to human IgG/Fc (DBA and MRL-Faslpr mice). An isotype control antibody (anti-IL-13 human IgG1 with the mutations in the Fc region identical to those of Ab-01 and Ab-02) was captured with FLAG-tagged recombinant human IL-13 (cynomolgus monkey) or with a mouse monoclonal antibody to human IgG/Fc (S-D rats). The bound anti-IL-21R or control antibody was detected with mouse anti-human IgG/Fc conjugated to either biotin (when anti-human IgG was used as a capture reagent) or to horseradish peroxidase (when IL-21R-H/F or IL-13 FLAG were used as capture reagents), followed by addition of enzyme substrates and measurement of optical densities (405 nm for 2,2′-azino-di (3-ethyl-benzthiazoline-6-sulfonate) (ABTS) or 450 nm for 3,3′,5,5′-tetramethylbenzidine (TMB)). Sample concentrations were determined by interpolation from a calibration curve that was fit using a 4 parameter logistic equation (Softmax Pro, version 4.3.1, Molecular Devices and Watson LIMS, version 7.0.0.01, Thermo Electron Corporation). The lower limit of quantitation (LLOQ) for anti-IL-21R was ∼33.4–66.8 ng/mL in rodents and 30.0 ng/mL in monkeys. The LLOQ for the control huIgG was ∼4 and 132 ng/mL for monkey and rat assays, respectively.
Pharmacokinetics calculations and simulations.
For rats and monkeys, PK parameters for individual animals were determined, while PK parameters in mice were determined based on the mean concentration profiles. A non-compartmental analysis module (Model 200 for IP and SC routes and Model 201 for IV route) of the pharmacokinetic software package WinNonlin, ver. 5.1 (Pharsight, Mountain View, CA) was used. The area under the serum concentration versus time curve (AUC) was calculated using the linear trapezoidal method. The slope of the apparent terminal phase was estimated by log-linear regression using at least 3 data points, and the terminal rate constant (λ) was derived from the slope. AUC0−∞ was estimated as the sum of the AUC0−t (where t is the time of the last measurable concentration) and Ct/λ. The apparent terminal half-life (t1/2) was calculated as 0.693/λ. Statistical analysis of differences in mean clearance values was performed using Student t test (Prism 5; GraphPad Software, Inc.,). Predictions of concentrations after a multiple dose regimens were conducted by nonparametric superposition module of the WinNonlin software using concentration-time profiles from the single dose PK studies.
An electrochemiluminescent paramagnetic bead assay for detection of anti-Ab-01 antibodies in serum in PK studies.
Serum samples were co-incubated with biotinylated-Ab-01 and ruthenylated-Ab-01 overnight. After incubation with streptavidin-coated paramagnetic beads, samples were placed in an M Series 384 Analyzer 2004 (BioVeris Corporation), a magnet was applied, unbound reactants were washed away, and emitted light was measured by photodetectors with the readout in response units (RU). Serum samples were diluted to 1:25 and 1:75, and those showing a response equal to or greater than twice the mean response of negative control serum samples (defined as the cutpoint RU) were considered positive. Positive samples were reanalyzed in a full dilution series to determine the titer (the dilution that would generate a response equal to the cutpoint RU). Results were reported as the log of the titer, with a lower limit of detection of 1.40 (corresponding to a minimum dilution of 1:25). This assay detects both neutralizing and non-neutralizing anti-Ab-01 antibodies.
Mouse lupus model study design.
Twelve week old male (first study) or eight week old female (second study) MRL-Faslpr mice (Jackson Laboratory, Bar Harbor, ME) were administered Ab-01, Ab-02, saline, or a control anti-human IL-13 antibody with the same triple mutant (L234A L235A G37A) human IgG1 constant region at 400 µg or 800 µg/mouse (10 or 20 mg/kg, as specified in the text), 3x/week intraperitoneally (IP) over 10 weeks and sacrificed at ∼3 days after the last dose. Serum samples were taken biweekly and examined for anti-dsDNA antibodies (as described below), for test article serum concentrations (using the ELISA described above for PK studies), and for antibodies to Ab-01 or Ab-02 using an ELISA in which Ab-01 or Ab-02 was used as a capture and an anti-mouse IgG-HRP (Southern Biotech, Birmingham, AL) was used as the detector. At the end of the study (week 10), kidneys were frozen in OCT medium, and kidney sections were examined for IgG deposits by immunohistochemistry. Urine was collected bi-weekly and tested for protein using Albustix test strips (Ames Co., London, UK). Statistical analyses of anti-dsDNA data and IgG deposit scores were performed using GraphPad Prizm 5 software package (GraphPad Software Inc., San Diego, CA).
ELISA for quantitation of anti-dsDNA in sera from MRL-Faslpr mice.
Anti-dsDNA IgG serum antibodies were measured by ELISA, as previously described.Citation18 Briefly, Immulon 1B plates (Thermo Labsystems, Franklin, MA) were UV irradiated overnight and then coated with 2 µg/mL calf thymus DNA (Sigma) for 1 hr at 25°C. Plates were blocked with PBS plus 1% BSA, diluted serum samples were added (starting at 1:100 dilution), and bound antibody was detected with HRP-conjugated goat antibodies directed against mouse IgG antibody (Southern Biotech). Plates were developed with TMB and optical densities were read at 450 nm. Arbitrary units of antibody were determined using standard positive control serum pooled from diseased MRL-Faslpr mice (SoftMax Pro software).
Immunohistochemistry for IgG deposits in the kidneys of MRL-Faslpr mice.
Deposits of IgG in the glomeruli (11 or 12 glomeruli per animal) were detected by incubating acetone-fixed 4-µm-thick cryostat sections of kidney in 10% normal goat serum for 30 min, followed by a 1-hr incubation with HRP-conjugated goat anti-mouse IgG (1/200; MP Biomedical Corporate Irvine CA), five washes, a 5-min incubation with 3,3′-diaminobenzidine (DAB), and counterstaining with hematoxylin. Immunohistochemical staining for IgG within glomeruli was scored blindly as none (0), slight (1), mild (2), moderate (3) or severe (4).
Figures and Tables
Figure 1 Neutralization of IL-21 dependent proliferation of BaF3 cells expressing IL-21R. Human IL-21R-transfected BaF3 cells (A) and murine IL-21R-transfected BaF3 cells (B) were treated with antibodies and human or murine IL-21, respectively, for 48 hours and their proliferation measured by CellTiter Glo. Antibodies tested were the parental 18A5 (solid circles), Ab-01 (open squares), Ab-02 (open triangles), Ab-03 (open circles), control IgG (X), and the human or murine IL-21R-Fc (solid squares).
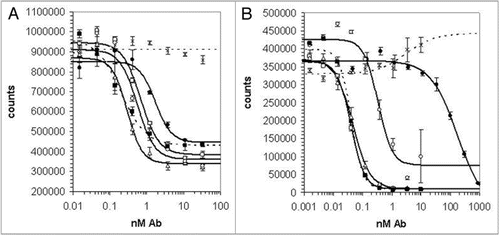
Figure 2 CDR3 sequences of optimized anti-IL-21R antibodies. The frequencies of different amino acids at each position of heavy chain CDR3 (A) or light chain CDR3 (B) in the 25 selected sequences with the highest potency are indicated by the height of the colored sections of each bar. The sequence of the 18A5 at the equivalent position is indicated below the graph. Blue lines show the sequences randomized in libraries VH-A, VH-B, VL-A and VL-B. CDR3 sequences of the clones chosen for detailed analysis are shown in (C).
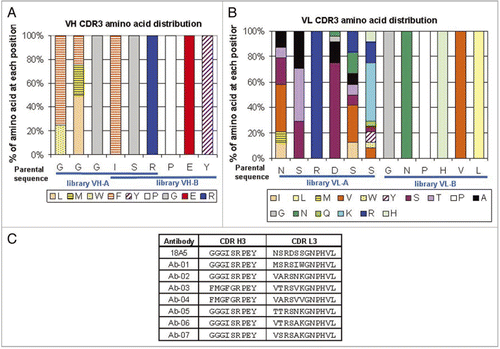
Figure 3 Serum concentration-time profiles of Ab-01 and Ab-02 following a single 10 mg/kg dose to MRL-Faslpr mice or Sprague-Dawley rats. Ab-01 (filled circles), Ab-02 (open circles), or an isotype control anti-human IL-13 antibody (open triangles in (B) only) were administered to ∼12-week old MRL-Faslpr mice (A, IPdose) or Sprague-Dawley rats (B, IV dose) and test article concentrations in serum were determined by ELISA, as described in the text. Individual concentration values below the limit of quantitation (LOQ of 33.4–66.8 ng/mL for anti-IL-21R antibodies and 132 ng/mL for an isotype control antibody) were treated as zero for calculations of the mean and standard deviation. Non-serial sampling design was used for mice (n = 6–9 per time point) and serial sampling design was used for rats (n = 4–6 per group). Time points at which all animals showed concentration values below the LOQ are indicated by ovals.
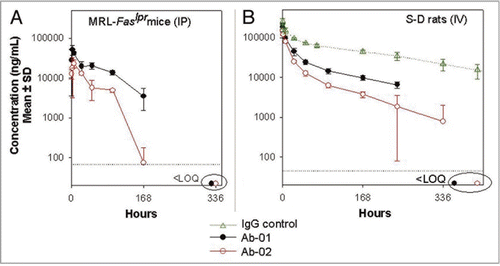
Figure 4 Serum concentration-time profiles of Ab-01 and Ab-02 following a single dose to cynomolgus monkeys. Ab-01 (filled circles in panels A and B and as indicated in panel C), Ab-02 (open circles in A and B), or an isotype control anti-human IL-13 antibody (open triangles in A) were administered to cynomolgus monkeys at indicated dose levels and routes (n = 3–4 per group), and test article concentrations in serum were determined by ELISA, as described in the text. Mean (A and B) or individual animal (C) concentrations are shown. Individual concentration values below the limit of quantitation (LOQ of 30 ng/mL for anti-IL-21R antibodies and 4 ng/mL for an isotype control) were treated as zero for calculations of the mean and standard deviation. Time points at which all (A) or specified (C) animals showed concentration values below the LOQ are indicated by ovals.
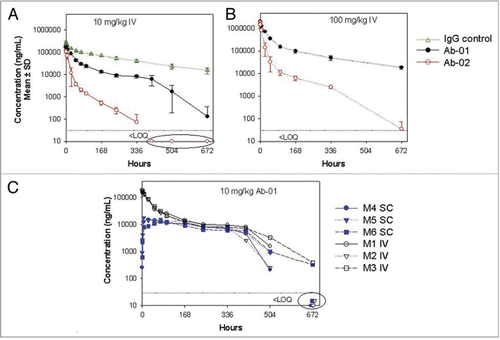
Figure 5 Median titer of anti-dsDNA antibodies in MRL-Faslpr mice treated with anti-IL-21R antibodies. Twelve week old MRL-Faslpr mice were administered Ab-01 (open triangles), Ab-02 (open circles), saline (filled diamonds), or an isotype control anti-human IL-13 antibody (filled squares) at 400 µg/mouse (10 mg/kg), 3x/week IP for 10 weeks and sacrificed at 3 days after the last dose. Serum was collected prior to dosing (week 0) and then at 2, 4, 6, 8 and 10 weeks post-dose, and mouse anti-dsDNA antibodies were monitored by ELISA, as described in the text. Limit of detection was 1,000 units/mL. Individual data values that were below the lower limit of detection were treated as 1,000 units/mL for calculations of the group median titers. *p < 0.05, based on a Mann-Whitney test using baseline, the saline group and the isotype control IgG group as a control.
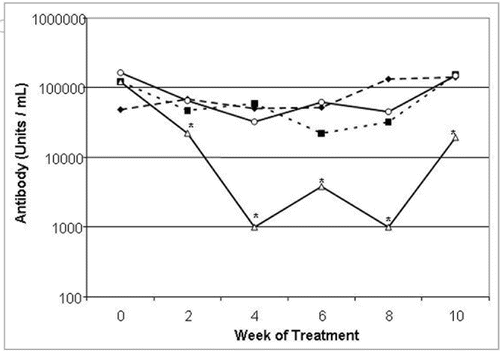
Figure 6 IgG deposits in the kidneys of MRL-Faslpr mice treated with anti-IL-21R antibodies. Twelve week old MRL-Faslpr mice were administered Ab-01, Ab-02, saline, or an isotype control anti-human IL-13 antibody at 400 µg/mouse (10 mg/kg), 3x/week IP for 10 weeks and sacrificed at 3 days after the last dose. IgG deposits in the kidney sections were assessed by immunohistochemistry and scored blindly on a scale of 0 (none) to 4 (severe) at the 10 week time point. Group mean scores (line) and individual animal scores (A), as wells as representative pictures (B) for each group are shown. *p < 0.05, based on a Mann-Whitney test using the saline and the isotype control IgG group as a control.
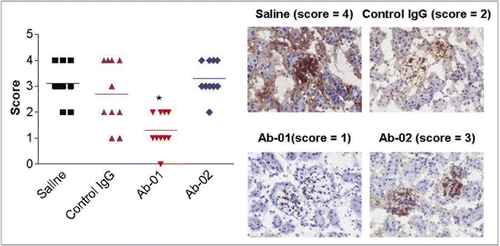
Table 1 Binding and neutralization properties of anti-IL-21 receptor antibodies
Table 2 Mean (±SD) PK parameters of Ab-01 and Ab-02 in mice, rats and monkeys
Additional material
Download Zip (516.2 KB)Acknowledgements
We thank Angela Robak, Jocelyn Sanford, Jimin Zhang, Ryan Jackobek, Jessica Targ, Paula Giampa, Kathy Heveron, Khetemcnee Lam, Adam Root, Richard Zollner and Stephane Olland for expression and purification of antibodies; Lora Haynes, Mostafa Ait-Zahra and Jan Kieleczawa for DNA sequencing support; Chris Shea, Nicole Duriga, Jamie Emerson, Marisa Fahey, Alison Joyce, Rosemary Lawrence-Henderson and Matthew Shepard for technical help with ELISA and anti-product antibody assays; Mark Ryan for neutralizing antibody assays, Tom Brown and Nancy Stedman for immunohistochemistry, Sean Keegan for data analysis, and Bilian Li for assistance with cell-based assays.
References
- Strand V, Kimberly R, Isaacs JD. Biologic therapies in rheumatology: lessons learned, future directions. Nat Rev Drug Discov 2007; 6:75 - 92
- Carter PJ. Potent antibody therapeutics by design. Nat Rev Immunol 2006; 6:343 - 357
- Lonberg N. Fully human antibodies from transgenic mouse and phage display platforms. Curr Opin Immunol 2008; 20:450 - 459
- Parrish-Novak J, Dillon SR, Nelson A, Hammond A, Sprecher C, Gross JA, et al. Interleukin 21 and its receptor are involved in NK cell expansion and regulation of lymphocyte function. Nature 2000; 408:57 - 63
- King C, Tangye SG, Mackay CR. T follicular helper (TFH) cells in normal and dysregulated immune responses. Annu Rev Immunol 2008; 26:741 - 766
- Vogelzang A, McGuire HM, Yu D, Sprent J, Mackay CR, King C. A fundamental role for interleukin-21 in the generation of T follicular helper cells. Immunity 2008; 29:127 - 137
- Odegard JM, Marks BR, DiPlacido LD, Poholek AC, Kono DH, Dong C, et al. ICOS-dependent extrafollicular helper T cells elicit IgG production via IL-21 in systemic autoimmunity. J Exp Med 2008; 205:2873 - 2886
- Jin H, Carrio R, Yu A, Malek TR. Distinct activation signals determine whether IL-21 induces B cell costimulation, growth arrest or Bim-dependent apoptosis. J Immunol 2004; 173:657 - 665
- Ozaki K, Kikly K, Michalovich D, Young PR, Leonard WJ. Cloning of a type I cytokine receptor most related to the IL-2 receptor beta chain. Proc Natl Acad Sci USA 2000; 97:11439 - 11444
- Spolski R, Leonard WJ. Interleukin-21: basic biology and implications for cancer and autoimmunity. Annu Rev Immunol 2008; 26:57 - 79
- Distler JH, Jungel A, Kowal-Bielecka O, Michel BA, Gay RE, Sprott H, et al. Expression of interleukin-21 receptor in epidermis from patients with systemic sclerosis. Arthritis Rheum 2005; 52:856 - 864
- Jungel A, Distler JH, Kurowska-Stolarska M, Seemayer CA, Seibl R, Forster A, et al. Expression of interleukin-21 receptor, but not interleukin-21, in synovial fibroblasts and synovial macrophages of patients with rheumatoid arthritis. Arthritis Rheum 2004; 50:1468 - 1476
- Li J, Shen W, Kong K, Liu Z. Interleukin-21 induces T-cell activation and proinflammatory cytokine secretion in rheumatoid arthritis. Scand J Immunol 2006; 64:515 - 522
- Ettinger R, Kuchen S, Lipsky PE. Interleukin 21 as a target of intervention in autoimmune disease. Ann Rheum Dis 2008; 67:83 - 86
- Caruso R, Fina D, Peluso I, Stolfi C, Fantini MC, Gioia V, et al. A functional role for interleukin-21 in promoting the synthesis of the T-cell chemoattractant, MIP-3alpha, by gut epithelial cells. Gastroenterology 2007; 132:166 - 175
- Fina D, Sarra M, Caruso R, Del Vecchio Blanco G, Pallone F, MacDonald TT, et al. Interleukin 21 contributes to the mucosal T helper cell type 1 response in coeliac disease. Gut 2008; 57:887 - 892
- Bubier JA, Bennett SM, Sproule TJ, Lyons BL, Olland S, Young DA, et al. Treatment of BXSB-Yaa mice with IL-21R-Fc fusion protein minimally attenuates systemic lupus erythematosus. Ann N Y Acad Sci 2007; 1110:590 - 601
- Herber D, Brown TP, Liang S, Young DA, Collins M, Dunussi-Joannopoulos K. IL-21 has a pathogenic role in a lupus-prone mouse model and its blockade with IL-21R.Fc reduces disease progression. J Immunol 2007; 178:3822 - 3830
- Young DA, Hegen M, Ma HL, Whitters MJ, Albert LM, Lowe L, et al. Blockade of the interleukin-21/interleukin-21 receptor pathway ameliorates disease in animal models of rheumatoid arthritis. Arthritis Rheum 2007; 56:1152 - 1163
- Fina D, Sarra M, Fantini MC, Rizzo A, Caruso R, Caprioli F, et al. Regulation of gut inflammation and th17 cell response by interleukin-21. Gastroenterology 2008; 134:1038 - 1048
- Kasaian MT, Tan XY, Jin M, Fitz L, Marquette K, Wood N, et al. Interleukin-13 neutralization by two distinct receptor blocking mechanisms reduces immunoglobulin E responses and lung inflammation in cynomolgus monkeys. J Pharmacol Exp Ther 2008; 325:882 - 892
- Dixon FJ, Andrews BS, Eisenberg RA, McConahey PJ, Theofilopoulos AN, Wilson CB. Etiology and pathogenesis of a spontaneous lupus-like syndrome in mice. Arthritis Rheum 1978; 21:64 - 67
- Reilly CM, Gilkeson GS. Use of genetic knockouts to modulate disease expression in a murine model of lupus, MRL/lpr mice. Immunol Res 2002; 25:143 - 153
- Bondensgaard K, Breinholt J, Madsen D, Omkvist DH, Kang L, Worsaae A, et al. The existence of multiple conformers of interleukin-21 directs engineering of a superpotent analogue. J Biol Chem 2007; 282:23326 - 23336
- Zhang JL, Foster D, Sebald W. Human IL-21 and IL-4 bind to partially overlapping epitopes of common gamma-chain. Biochem Biophys Res Commun 2003; 300:291 - 296
- Ozaki K, Spolski R, Feng CG, Qi CF, Cheng J, Sher A, et al. A critical role for IL-21 in regulating immunoglobulin production. Science 2002; 298:1630 - 1634
- Zhou J, Pop LM, Ghetie V. Hypercatabolism of IgG in mice with lupus-like syndrome. Lupus 2005; 14:458 - 466
- Lloyd C, Lowe D, Edwards B, Welsh F, Dilks T, Hardman C, et al. Modelling the human immune response: performance of a 1,011 human antibody repertoire against a broad panel of therapeutically relevant antigens. Protein Eng Des Sel 2009; 22:159 - 168
- Griffiths AD, Williams SC, Hartley O, Tomlinson IM, Waterhouse P, Crosby WL, et al. Isolation of high affinity human antibodies directly from large synthetic repertoires. EMBO J 1994; 13:3245 - 3260
- Hawkins RE, Russell SJ, Winter G. Selection of phage antibodies by binding affinity. Mimicking affinity maturation. J Mol Biol 1992; 226:889 - 896
- Cummins E, Luxenberg DP, McAleese F, Widom A, Fennell BJ, Darmanin-Sheehan A, et al. A simple high-throughput purification method for hit identification in protein screening. J Immunol Methods 2008; 339:38 - 46