Abstract
Antagonist antibodies targeting CD28 have been proposed as an alternative to the use of CD80/86 antagonists to modulate T cell responses in autoimmunity and transplantation. Advantages would be the blockade of CD28-mediated co-stimulatory signals without impeding the co-inhibitory signals dependent on CD80 interactions with CTLA-4 and PD-L1 that are important for the control of immune responses and for the function of regulatory T cells. Anti-CD28 antibodies are candidate antagonists only if they prevent access to the CD80/86 ligands without simultaneously stimulating CD28 itself, a process that is believed to depend on receptor multimerization. In this study, we evaluated the impact of different formats of a potentially antagonist anti-human CD28 antibody on T cell activation. In particular, we examined the role of valency and of the presence of an Fc domain, two components that might affect receptor multimerization either directly or in the presence of accessory cells expressing Fc receptors. Among monovalent (Fab’, scFv), divalent (Fab’2), monovalent-Fc (Fv-Fc) and divalent-Fc (IgG) formats, only the monovalent formats showed consistent absence of induced CD28 multimerization and absence of associated activation of phosphoinositol-3-kinase, and clear antagonist properties in T cell stimulation assays. In contrast, divalent antibodies showed agonist properties that resulted in cell proliferation and cytokine release in an Fc-independent manner. Conjugation of monovalent antibodies with polyethylene glycol, α-1-antitrypsin or an Fc domain significantly extended their in vivo half-life without modifying their antagonist properties. In conclusion, these data indicate that monovalency is mandatory for maintaining the antagonistic activity of anti-CD28 monoclonal antibodies.
Introduction
The CD28-CD80/86-CTLA4 triad of costimulatory molecules forms a molecular rheostat that controls effector (Teff) and regulatory (Treg) T cell activity.Citation1 Antagonists of CD80/86 are potent immunosuppressors that are indicated for use in rheumatoid arthritis and kidney transplantation.Citation2,Citation3 CD28-CD80/86 interactions stimulate and CTLA4-CD80/86 interactions dampen T cell activation. Thus, it has been suggested that selective CD28 antagonists might show superiority by preventing only the CD28-CD80/86 interactions and not the CTLA4-CD80/86 interactions that are mandatory for the function of Treg cells, thereby potentially promoting immune tolerance.Citation4 Recent identifications of the costimulatory CD28-ICOSLCitation5 and coinhibitory CD80-PDL1Citation6 interactions reinforced the idea that CD28 might be a better therapeutic target to promote immune tolerance, and this has been demonstrated experimentally in both autoimmunity and transplantation models.Citation7-Citation11
Directly targeting CD28 with antibodies, however, poses two problems. First, the target epitope may not lie inside the C”D basolateral domain of CD28, since antibodies against that domain present superagonist activities resulting in a non-physiological, T cell receptor independent, polyclonal activation of T cells mimicking the action of superantigens.Citation12,Citation13 Such antibodies directed against the C”D basolateral domain of CD28 caused a dramatic accident in a Phase 1 trial in 2006.Citation14 Second, even when the target epitope lies outside the C”D domain of CD28, antibodies might cross-link CD28 and mimic its physiological role, which in the presence of antigen, is the reinforcement of TCR second messengers leading to T cell activation. This effect has been attributed to the divalency of antibodies in their IgG form, CD28 being itself a homodimeric receptor,Citation15 and to the engagement of antibody Fc domains by FcR expressed by accessory cells.Citation16 It has been shown that anti-CD28 IgG antibodies with mutations in the Fc domain, minimizing interactions with FcR, still possess agonist properties, indicating that interaction with Fc receptors might not be the dominant mechanism generating agonist activity.Citation16
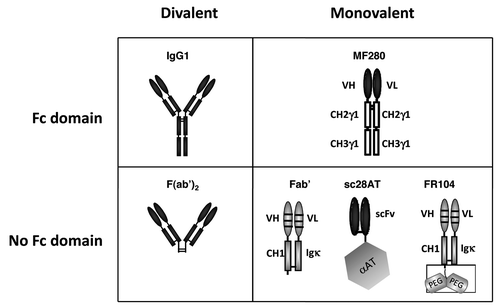
To decipher the respective roles of valency vs. Fc-FcR interactions in the antagonist/agonist properties of anti-CD28 antibodies, we compared different constructions of the same parental anti-CD28 antibody. We selected CD28.3, an anti-human CD28 monoclonal antibody (mAb) that overlaps with the MYPPPY epitope of CD28 known to be important for the interaction with CD80/86,Citation11 therefore representing a potential antagonist antibody. We compared CD28.3 in the divalent IgG or F(ab’)2 formats with monovalent Fab’, scFv or a novel VH/VL-Fc construction combining a monovalent paratope with the presence of an IgG1 Fc domain (), and evaluated antagonist and agonist activities in cell-based assays. The data formally established that for this mAb, valency is a dominant parameter that controls antagonist properties and that the presence of an Fc receptor per se does not influence the antagonist properties of monovalent antibodies.
Figure 1. Schematics of the antibodies used in this study, all derived from the CD28.3 anti-human CD28 mAb. IgG1, murine original mAb in its divalent IgG1 format. F(ab’)2, product of the pepsin digestion of the CD28.3 mAb. MF280, novel monovalent construction consisting in a chimeric heterodimer of VH-CH2-CH3 and VL-CH2-CH3 chains. Fab’, humanized recombinant antibody fragment produced in CHO cell supernatant. Sc28AT, recombinant fusion antibody consisting in the assembly of a scFv with human α-1-antitrypsin. FR104, humanized Fab’ modified by pegylation with a 2 x 20 kDa polyethylene glycol moiety branched at the c-terminal end of the heavy chain.
Results
Construction and expression of monovalent VH/VL-Fc fusion antibodies
In the search for a new antibody format that could combine a monovalent paratope with the presence of an IgG Fc domain, we hypothesized that independent production of antibody variable heavy and light chain domains in genetic fusion with an IgG Fc domain might lead to dimerization and the formation of an immunologically active monovalent antibody. We first individually fused cDNA corresponding to the VH and VL domains of the CD28.3 antibody to the CH1-hinge-CH2-CH3 cDNA of human IgG1. Co-transfection of the 2 constructs into Cos cells, however, did not result in the synthesis of immunologically active antibodies (data not shown). Next we removed the CH1 domain from the same constructs and observed that the resulting VH-Fc (42.4 KDa) and VL-Fc (41.7 KDa) proteins presented anti-CD28 binding activity (). This monovalent antibody was named MF280. Cells transfected with either VH-Fc or VL-Fc only expressed the corresponding chain, but did not produce immunologically active antibodies (data not shown). MF280 presented a stable anti-CD28 immune reactivity over at least 5 d. That the Fc domain of MF280 was actually functional and could be recognized by Fcγ receptors was confirmed by ELISA using recombinant human Fcγ RI/CD64 immobilized on plastic (R&D Systems; data not shown). We also fused VH and VL domains with the CH2-CH3 domains of human IgG4 to create MF280-G4. The idea was to minimize the biological function of the Fc domain besides its interaction with neonatal Fc Receptors. In this construct, the hinge region was still of the IgG1 type to prevent Fab-arm exchange with endogenous IgG4 antibodies, a phenomenon attributed to the dissociation properties of the IgG4 hinge domain.Citation17 MF280-G4 could also be expressed in and purified from eukaryotic cells, resulting in immunologically active antibodies. By gel filtration analysis, however, we observed that whereas MF280 was mostly monovalent, MF280-G4 contained a significant amount of aggregates and was therefore excluded from further studies (data not shown). We did not consider fusions with Fc domains of the IgG2 isotype because they are described to form dimers in vivo by disulfide rearrangement in the hinge.Citation18,Citation19
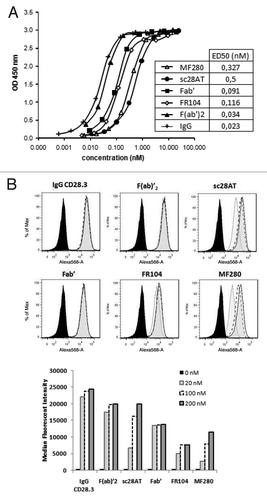
Figure 2. Binding analysis of anti-CD28 antibodies. (A) Assessment by ELISA on immobilized CD28-Fc of MF280 (Δ), sc28AT (●), Fab’ (■), FR104 (◊), F(ab)’2 (▲) and IgG (+). Revelation was performed with a rabbit antibody against VH/VL domains of the CD28.3 mAb plus peroxidase-labeled goat anti-rabbit antibodies and revealed by colorimetry at 450nm using TMB substrate. ED50 is the concentration of the indicated antibody to reach 50% of the signal in this assay. One experiment representative of three is shown. (B) Assessment of binding by flow cytometry on target Jurkat T cells of indicated antibodies at 200 nM (gray-tinted, black line), 100 nM (black dotted line), 20 nM (light-gray-tinted, gray line) and without antibody (solid black line).
Characterization of monovalent and divalent anti-CD28 mAbs
Binding activity of the CD28.3 anti-CD28 antibody in its different formats was evaluated by ELISA (), plasmon resonance () and flow cytometry (). Whereas divalent antibodies [IgG and F(ab’)2] presented a comparable ED50 of 0.03 nM, the binding of monovalent Fab fragments was reduced by ca. two-fold, reflecting the effect of valency on affinity. Conjugation of a bi-branched 2 × 20 kDa polyethylene glycol moiety to the C-terminus of the heavy chain of the Fab’ fragment in FR104 did not modify the ED50 measured by ELISA. Two other monovalent formats, MF280 and sc28AT, presented much lower binding properties, with an ED50 of 0.3 and 0.5 nM, respectively, indicating that the conformation of these antibodies was probably suboptimal. MF280 and sc28AT also presented a lower affinity by Biacore assay (). Interestingly, significant differences in binding affinity between Fab’ and FR104 detected by Biacore did not translate into different binding properties measured by ELISA. This might be due to different association (Kon) and similar dissociation (Koff) constants between these two molecules, presumably caused by the presence of a PEG moiety that can interfere with the chip and decrease association. Flow cytometry using CD28+ Jurkat T cells as targets () and Raji B cells as negative controls (not shown) revealed the specificity of the antibodies. In their IgG, F(ab)’2, Fab’ and FR104 formats, the mean fluorescence intensity remained similar when assessed in a dose ranging from 20 to 200 nM. In contrast, for sc28AT and MF280 the mean fluorescence intensity dropped more than 2-fold at 20 nM, confirming the ELISA data showing lower ED50 for these two formats. The weaker signal obtained with FR104 at any concentration was most probably due to a reduced access to the secondary antibody caused by pegylation.
Table 1. Surface plasmon resonance analysis of interaction with recombinant CD28
Induction of CD28 multimerization
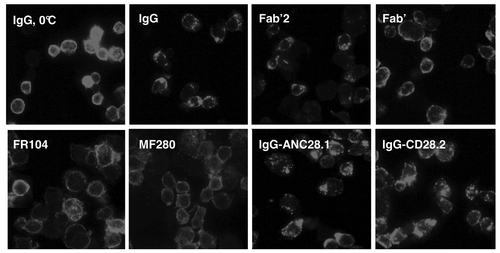
Anti-CD28 antibodies might present both antagonist and agonist properties because, although they can inhibit CD28/B7 interaction, they could also directly alter CD28 conformation by inducing multimerization. To visualize the action of antibody-induced CD28 receptor multimerization, we incubated CD28-positive Jurkat T cells with anti-CD28 antibodies and revealed capping formation by immunofluorescence. Anti-CD28 antibodies in their divalent IgG or Fab’2 forms induced formation of CD28 clusters on T cell membranes within an hour at 37°C, but not at 0°C. In contrast, monovalent Fc-free antibodies [Fab’, Fab’-PEG (FR104) and monovalent-Fc (MF280)] antibodies did not induce formation of CD28 clusters (). The superagonist anti-CD28 mAb ANC28.1, as well as the agonist CD28.2 mAb, induced a strong multimerization ().
Figure 3. CD28 capping induction by anti-CD28 antibodies. (A) Jurkat T cells expressing CD28 were incubated for 1h with 20µg/ml of indicated antibodies at 0 or 37°C. After washing, cells were fixed in 0.5% paraformaldehyde, centrifuged onto glass slides, immunolabeled with anti-CD28.3 VH/VL antibodies plus FITC-labeled donkey anti-rabbit antibodies and examined by epifluorescence.
Activation of the CD28 signaling pathway
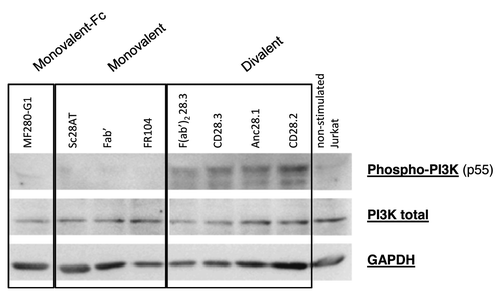
To study to what extent engagement of CD28 with anti-CD28 antibodies resulted in activation of the CD28 downstream signaling pathway, we stimulated Jurkat T cells with immobilized anti-CD3 antibodies plus anti-CD28 antibodies in their different formats and assessed PI3K phosphorylation (). The results showed that the four divalent anti-CD28 antibodies induced phosphorylation of the p55α subunit of PI3K after anti-CD3 activation of T lymphocytes, and removal of the Fc domain in the F(ab’)2 format did not abolish activation. Monovalent antibodies without an Fc domain or monovalent-Fc antibodies (MF280) were consistently devoid of agonist activity.
Figure 4. Activation of PI3K pathway with divalent or monovalent anti-CD28 mAbs. (A) Expression of Phospho-PI3K, total PI3K and GAPDH was assessed by Western Blot in lysate of Jurkat T cells stimulated 5 min. at 37°C with 10 µg/ml of each indicated mAb in the liquid phase plus immobilized anti-CD3 antibodies. Results are representative of 4 independent experiments.
Activation of T cell proliferation and cytokine synthesis
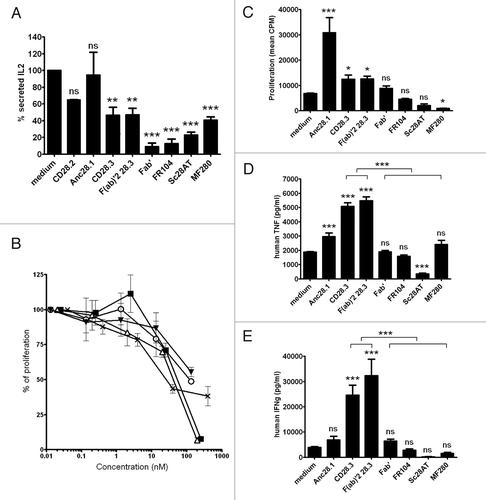
To better understand the relative importance of antibody valency and presence of an Fc receptor, we tested monovalent and divalent anti-CD28 antibodies, with or without Fc domains, in activation assays. First, we stimulated Jurkat T cells with Raji B cells and Staphylococcus enterotoxin E (SEE) superantigens (recognized by the TCR of Vβ8-type Jurkat cells). In these conditions, T cells consistently secreted IL-2 in a costimulation-dependent manner. Monovalent, Fc-free antibodies (Fab’, sc28AT or Fab’-PEG) significantly inhibited IL-2 secretion in this assay, whereas superagonist (ANC28.1) or agonist (CD28.2) antibodies failed to modulate IL-2 secretion. MF280 Fc-fusion monovalent antibodies and divalent antagonist antibodies with (CD28.3) or without (Fab’2) an Fc domain also inhibited IL-2 secretion, although to a lesser extent (). We next tested some of our antibodies in mixed lymphocyte reactions. Monovalent FR104 and, to a lesser extent, MF280 dose-dependently inhibited proliferation of alloreactive T cells. Divalent antibodies (IgG, Fab’2) also inhibited alloreactivity but only when added at higher concentrations (). We then tested antagonist/agonist properties in an assay where human PBMC were stimulated with immobilized anti-CD3 antibodies. T cell proliferation was measured 3 d after addition of anti-CD28 antibodies () whereas cytokines were measured in the supernatant after 24h (). As expected, superagonist Anc28.1 and divalent CD28.3 antibodies induced T cell proliferation. Superagonist Anc28.1 only moderately induced release of TNF and did not induce a significant increase of IFNg, which might be due to the relatively late timing at which we assessed our supernatants (whereas superagonist antibodies induced cytokine release in man within 2 hCitation14). Interestingly, divalent Fc-free Fab’2 antibodies also increased proliferation and cytokine secretion. In contrast, monovalent Fc-free antibodies (sc28AT, Fab’ and FR104) and monovalent-Fc antibodies (MF280) did not elicit cytokine synthesis or proliferation, and even reduced the basal activation level induced by anti-CD3 antibodies. These results indicated that monovalency correlates with a consistent antagonist behavior and the presence of an Fc domain does not modify the antagonist properties of monovalent antibodies.
Figure 5. Agonist or antagonist properties of anti- CD28 antibodies. (A) IL-2 secretion by Jurkat T cells stimulated for 2 d with SEE superantigens presented by Raji B cells, after addition of 10 µg/ml of the indicated mAb. (B) Mixed-lymphocyte reactions measured after 5 d with human PBMC and addition of MF280 (■), FR104 (x), F(ab)’2 (Δ), IgG CD28.3 (o) and IgG CD28.2 (▼) at different concentrations. (C) Proliferation of human PBMC maintained for 3 d in culture wells coated with anti-CD3 antibodies, and in the presence of 10 µg/ml of indicated mAbs in the liquid phase. (D and E) TNFα and (E) IFNγ cytokines were measured after 24h in the supernatant of PBMC stimulated as in condition C. Data were mean ± SEM of three experiments. *p < 0.05, **p < 0.01, ***p < 0.001. ns, not significant.
Pharmacokinetics in mice
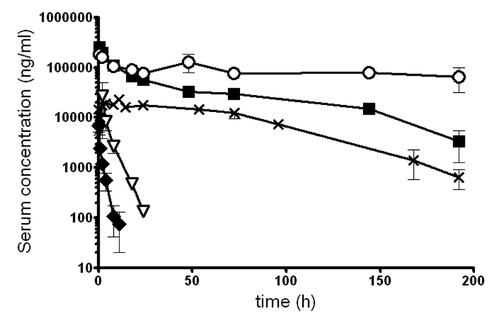
To understand which antibody format could be used in vivo to antagonize CD28, we performed 1-week pharmacokinetic analyses in mice after bolus i.v. injections (in the knowledge that these antibodies do not cross-react with murine CD28). As expected, Fab’ fragments presented a limited half-life of only 1.9 h. The scFv-AT construction presented a longer half-life, albeit limited to 3.5 h. Fab’-PEG monovalent antibodies (FR104) presented an elimination half-life of 42 h, illustrating the previously reported effect of pegylation on elimination.Citation20 The impact of the Fc fusion was very clear since MF280 presented an elimination half-life of 83 h, which was still inferior to the elimination profile presented by the original CD28.3 mouse IgG1 ().
Discussion
It has been previously shown that antagonist antibodies targeting CD28 down-modulate immune responses in rodents, primates and humanized murine models of autoimmunity and transplantation, and synergize with regulatory T cells to promote immune tolerance.Citation7,Citation8,Citation10,Citation11,Citation21-Citation27 Given the dramatic events that followed the administration to healthy volunteers of TGN1412, a superagonist anti-CD28 mAb that stimulated polyclonal T cell activation and massive cytokine release,Citation14 it appeared mandatory to pursue the development of anti- CD28 antibodies only if they present antagonist properties and do not induce cytokine release by target human T cells. Attempts were thus made to engineer the Fc portion of the heavy chain to prevent binding to FcγR and dampen T cell activation mediated by accessory cells, but the resulting antibody still presented residual agonist properties.Citation16 Other reports showed that monovalent fragments from the CD28.3 anti-CD28 antibody did not induce activation of target T cells in vitro or in vivo,Citation11,Citation26 suggesting that monovalency was a critical parameter controlling the antagonist activity of anti-CD28 antibodies.
To understand the parameters controlling T cell activation mediated by anti-CD28 antibodies, our study tested the respective impact of valency and the presence of an IgG Fc domain on human T cell proliferation and cytokine release and revealed that the presence of an Fc domain per se is not detrimental. Rather, valency obviously controls agonist activity. The impact of an Fc domain in a mAb can easily be studied by comparing activity of the IgG with that of F(ab’)2 fragments obtained after pepsin digestion. The impact of monovalency can also be assessed by comparing F(ab’)2 fragments with Fab fragments obtained after papain digestion or with recombinant Fab’ fragments. So far, the consequences of the presence of an Fc domain in a monovalent antibody have not been evaluated, despite the theoretical advantage of enhancing the in vivo half-life of monovalent antibodies by expressing an Fc domain and therefore making it possible to use them therapeutically. Examples of applications are those where a cell surface receptor needs to be blocked, without being multimerized, such as with anti-CD3 antibodiesCitation28 or anti-CD28 antibodies.Citation15
We therefore constructed MF280, a novel antibody format combining a monovalent paratope from the CD28.3 mAb with an Fc domain. This was made possible by expressing two different chains containing VH-CH2-CH3 and VL-CH2-CH3 domains that dimerize in vivo to form a VH/VL-Fc heterodimeric protein. We tried CH2-CH3 domains from human IgG1 and IgG4 and found that IgG4 resulted in the formation of substantial aggregates, precluding the use of MF280-G4 as monovalent binders. The affinity of MF280, as assessed by Biacore, was lower than that of the corresponding recombinant Fab’ fragment, which indicated that the paratope conformation was probably suboptimal. The difference, however, was mainly due to a lower association constant with a similar dissociation constant, suggesting that these antibodies might show comparable biological activities after engagement with their target. This situation was similar to that observed with sc28AT, a previously developed monovalent antibody combining a monovalent scFv from the CD28.3 mAb in molecular fusion with a molecule of α-1-antitrypsin.Citation15 Indeed, in binding assays MF280 behaved like sc28AT, and in cell-based assays MF280 and sc28AT showed similar inhibitory properties compared with Fab’ fragments. This might be also explained by the fact that the affinity of the CD28-CD80/86 interactions occurring during contacts between T cells and antigen presenting cells is several orders of magnitude lower than the affinity of anti-CD28 antibodies (CD28-CD80/CD86 affinities are 4 × 10−6 and 2 × 10−5 KD (M), respectivelyCitation29). In vivo, the elimination half-life of Fab’ and scFv fragments is usually very short, which makes it difficult to use such antibodies to antagonize molecular interactions in the long-term. Various molecular fusions have been used to extend the molecular weight of an antibody fragment, including albumin,Citation30 α-1-antitrypsin,Citation15 and polyethylene glycol.Citation20 These approaches are successful at extending the in vivo half-life because they increase the molecular weight of the complex above the glomerular filtration threshold. Protein fusion with an Fc domain extends the in vivo half-life even more due to Fc domain recognition by neonatal Fc receptors (FcRn) expressed on endothelial cells, which actively recycles serum IgG. In our mouse pharmacokinetic analysis, we could indeed observe a considerable extension of the half-life of Fab’ fragments after pegylation, greater than the advantage conferred by genetic fusion of a scFv antibody fragment with αAT in sc28AT. We also noted that the presence of an Fc domain in the MF280 antibody extended its half-life even more, to a value approaching that of a natural IgG. This analysis was only indicative, however, since other factors such as glycosylation are known to influence antibody half-life and since the antibodies that are compared here were produced in different systems (recombinant and hybridoma origins) that may not glycosylate proteins to a similar extent. Overall, our results suggest that the MF280 format would fulfill criteria for becoming a drug candidate (i.e., efficacy in dampening CD28-dependent T cell activation, absence of agonist activity for human T cells and prolonged in vivo half-life). These results also show that addition of an Fc domain to a monovalent antibody, such as in MF280 where we could observe that it actually bound to FcγR, did not result in target cell activation even in the presence of PBMC-containing FcR-positive cells (such as B cells and monocytes). This suggests that engagement of Fc domains by FcR, at least in vitro, cannot significantly mimic the action of divalent antibodies; however, the in vivo occurrence of Fc-dependent activities such as complement-dependent cytotoxicity and antibody-dependent cellular cytotoxicity cannot be excluded by the current study.
The multimerization and activation assays clearly showed a correlation between antibody-mediated CD28 aggregation and T cell activation. Divalent antibodies induced CD28 capping and PI3k phosphorylation and also induced T cell proliferation and cytokine release, whereas monovalent antibodies were negative in these tests (–). Surprisingly, in mixed lymphocyte reactions all antibodies eventually became inhibitors when their concentration was increased. This might be because, in this assay, alloreactive T cells need to be stimulated through formation of immune synapses and CD28-CD86 interactions are required for synapse stabilization.Citation31 Thus, our data highlight the importance of a monovalent format for the antagonist function of anti-CD28 antibodies, but this rather applies to T cell activation (cytokine synthesis) than to proliferation in response to alloreactive antigen-presenting cells. Indeed, in a humanized mouse model where infused human T cells become activated as a result of their xenoreactivity, we have previously shown that both monovalent and IgG anti-CD28 antibodies (FR104 and IgG CD28.3) blocked T cell expression of activation markers, but only the monovalent format blocked cytokine synthesis.Citation26
In summary, we have shown that the therapeutic blockade of the CD28 receptor using antagonist antibodies requires the antibodies to be used in their monovalent form to prevent target T cell activation driven by CD28 multimerization in the presence of a TCR stimulus. Addition of an IgG1 Fc domain, intended to extend the in vivo half-life, did not modify the antagonist properties of monovalent binders in vitro. These findings contribute to understanding the appropriate antibody format necessary to safely antagonize CD28 in humans.
Materials and Methods
Recombinant antibodies and fragments
The Anc28.1 superagonist anti-CD28 antibody was from Calbiochem (Merck). The mouse anti-human CD28 hybridoma CD28.2 (IgG1) and CD28.3 (IgG1) were provided by Dr. D. Olive (Inserm UMR 1068, Marseille, France). F(ab’)2 antibody fragments were prepared using the Pierce Mouse IgG1 Fab and F(ab’)2 Preparation Kit (Perbio Science France) and Sc28AT, a single-chain Fv from the CD28.3 antibody linked to human α-1-antitrypsin, was prepared as previously described.Citation15 Humanized Fab’ fragments from the CD28.3 mAb and their pegylated versions (FR104) were obtained from Effimune and have been previously described.Citation26 In summary, the humanized Fab’ fragments from the CD28.3 mAb were produced by transfected GS-CHO mammalian cells and optionally conjugated with a Y-shaped 40 kDa polyethylene glycol molecule (NOF) at the C-terminal cysteine end of the VH chain. The MF280 construction (Fv-IgG1 Fc format) was obtained by introducing synthetic VH and VL cDNA fragments of the CD28.3 mAb independently into the EcoRV sites of two pFusehIgG1e2fc2 eukaryotic expression plasmids (Cayla Invivogen). An Fv-IgG4 Fc construct was also generated from the CD28.3 mAb by synthesizing VH-γ1hinge- γ4CH2-CH3 and VL- γ1hinge- γ4CH2-CH3 cDNAs (NCBI accession #K01316.1) inserted sequentially into the pCIneo plasmid (Promega). MF280 was secreted into the supernatant of Cos cells after co-transfection with VH-Fc and VL-Fc plasmids and purified by cation exchange chromatography on HiTrap SP XL columns (GE Healthcare). MF280-G4 was secreted into the supernatant of cloned CHO cells stably transfected with the MF280-G4-pCIneo plasmid and purified by anion exchange chromatography on Mustang Q Coins (Pall). Details and nucleotide sequences for the expression of MF280 can be found in the European patent application #20110313135.
Enzyme-linked immunosorbent assay
Recombinant human CD28-Fc (R&D Systems) was used at 2 µg/ml in carbonate buffer (pH 9.2) to coat 96-well microtiter plates (Maxisorp Nunc, VWR) overnight at 4°C. Reactive sites were blocked with 1% bovine serum albumin (BSA; Sigma Aldrich) in 0.1% Tween-PBS for 2 h at 37°C. Anti-CD28 antibody samples were incubated for 2 h at 37°C and detected by polyclonal rabbit anti-scFv28 antibody (Effimune) at 0.25 µg/ml plus peroxidase-labeled goat anti-rabbit antibodies, revealed by colorimetry using TMB substrate and read at 450nm.
Biosensor affinity measurement
CD28-Fc recombinant protein (R&D Systems) was immobilized onto a CM5 sensor chip (GE Healthcare). Analysis was performed with a BIAcore 2000 (Biacore, GE Healthcare) by the BiogenOuest IMPACT academic platform. Antibodies were applied at concentrations ranging from 3.9 to 500 nM with a flow rate of 40 µl/min. An association period of 4 min. was followed by a dissociation period of 10 min. The binding constant was analyzed using the BIAeval4.1 software (Biacore, GE Healthcare).
Flow cytometry
CD28+ Jurkat T cells and CD28- Raji B cells were incubated with anti-hCD28 antibodies for 30 min at 4°C and revealed with polyclonal rabbit anti-scFv28 antibodies (Effimune) plus polyclonal PE-donkey anti-rabbit antibodies (Jackson ImmunoResearch).
Cell-based assays
To assess IL-2 production by T cells, we stimulated 105 Jurkat T cells with 2.10Citation4 Raji B cells in microtiter plates in the presence of staphylococcus enterotoxin E (5 ng/ml) for 48 h at 37°C and 5% CO2 in complete medium (RPMI-1640 medium supplemented with L-glutamine, sodium pyruvate, Hepes, antibiotics and 10% heat-inactivated FCS, all from Gibco, Invitrogen). IL-2 secretion was evaluated in the supernatant with the Max Set Deluxe Human IL-2 Elisa Kit (Biolegend).
Mixed lymphocyte reactions were performed with PBMCs from healthy donors incubated with allogeneic irradiated PBMCs (105 cells per well of each cell type) and anti-human CD28 mAbs for 5 d at 37°C and 5% CO2 in complete medium.
Proliferation assays were performed using human PBMCs (105 cells per well) in microtiter plates coated with 1 µg/ml anti-CD3 antibodies (OKT3) and stimulated with soluble anti-CD28 mAbs for 3 d. After 24 h, 50 µl supernatant was collected to measure IFNγ and TNFα concentration with Human IFNγ and TNFα Cytokine Elisa Kits (BD Biosciences). Cells were pulsed with 1 µCi of [3H] thymidine during the final 8 h of culture and evaluated in a scintillation counter.
Capping and immunofluorescence
Jurkat T cells (3.10Citation5 cells) were incubated at 37°C for 1 h with 20µg/ml of anti-CD28 mAbs in complete culture medium. Alternatively, cells were incubated with the same antibodies at 0°C in complete culture medium containing 0.01% sodium azide. After washing, cells were fixed in 0.5% paraformaldehyde, centrifuged onto glass slides, immunolabeled and examined by epifluorescence.
Western blotting
CD28 signaling pathways were studied from the lysates of Jurkat T cells stimulated for 5min at 37°C with OKT3 antibodies immobilized on plastic at 1µg/ml in PBS plus 10 µg/ml soluble anti-human CD28 antibodies. Western blots were revealed with Phospho-PI3K p85, PI3 kinase p85, Phospho-Akt and Akt antibodies (Cell Signaling Technology) at 1/1000 in 1% BSA-TBS (overnight at 4°C) followed by polyclonal goat anti-rabbit horseradish peroxidase-labeled antibody (Cell Signaling Technology) at 1/1000 for 1.5 h at room temperature, or with GAPDH antibody (Santa Cruz) followed by polyclonal goat anti-mouse horseradish peroxidase-labeled antibody (Jackson ImmunoResearch). Membranes were revealed by chemiluminescence using the LAS-3000 imaging system (Fujifilm).
Animals and treatments
Pharmacokinetics studies of anti-CD28 antibodies were performed on 6-week-old female Swiss mice injected with 4 mg/kg into the right tail vein. Blood samples were collected at different times (0, 0.5, 2, 4, 8, 12, 24 h and then daily) from the left tail vein and ELISA was used to measure antibody concentration in mouse sera. The data were analyzed by Siphar software (Simed) with the use of a 2-compartment model. Experiments performed in mice complied with the recommendations of the Institutional Ethical Guidelines of the Institut National de la Santé Et de la Recherche Médicale.
Statistical analyses
Variables were expressed as mean ± SEM and were compared when appropriated using the ANOVA nonparametric test. p values less than 0.05 were considered statistically significant. All statistical analyses were performed with GraphPad Prism (GraphPad Software).
Acknowledgments
The authors thank Prof. Hervé Watier and Prof. Gilles Paintaud, (Université Paul Sabatier, Tours, France) for fruitful discussions during the conception of MF280 and for assistance in the interpretation of our pharmacokinetic data, and thank Mike Maillasson (BiogenOuest IMPACT academic platform, Nantes, France) for the Biosensor analyses. The authors received funding from the Fondation de la Recherche Médicale (FRM grant #DIM20081013832; Paris, France), the Agence Nationale de la Recherche (ANR grant #09-BIOT-013-02; Paris, France) and the European project TRIAD (EU-FP7-Health program EC-GA N◦281493 ; www.TRIAD-CD28.eu). This work was also supported by the Progreffe and Centaure foundations (France).
Disclosure of Potential Conflicts of Interest
M.C., N.P., N.D. and B.V. have conflicts of interest to disclose as described by mAbs. B.V. is a shareholder in Effimune, a company developing CD28 antagonists. C.M., N.P. and N.D. are an employee of Effimune. F.C., B.M. and G.B. have no conflicts of interest to disclose.
References
- Poirier N, Blancho G, Vanhove B. A more selective costimulatory blockade of the CD28-B7 pathway. Transpl Int 2011; 24:2 - 11; http://dx.doi.org/10.1111/j.1432-2277.2010.01176.x; PMID: 20964725
- Nogid A, Pham DQ. Role of abatacept in the management of rheumatoid arthritis. Clin Ther 2006; 28:1764 - 78; http://dx.doi.org/10.1016/j.clinthera.2006.11.020; PMID: 17212998
- Vincenti F, Larsen CP, Alberu J, Bresnahan B, Garcia VD, Kothari J, et al. Three-year outcomes from BENEFIT, a randomized, active-controlled, parallel-group study in adult kidney transplant recipients. Am J Transplant 2012; 12:210 - 7; http://dx.doi.org/10.1111/j.1600-6143.2011.03785.x; PMID: 21992533
- Poirier N, Blancho G, Vanhove B. CD28-specific immunomodulating antibodies: what can be learned from experimental models?. Am J Transplant 2012; 12:1682 - 90; http://dx.doi.org/10.1111/j.1600-6143.2012.04032.x; PMID: 22471377
- Yao S, Zhu Y, Zhu G, Augustine M, Zheng L, Goode DJ, et al. B7-h2 is a costimulatory ligand for CD28 in human. Immunity 2011; 34:729 - 40; http://dx.doi.org/10.1016/j.immuni.2011.03.014; PMID: 21530327
- Butte MJ, Keir ME, Phamduy TB, Sharpe AH, Freeman GJ. Programmed death-1 ligand 1 interacts specifically with the B7-1 costimulatory molecule to inhibit T cell responses. Immunity 2007; 27:111 - 22; http://dx.doi.org/10.1016/j.immuni.2007.05.016; PMID: 17629517
- Haspot F, Séveno C, Dugast AS, Coulon F, Renaudin K, Usal C, et al. Anti-CD28 antibody-induced kidney allograft tolerance related to tryptophan degradation and TCR class II B7 regulatory cells. Am J Transplant 2005; 5:2339 - 48; http://dx.doi.org/10.1111/j.1600-6143.2005.01018.x; PMID: 16162181
- Raychaudhuri SP, Kundu-Raychaudhuri S, Tamura K, Masunaga T, Kubo K, Hanaoka K, et al. FR255734, a humanized, Fc-Silent, Anti-CD28 antibody, improves psoriasis in the SCID mouse-psoriasis xenograft model. J Invest Dermatol 2008; 128:1969 - 76; http://dx.doi.org/10.1038/jid.2008.38; PMID: 18337836
- Perrin PJ, June CH, Maldonado JH, Ratts RB, Racke MK. Blockade of CD28 during in vitro activation of encephalitogenic T cells or after disease onset ameliorates experimental autoimmune encephalomyelitis. J Immunol 1999; 163:1704 - 10; PMID: 10415078
- Zhang T, Fresnay S, Welty E, Sangrampurkar N, Rybak E, Zhou H, et al. Selective CD28 blockade attenuates acute and chronic rejection of murine cardiac allografts in a CTLA-4-dependent manner. Am J Transplant 2011; 11:1599 - 609; http://dx.doi.org/10.1111/j.1600-6143.2011.03624.x; PMID: 21749640
- Poirier N, Azimzadeh AM, Zhang T, Dilek N, Mary C, Nguyen B, et al. Inducing CTLA-4-dependent immune regulation by selective CD28 blockade promotes regulatory T cells in organ transplantation. Sci Transl Med 2010; 2:17ra10; http://dx.doi.org/10.1126/scitranslmed.3000116; PMID: 20371478
- Lühder F, Huang Y, Dennehy KM, Guntermann C, Müller I, Winkler E, et al. Topological requirements and signaling properties of T cell-activating, anti-CD28 antibody superagonists. J Exp Med 2003; 197:955 - 66; http://dx.doi.org/10.1084/jem.20021024; PMID: 12707299
- Arad G, Levy R, Nasie I, Hillman D, Rotfogel Z, Barash U, et al. Binding of superantigen toxins into the CD28 homodimer interface is essential for induction of cytokine genes that mediate lethal shock. PLoS Biol 2011; 9:e1001149; http://dx.doi.org/10.1371/journal.pbio.1001149; PMID: 21931534
- Suntharalingam G, Perry MR, Ward S, Brett SJ, Castello-Cortes A, Brunner MD, et al. Cytokine storm in a phase 1 trial of the anti-CD28 monoclonal antibody TGN1412. N Engl J Med 2006; 355:1018 - 28; http://dx.doi.org/10.1056/NEJMoa063842; PMID: 16908486
- Vanhove B, Laflamme G, Coulon F, Mougin M, Vusio P, Haspot F, et al. Selective blockade of CD28 and not CTLA-4 with a single-chain Fv-alpha1-antitrypsin fusion antibody. Blood 2003; 102:564 - 70; http://dx.doi.org/10.1182/blood-2002-08-2480; PMID: 12649149
- Shiao SL, McNiff JM, Masunaga T, Tamura K, Kubo K, Pober JS. Immunomodulatory properties of FK734, a humanized anti-CD28 monoclonal antibody with agonistic and antagonistic activities. Transplantation 2007; 83:304 - 13; http://dx.doi.org/10.1097/01.tp.0000251426.46312.d5; PMID: 17297405
- Labrijn AF, Buijsse AO, van den Bremer ET, Verwilligen AY, Bleeker WK, Thorpe SJ, et al. Therapeutic IgG4 antibodies engage in Fab-arm exchange with endogenous human IgG4 in vivo. Nat Biotechnol 2009; 27:767 - 71; http://dx.doi.org/10.1038/nbt.1553; PMID: 19620983
- Liu YD, Chen X, Enk JZ, Plant M, Dillon TM, Flynn GC. Human IgG2 antibody disulfide rearrangement in vivo. J Biol Chem 2008; 283:29266 - 72; http://dx.doi.org/10.1074/jbc.M804787200; PMID: 18713741
- Yoo EM, Wims LA, Chan LA, Morrison SL. Human IgG2 can form covalent dimers. J Immunol 2003; 170:3134 - 8; PMID: 12626570
- Chapman AP, Antoniw P, Spitali M, West S, Stephens S, King DJ. Therapeutic antibody fragments with prolonged in vivo half-lives. Nat Biotechnol 1999; 17:780 - 3; http://dx.doi.org/10.1038/11717; PMID: 10429243
- Yu XZ, Bidwell SJ, Martin PJ, Anasetti C. CD28-specific antibody prevents graft-versus-host disease in mice. J Immunol 2000; 164:4564 - 8; PMID: 10779758
- Silver PB, Hathcock KS, Chan CC, Wiggert B, Caspi RR. Blockade of costimulation through B7/CD28 inhibits experimental autoimmune uveoretinitis, but does not induce long-term tolerance. J Immunol 2000; 165:5041 - 7; PMID: 11046033
- Dengler TJ, Szabo G, Sido B, Nottmeyer W, Zimmerman R, Vahl CF, et al. Prolonged allograft survival but no tolerance induction by modulating CD28 antibody JJ319 after high-responder rat heart transplantation. Transplantation 1999; 67:392 - 8; http://dx.doi.org/10.1097/00007890-199902150-00009; PMID: 10030284
- Guillonneau C, Séveno C, Dugast AS, Li XL, Renaudin K, Haspot F, et al. Anti-CD28 antibodies modify regulatory mechanisms and reinforce tolerance in CD40Ig-treated heart allograft recipients. J Immunol 2007; 179:8164 - 71; PMID: 18056359
- Haspot F, Villemain F, Laflamme G, Coulon F, Olive D, Tiollier J, et al. Differential effect of CD28 versus B7 blockade on direct pathway of allorecognition and self-restricted responses. Blood 2002; 99:2228 - 34; http://dx.doi.org/10.1182/blood.V99.6.2228; PMID: 11877302
- Poirier N, Mary C, Dilek N, Hervouet J, Minault D, Blancho G, et al. Preclinical efficacy and immunological safety of FR104, an antagonist anti-CD28 monovalent Fab' antibody. Am J Transplant 2012; 12:2630 - 40; http://dx.doi.org/10.1111/j.1600-6143.2012.04164.x; PMID: 22759318
- Jang MS, Pan F, Erickson LM, Fisniku O, Crews G, Wynn C, et al. A blocking anti-CD28-specific antibody induces long-term heart allograft survival by suppression of the PKC theta-JNK signal pathway. Transplantation 2008; 85:1051 - 5; http://dx.doi.org/10.1097/TP.0b013e31816846f6; PMID: 18408588
- Clark M, Bindon C, Dyer M, Friend P, Hale G, Cobbold S, et al. The improved lytic function and in vivo efficacy of monovalent monoclonal CD3 antibodies. Eur J Immunol 1989; 19:381 - 8; http://dx.doi.org/10.1002/eji.1830190224; PMID: 2522881
- Poirier N, Blancho G, Vanhove B. Alternatives to calcineurin inhibition in renal transplantation: belatacept, the first co-stimulation blocker. Immunotherapy 2010; 2:625 - 36; http://dx.doi.org/10.2217/imt.10.57; PMID: 20874646
- Smith BJ, Popplewell A, Athwal D, Chapman AP, Heywood S, West SM, et al. Prolonged in vivo residence times of antibody fragments associated with albumin. Bioconjug Chem 2001; 12:750 - 6; http://dx.doi.org/10.1021/bc010003g; PMID: 11562193
- Andres PG, Howland KC, Dresnek D, Edmondson S, Abbas AK, Krummel MF. CD28 signals in the immature immunological synapse. J Immunol 2004; 172:5880 - 6; PMID: 15128767