Abstract
The use of antibody-drug conjugates (ADCs) as a therapeutic platform to treat cancer has recently gained substantial momentum. This therapeutic modality has the potential to increase the efficacy and reduce the systemic toxicity associated with current therapeutic regimens. The efficacy of ADCs, however, relies on the proper exploitation of intracellular sorting dynamics of the antigen as well as the specificity, selectivity and pharmacokinetic properties of the antibody itself. Our understanding of endocytosis and endosomal trafficking of receptors has appreciably increased in recent years, as improvements in the assays used to study these events have resolved many of the molecular mechanisms regulating these processes. As a result, we now have the knowledge necessary to exploit these pathways efficiently to improve the efficacy of antibody-based therapy. This review discusses some recent studies that have explored how endo/lysosomal dynamics can affect the efficacy of engineered therapeutic antibodies, including ADCs.
Introduction
Cells continuously internalize their surface receptors through receptor-mediated endocytosis. When these internalized receptors incorporate into endosomes, they are trafficked throughout a complex array of recycling or degradative pathways. Since the discovery of this process, there has been a great deal of emphasis put on identifying ways to efficiently harness receptor-mediated endocytosis as part of a therapeutic strategy through the use of engineered antibody conjugates and other biologic modalities. This idea has gained substantial momentum recently, as our knowledge of the endo/lysosomal system and our ability to engineer antibodies and select appropriate targets has increased. Monoclonal antibodies (mAbs) can achieve selective cytotoxic effects against tumors that overexpress a particular target. This result can be achieved through multiple mechanisms depending on the therapeutic platform used. The mainstay of cancer biologic therapies has concerned naked antibodies, but with advances in antibody engineering, antibodies conjugated to toxic payloads have become increasingly prevalent. Unconjugated mAbs (also referred to as ‘naked’) do not have toxic payloads attached to them. Typically, they can act through a number of different mechanisms including receptor downregulation, induction of apoptosis through inhibition of receptor-linked signaling pathways, antibody-dependent cell-mediated cytotoxicity or complement-dependent immunocytotoxicity.Citation1 Alternatively, conjugated mAbs utilize receptor internalization and act as a carrier to deliver the toxic payload to the cancer cell. The more recently developed ADCs require the successful delivery of the ADC to the lysosomal compartment for proper release of the toxic payload to the cell.Citation2 Accordingly, a more comprehensive understanding of the molecular mechanisms governing intracellular trafficking, the nuances involved in designing effective elements of the ADC, and the biological interactions that occur between an ADC and a tumor mass is needed for the successful development of efficacious ADCs. Here, we review recent studies which have explored the ways an antibody can be designed to exploit certain aspects of the endolysosomal system, how engineered antibodies interact with a tumor mass and the biological implications of the chemistry involved in the design of an ADC.
Receptor-Mediated Endocytosis and Intracellular Trafficking Dynamics
Molecules can be internalized from the surface of eukaryotic cells through a wide array of mechanisms. These include clathrin-independent mechanisms such as phagocytosis, macropinocytosis and caveolin-dependent endocytosis or clathrin-dependent mechanisms such as receptor-mediated endocytosis.Citation3,Citation4 Clathrin-dependent endocytosis is the best characterized and predominant mechanism for the internalization of cell surface receptors and thus provides an ADC with a cell specific entry mechanism.Citation3,Citation4 Clathrin-mediated endocytosis commences with the recruitment of adaptor proteins, accessory proteins and a clathrin polymeric lattice to phosphatidylinositol-4,5-bisphosphate-enriched plasma membrane regions.Citation5 The clathrin adaptors function to select the cargo proteins that will be internalized; the adaptor protein most commonly found to regulate receptor internalization is adaptor complex 2 (AP2), which binds to short linear tyrosine- and dileucine-based sequences on the cytoplasmic tails of receptors.Citation6 Once receptors are selected by adaptor proteins for internalization, clathrin moves from the cytoplasm to adaptor protein-enriched regions of the membrane; the subsequent polymerization of clathrin causes membrane displacement and the formation of the budding vesicle.Citation7 Liberation of the budding vesicle from the plasma membrane is mediated, in part, by the large GTPase, dynamin (Dyn). Dyn is recruited by Bin–Amphiphysin–Rvs domain-containing proteins, such as amphiphysin, endophilin and sorting nexin 9, which interact with Dyn’s proline-rich regions through SRC homology 3 domains.Citation8-Citation10 The precise mechanism of vesicle release is presently unclear, but Dyn undergoes a GTP hydrolysis-dependent conformational change that likely helps to mediate scission.Citation11-Citation13 Once individual vesicles are liberated from the plasma membrane, they fuse with each other in the cytoplasm and form the early endosome.
The endosome is an extraordinarily complex and compartmentalized system of proteins and lipids functioning in concert to regulate the intracellular distribution of internalized proteins (). Endosomes send cargo through two distinct pathways. The first is cargo recycling that can result in the trafficking of the receptor back to the plasma membrane.Citation14 The second pathway that internalized cargo can traverse is endolysosomal degradation. This route is entered when internalized cargo are retained in a maturing endosome (rather than being recycled back to the plasma membrane) until finally being delivered to the lysosome.
Figure 1. Intracellular trafficking through the endo/lysosomal system. The internalized cargo is initially contained within endocytotic vesicles which fuse together to become the early endosome. Cargo can be recycled directly back to the plasma membrane from the early endosome through the “short recycling loop” or retained within the maturing endosome. As the early endosome matures, cargo can be trafficked through the trans-golgi network for repackaging or trafficked back to the plasma membrane through the “long recycling loop.” As the early endosome matures to multivesicular bodies (MVB), the retained cargo is internalized into intraluminal vesicle (ILVs) formed within the fluid phase of the MVB and subsequently delivered to the lysosome. A biochemical event that occurs during the maturation of an early endosome to an MVB is the RAB5 to Rab7 switch. Although the precise role that the Rab5 to Rab7 switch is unknown, it serves as a reliable marker for the transition from an early endosome to a late endosome.
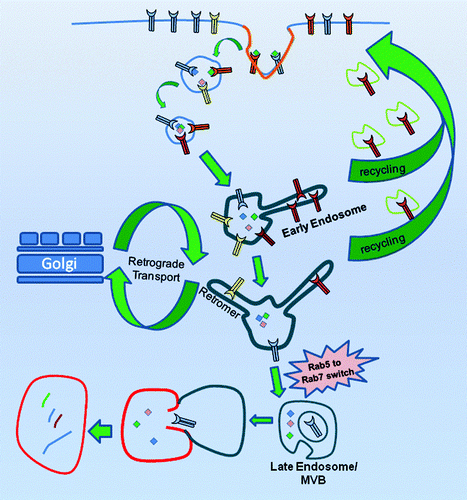
The early endosome (EE) is the organelle within a cell that receives the incoming endocytosed cargo and fluid from the plasma membrane.Citation15 EEs are mildly acidic (pH 5.9–6.8) and serve as the initial sorting stations for internalized cargo.Citation16-Citation18 While cargo can be recycled back to the plasma membrane directly from the EE through the “short recycling loop,” a discrete endosomal structure called the recycling endosome has been identified which recycles cargo through the “long recycling loop.”Citation19-Citation22 Additionally, cargo can be sent to the Golgi complex for re-packaging through the EE-associated retromer complex.Citation22
Before delivering its cargo to the lysosome, maturing EEs transition into late endosomes (LE), also known as multivesicular bodies (MVBs).Citation23 Endosomal maturation to LEs is characterized by an increase in luminal acidification, movement to the perinuclear space and the formation of intraluminal vesicles (ILVs), which are vesicles containing cargo proteins that bud off of the LE membrane inwardly into the LE lumen.Citation24,Citation25 Once ILVs are formed, the proteins within are delivered to the lumen of lysosomes where they are degraded.
Engineering Antibodies to Exploit the Endo/Lysosomal System
The ideal tumor target for an ADC has the following features: (1) the antigen is abundantly and exclusively expressed on the target cell; (2) it undergoes minimal secretion since secreted receptors can bind the antibody in the circulation, thus limiting exposure to the target cell; (3) it possesses an appropriate rate of endocytosis; and (4) it undergoes an appropriate intracellular trafficking route for the desired outcome. Recent advances in antibody engineering may allow investigators to design efficacious ADCs against targets that meet most of these criteria, but are otherwise unattractive because their particular intracellular trafficking characteristics are not conducive to efficient ADC delivery to the lysosome. Hence, there are now a few instances of antibodies have been engineered to possess high antigen binding affinity at a pH of 7.4 (extracellular pH) with an increased off-rate at a pH of 6.0 (endosomal pH). This approach ensures increased dissociation of the antibody from the receptor within the endosome so that it becomes an independent entity within the endosomal sorting systems. While these particular studies were done to increase the efficacy of naked antibodies, one may envisage that this mode of engineering could be utilized differently depending on the intracellular trafficking dynamics of the receptor being targeted and the particular antibody- based therapeutic platform used.
Receptors such as the transferrin receptor, LDL-receptor, the metabotropic glutamate receptor 5, integrin receptors and HER2 continuously recycle back to the plasma membrane immediately after cellular internalization.Citation26-Citation30 When developing an ADC against receptors such as these, one could plausibly attempt to engineer antibody dissociation within the endosome in an attempt to reduce the likelihood that the ADC will cycle back out of the cell with its receptor (). While this concept has not been demonstrated for ADCs, ligands such as tumor growth factor α and transferrin can indeed dissociate from their receptor within the endosome, avoid recycling out of the cell and traffic to the lysosome for degradation.Citation27,Citation31-Citation33 This mechanism would require the absence of the neonatal Fc receptor (FcRn) when using an ADC, as FcRn is a receptor present within the fluid phase of endosomes which can transport IgG out of the endosomes to the cell surface.Citation34
Figure 2. Possible Intracellular trafficking routes of Antibody-Drug Conjugates. If an ADC binds to a receptor which undergoes continual recycling back to the plasma membrane, there is the potential that the ADC may be recycled back to the plasma membrane without delivering the toxic payload, as depicted on the right trafficking route. In certain cell types, the presence of the FcRn can complicate this process, as FcRn binds IgGs and recycles them back to the plasma membrane; as depicted on the right trafficking route. However, in the absence of FcRn, dissociation of the ADC from its receptor within the endosome may lead to lysosomal trafficking of the ADC, where it can release its toxic payload to the cell, as depicted on the left trafficking route.
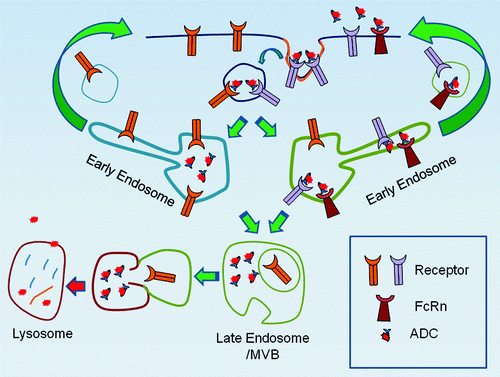
Alternatively, receptors such as epidermal growth factor receptor 1, interleukin-2β, β2- adrenergic receptor and E-cadherin are principally trafficked to the lysosome for degradation after internalization.Citation35-Citation38 Under these conditions, antibody dissociation from its receptor within the endosome would allow the antibody to escape lysosomal degradation by binding to FcRn and returning to the cell surface, effectively increasing the serum half-life of the particular mAb. Accordingly, these IgGs are rescued from degradation via FcRn and potentially re-bind their antigen at the cell surface.Citation39
Two recent studies employed a mutagenic strategy to engineer pH-dependent binding affinity to increase serum clearance half-life. The first study sought to modify tocilizumab (TCZ), a humanized IgG antibody targeting the interleukin-6 receptor (IL-6R); TCZ is marketed for the treatment of moderate to severe rheumatoid arthritis.Citation40 The IL-6R undergoes a high rate of membrane turnover, which results in a high rate of TCZ cellular clearance through lysosome mediated degradation and a consequential decrease in plasma elimination half-life. Consequently, the investigators of this study reduced the binding affinity of TCZ to IL-6R at the endosomal pH 6.0 without affecting binding affinity at the cell surface pH of 7.4. This approach allowed TCZ to dissociate from IL-6R in the endosome to become unlinked from the lysosomal degradation pathway that IL-6R enters and bind FcRn to recycle back to the cell surface.Citation41 To reengineer the binding affinity of TCZ at pH 6.0, the investigators utilized a histidine scanning approach.Citation41 This involves mutating critical amino acids within the antigen binding interface to histidine residues; histidine has a pKa of 6.0 and thus its presence within a binding interface can have a dramatic effect on the pH dependency of the binding affinity. One mutant, which contained Y27H and S31H mutations on the heavy chain and Y32H and R53H mutations on the light chain of the antibody (clone PH2), reduced IL-6R binding affinity by ~4-fold at pH 6.0 while actually increasing the affinity at pH 7.4.Citation41 However, increased dissociation from its target at pH 6.0 alone will not always save an antibody from lysosomal degradation, as retention within the fluid phase of the endosome will result in trafficking to the lysosome. Accordingly, when the pharmacokinetics of PH2 and TCZ were compared in mice that were administered a single intravenous dose of 25 mg/kg, there was no significant increase in plasma antibody concentration of PH2 over TCZ when tracked over a 35 d period.Citation41 As a result, PH2 was engineered to possess an increased binding affinity for FcRn at pH 6.0 (PH2-FcRn); this reduced lysosomal accumulation and successfully increased the serum half-life 4 fold for the antibody in vivo (1 week for PH2, 4 weeks for PH2-FcRn).Citation41
In a different study, investigators sought to re-engineer an antibody directed against proprotein convertase subtilisin kexin type 9 (PCSK9);Citation42 PCSK9 promotes the degradation of low density lipoprotein (LDL) receptor, thus increasing serum levels of LDL-cholesterol. Accordingly, antibodies targeted to PCSK9, such as J10 and the affinity matured J16, can be used to effectively lower LDL. Similar to the previous study discussed, the antibody studied, J16, undergoes significant lysosome mediated degradation.Citation42 Therefore, these investigators also sought to decrease J16 binding affinity at the endosomal pH of 6.0 without affecting its binding affinity at the plasma pH of 7.4. The investigators also utilized a histidine scanning approach to generate the J17 mutant antibody that contained histidine substitutions in complementarity determining region (CDR)-1 (S30H) and CDR2 (S50H) of the light chain and CDR2 (S52H) of the heavy chain.Citation42 Surface plasmon resonance studies revealed that J17 possesses a 9.2 fold lower binding affinity at pH 6.0 compared with pH 7.4; most of this pH-dependent effect comes from the difference in the rate of dissociation of the complex.Citation42
When the pharmacokinetics of J16 and J17 were compared in wild-type cynomolgus monkeys treated with a single intravenous injection of either J16 or J17 (1.5 mg/kg), there was a substantial increase in antibody plasma elimination half-life of J17 (t1/2: 7.4 d) over J16 (T1/2: 0.9 d).Citation42 Furthermore, when either J10 or J17 were injected into FcRn −/− mice, there was no difference in the serum half-life of either antibody, supporting the notion that FcRn plays a pivotal role in increasing the serum half-life of J17; no pharmacokinetic comparisons between J16 and J17 in FcRn −/− mice were reported in this study.Citation42 Confocal microscopy and colocalization analysis revealed that J17 co-localized with LAMP1 to an ~15% lower extent than J10, supporting the hypothesis that the escape from lysosomal degradation of J17 helped increase serum half-life; no direct comparison of the lysosomal colocalization characteristics of J16 and J17 was reported in this study.Citation42
Not surprisingly, histidine-regulated binding affinity is a concept found in nature; however, one of the documented studies of this characteristic relies on histidines placed both on the antibody and its receptor. Human prolactin (PRL), a member of the family of hematopoietic cytokines, binds to the PRL receptor (PRLR) in a pH-dependent manner; affinity of PRL for PRLR decreases 500-fold as the pH decreases from pH 8 to 6.Citation43,Citation44 To investigate the molecular mechanism by which this occurs, Kullkarni and colleagues sought to attribute pH dependence of PRL binding to the effect of individual His residues within the high affinity PRL-PRLR interface.Citation45 These histidines in PRL (His-27, His-30 and His-180), which are located within the high affinity binding interface, appear to act cooperatively in creating pH-dependent binding characteristics, as no single mutation drastically changed the pH affinity profile of PRL. This study revealed, however, that the pH dependent regulation of the PRL:PRLR interaction depends critically on His-188 in the PRLR.Citation45 Importantly, the binding of human growth hormone (a disparate and natural binding partner for PRLR) to the PRLR is pH-independent, demonstrating specificity for pH-dependency within the same receptor.Citation45 High resolution crystallographic structures of the binding interfaces of the PRLR in complex with the WT and all possible single-site His mutants revealed the importance of electrostatic forces in regulating the pH dependence rather than conformational effects.Citation45
Balancing Receptor Kinetics, Binding Affinity, Molecular Size and Tumor Penetration of Engineered Antibodies
Antibodies have also been reengineered to achieve increased antigen binding affinity at the cell surface. These particular modifications, in some circumstances, may prove to be obstructive because increasing binding affinity may adversely affect biology and intracellular targeting. Accordingly, high affinity driven by a fast on-rate and slow off rate may inhibit diffusion throughout the entire tumor mass after extravasation and lead to an accumulation of antibodies at perivascular regions of the tumor where they are internalized and catabolized by those cells.Citation46 Furthermore, the molecular size of the biological modality used and the internalization kinetics of the tumor antigen targeted can also drastically impact the degree of tumor penetration of an ADC. This concept is known as the “binding site barrier” effect and was proposed over two decades ago.Citation47-Citation49 The binding site barrier effect suggested that: (A) greater antigen density; (B) higher antibody affinity; and (C) faster antibody internalization and metabolism by cells would increase the “barrier” effect for antibodies binding to antigens on the tumor, limiting the number of available diffusible molecules available for penetration deeper into the tumor mass.Citation47,Citation49,Citation50 Thus, there is an appropriate range of antibody affinity and molecular size required to balance specificity, selectivity, pharmacokinetic properties, but also tumor accumulation and penetration.
Cell surface receptors exhibit a broad range of basal and antibody-induced internalization rates and this can have a dramatic effect on ADC efficacy. For example, when Ingle et al. compared the efficacy of ADCs targeting CD19 and CD21, which are cell surface receptors present on normal and tumorigenic B-cells, they found that CD21 does not appreciably internalize upon antibody binding, even when expressed at very high levels.Citation51 Furthermore, while CD19 did undergo endocytosis and efficiently internalized anti-CD19 ADCs, it only did so in the absence of CD21.Citation51 Hence, CD21 and CD19 form a complex on the surface of B cells where CD21 prevents the internalization of CD19.Citation51 The results of this study underscore the need to comprehensively understand the molecular mechanisms governing receptor endocytosis and intracellular trafficking properties of the respective target when designing an ADC.
The rate of receptor endocytosis can also affect ADC efficacy through modifications in tumor penetration. Ackerman et al. compared how a difference in the rate of receptor internalization affects the distance to which the antibodies M85151a and M111147 can penetrate a tumor spheroid.Citation52 M85151a and M111147 are antibodies which both bind to carcinoembryonic antigen (CEA), however they possess an ~3-fold difference in internalization rates (M85151a t1/2 is 5 h and M111147 t1/2 is 13 h) due to the fact that M85151a recognizes two epitopes and thus cross-links CEA, leading to more rapid rate of internalization.Citation52,Citation53 When LS174T spheroids were incubated with each antibody, the slower internalizing M111147 antibody penetrated much deeper within the spheroid tumor than did the more rapid internalizing M85151a antibody.Citation52 Thus, when developing an ADC, the target selection process must include a comprehensive analysis of receptor internalization kinetics and how the receptor internalization kinetics of the receptor will affect ADC delivery to the entire tumor mass.
Antibody affinity for the receptor also has an effect on tumor penetration. This notion is demonstrated by the differences in the tumor penetration efficacy of the anti-human epidermal growth factor receptor 2 (HER2) IgGs trastuzumab (Herceptin®) and C6.5 (in IgG format, C6.5-IgG). Although both C6.5 and trastuzumab bind to HER2, they do so at distinct sites and do not compete for binding.Citation54 Trastuzumab (KD of 0.1 × 10−10 M) can penetrate SK-OV-3 tumors xenografts to a distance ~20% less than C6.5-IgG (KD of 2.7 × 10−8 M),Citation46 suggesting that C6.5-IgG may be more effective in vivo, but published results of the direct comparison of the cancer cell toxicity between trastuzumab and C6.5-IgG were not found. Nevertheless, given that trastuzumab has a koff of 3 × 10−4 s−1 and C6.5 has a koff of 6 × 10−3 s−1, it is possible that a faster off rate allows C6.5 to penetrate solid tumors more than trastuzumab due to a diminished binding site barrier effect
To further explore how antibody binding affinity affects solid tumor accumulation and penetration, Rudnick and colleagues tested the solid tumor uptake and penetration characteristics of C6.5-IgG and three affinity altered mutant IgGs (G98A, ML39, H3B1).Citation46 These particular antibodies have intrinsic KD values ranging from 10−7 M to 10−10 M.Citation55 These affinity variants were generated by mutating the residues of C6.5 within the CDR regions located within the center of the antibody:antigen binding interface.Citation55 The substantial variations in KD values were primarily driven by large changes in the koff rate, as kon rates for the variants generated were not significantly different from the C6.5 wild-type antibody.Citation56 To determine the differences in tumor accumulation characteristics of these IgGs, 20 µg radioiodinated IgG of each variant was injected intravenously into SCID mice carrying subcutaneous SK-OV-3 (HER2 overexpressing, human ovarian carcinoma) tumor xenografts.Citation46 Twenty-four and 120 h after IgG injection, the mice were euthanized and the amount of radiolabeled IgG present within the tumor mass, the surrounding tissue and in circulation was determined by a gamma counter.Citation46 The results showed a correlation between binding affinity and tumor accumulation with the highest affinity IgG (MH3B1, KD 1 × 10−10 M) having the lowest tumor accumulation at both 24 and 120 h.Citation46 In order to examine how binding affinity affects the distance to which an IgG can penetrate a tumor after extravasation, 500 µg of unlabeled IgG was administered by intraperitoneal injection to SCID mice harboring established SK-OV-3 tumors and the distance that each IgG variant traveled from the blood vessels through the tumor was assessed by staining for HER2, tumor vasculature (CD31) and human IgG after the injection.Citation46 The results from this experiment confirmed that IgGs with a slower off rate failed to efficiently penetrate into the tumor.Citation46 Subsequent analyses revealed that the IgGs with slow off rates were internalized and catabolized by the perivascular tumor cells, and thus were eliminated before reaching other areas of the tumor.Citation46
When Adams et al. studied the tumor penetration characteristics of C6.5 and its affinity variants in single chain variable fragment (scFv) format rather than a full-length IgG, they found that increased binding affinity still correlated with a decrease in tumor accumulation and the ability to penetrate a tumor, although this characteristic was less pronounced in scFv format.Citation57 To assess the magnitude that these scFvs can accumulate within a tumor, 20 µg of radioiodinated scFv of each variant was injected into mice harboring SK-OV-3 human ovarian carcinoma xenografts.Citation57 Twenty four hours after scFv injection, the mice were sacrificed and the amount of radiolabeled scFv present in the tumors was determined by a gamma counter.Citation57 A KD of at least 1 × 10−7 M was required for any appreciable accumulation within the tumor.Citation57 While overall scFv accumulation within the tumor positively correlated with lower KD values in the range of 3 × 10−7 M to 1 × 10−9 M, tumor accumulation reached a plateau and no further scFv accumulation was observed with variants possessing a KD below 1 × 10−9 M.Citation57 Additionally, 100 µg of low affinity (3 × 10−7 M) or high affinity (1 × 10−11 M) scFv was injected into mice bearing SK-OV-3 tumor xenografts and the distance that each scFv variant traveled from the blood vessels through the tumor was assessed by staining for tumor vasculature (anti-CD31) and scFv (a rabbit polyclonal antibody specific for scFv molecules was used).Citation57 Twenty-four hours after injection, the high affinity scFv was present only in nearby blood vessels within the tumor mass while the low affinity scFv was dispersed throughout the vascularized regions of the tumor.Citation57 Thus, while the smaller anti-HER2 scFv molecules exhibited affinity-based restrictions in tumor accumulation and penetration, it was to a lesser degree than the larger IgG molecules.Citation57
Zahnd et al. came to a different conclusion when studying the correlation between binding affinity and overall tumor accumulation of the anti-HER2 designed ankyrin repeat proteins (DARPins).Citation58 DARPins are 14.5 kDa molecules (and therefore smaller than either the ~150 kDa IgG or ~25 kDa scFv) containing an NH2-terminal capping repeat, two internal repeats carrying the binding residues and a COOH-terminal capping repeat.Citation58 In this study, anti-HER2 DARPins with affinities ranging from 9 × 10−11 M to 2 × 10−7 M were labeled with 99mTc(CO)3 on the NH2-terminal His-tag and were intravenously injected (single dose of 8–10 µg DARPins) in mice bearing human ovarian carcinoma SK-OV-3 xenografts at the lateral flanks.Citation58 The mice were sacrificed at various time points over the course of three days after DARPin injection and the level of radioactivity retained within the tumor was measured in a gamma scintillation counter.Citation58 While these DARPins have a rapid serum elimination half-life (< 3 min), tumor accumulation was proportional to DARPin affinity for HER2, with the highest affinity DARPin (KD 9 × 10−11 M) accumulating the most within the tumor mass.Citation58 The tumor accumulation of DARPins modified by the addition of PEG20 was also analyzed in mice harboring human ovarian carcinoma SK-OV-3 xenografts at the lateral flanks.Citation58 While the PEGylated DARPins had an increased serum half-life (~19 h), the addition of PEG20 to the DARPins eliminated most of the correlation between binding affinity and tumor accumulation; with the exception of the lowest affinity anti-HER2 PEGylated DARPin (KD: 2 × 10−7 M), which had negligible tumor accumulation, there was little difference in the magnitude of accumulation of any other PEGylated anti-HER2 DARPins.Citation58 Thus, while there was a clear correlation between binding affinity and tumor accumulation of the smaller, unmodified DARPins, increasing the DARPIn hydrodynamic radius through PEGylation eliminated this correlation.
While it is somewhat difficult to reconcile these seemingly contradictory findings, given that all three studies utilized a biological moiety of different molecular size, it is plausible that there is a complex interplay between molecular weight, hydrodynamic radius and binding affinity that contribute to tumor accumulation and penetration characteristics. Thus, for much smaller molecules such as DARPins, which diffuse through a tumor easily by virtue of their size, an increased binding affinity may lead to increased tumor accumulation. However, as molecules increase in size and become inherently more difficult to diffuse through a tumor, an increased binding affinity influences this penetration restriction. Thus, because DARPins are nearly half the size of a scFv, perhaps there is a critical molecular weight somewhere between 15kDa and 25/30 kDa in which binding affinity begins to influence tumor penetration. Another explanation for these results may be that DARpins have a different epitope on HER2 compared with C6.5 and trastuzumab, which may lead to a different internalization rate and less catabolism. Future experiments aimed at addressing these questions may be revealing.
To better understand how both molecular size and binding affinity affect tumor accumulation, Schmidt and Wittrup developed a mathematical model and utilized data available in the literature to predict how binding affinity and molecular size influence tumor accumulation.Citation59 The model predicts that intermediate-sized proteins (~25 kDa) accumulate within tumors the least, whereas both smaller and larger biological moieties accumulate to a greater degree within a tumor.Citation59 IgGs are predicted to attain significantly higher levels of tumor accumulation due to both a slower serum clearance half-life and FcRn mediated retrieval from lysosomal degradation.Citation59 The model also predicted that smaller proteins require antigen binding affinities on the order of 10−9 M to 10−12 M to achieve significant tumor accumulation while larger proteins, such as an IgG can possess antigen binding affinity as low as 10−7 M and achieve substantial tumor accumulation.Citation59
Antibody Conjugation: Implications for Linker Chemistry and the Conjugate
Another factor that affects the efficacy of an ADC is the efficient release of the drug from the antibody into the cell cytosol. This is achieved through the disruption of the chemical linkage between the antibody and the toxic payload. Thus, the choice of the linker used may have an impact on ADC efficacy and several different types have been synthesized. Acid-labile hydrazone linkers are designed to be stable within the neutral pH extracellular environment, but become cleaved within the low pH environment of intracellular endosome and lysosome compartments.Citation60 Peptide linkers, such as citruline-valine, are designed to be selectively cleaved by lysosomal proteases (e.g., cathepsin or plasmin).Citation61 Another linker that has been developed is disulfide-based which is selectively cleaved in the reductive environment of the cell cytosol.Citation61 Non-cleavable linkers, such as thioether or amide bonds, have been utilized more recently and are intended to retain stability throughout the plasma and most of the intracellular space. Thus, when using a non-cleavable linker, liberation of the payload relies on the degradation of the antibody within the lysosome, however it is important to note that thioether linkers can be less stable in the plasma due to thiol exchange reactions.Citation62 ADCs with cleavable disulfide linkers, such as the disulfide linker N-succinimydyl 4-(2-pyridyldithio)-pentanoate (SPP), can form lipophilic drug metabolites within the lysosome which possess the ability to exit the target cell and re-enter neighboring cells which may lead to improved efficacy through bystander killing of neighboring tumor cells, but can also cause off-target toxicity.Citation60,Citation63 Alternatively, ADCs possessing a thioether linker, such as succinimidyl-4-(N-maleimidomethyl) cyclohexane-1-carboxylate (SMCC), form charged drug metabolites within the lysosome that cannot freely diffuse across the plasma membrane of a neighboring cell.
The efficacy of trastuzumab linked to the maytansinoid DM1 by a reducible disulfide linker (T-SPP-DM1) or a non-cleavable thioether linker (T-MCC-DM1) has been compared by multiple groups. In the first study, mice bearing MMTV-HER2 Fo5 mammary tumor transplants were treated with a single intravenous injection (10 mg/kg) of T-SPP-DM1, T-MCC-DM1 or vehicle and tumor volume was monitored for 21 d.Citation64 While both T-SPP-DM1 and T-MCC-DM1 delayed tumor growth compared with mice treated with vehicle, MCC-DM1 was significantly better at inhibiting the tumor growth; tumor volume reached a size of ~800 mm3 by day 10 in vehicle treated mice, tumor volume reached a size of ~900 mm3 by day 21 in T-SPP-DM1 treated mice, and tumor volume reached a size of ~500 mm3 by day 21 in T-MCC-DM1 treated mice.Citation65 T-MCC-DMI was also better tolerated by female Sprague-Dawley rats in these studies; rats treated with single doses of either 25 or 50 mg/kg T-MCC-DM1 showed no appreciable weight loss over a 5 d period, while rats treated with a single dose of 25 mg/kg T-SPP-DM1 exhibited a 10% reduction in total body weight by day 5.Citation65 The efficacy differences observed between T-SPP-DM1 and T-MCC-DM1 may have been caused by either inefficient reduction of disulfide bond linking SPP to the antibody and hence release of the payload to the cell or by the formation of lipophilic metabolites and the subsequent exit of the linker/payload from the tumor cell. The latter explanation could be supported by evidence from the study showing weight loss in mice treated with T-SPP-DM1 which is indicative of off-target toxicity.
A subsequent study by Erickson et al. reported no statistical difference between the in vivo efficacy of T-SPP-DM1 and T-MCC-DM1 against BT-474EEI tumors.Citation66 In this study, nude XID mice harboring BT-474EEI xenografts on their mammary fat pads were treated with a single intravenous injection of 3–18 mg/kg of T-SPP-DM1 or T-MCC-DM1. Over a 38 d period, the tumor volume was reduced in similar proportions after either T-SPP-DM1 or T-MCC-DM1 treatment.Citation66 The reasons for the disparate results of these two studies may lie within the inherent biological differences that exist between the two tumors targeted. For example, differences in HER2 densities, endo/lysosomal dynamics and other biological differences present within the two tumors may have drastic effects on the efficacy of a given ADC.
Summary and Perspectives
Our current understanding of the nuances involved in ADC design has led to some success within the clinic. As of early October 2012, there were at least 22 ADCs in clinical evaluationCitation67 and one ADC, brentuximab vedotin is currently approved by the FDA and marketed for treatment in anaplastic large cell lymphoma and Hodgkin lymphoma.Citation68,Citation69 Brentuximab vedotin is an anti-CD30 mAb conjugated to monomethyl auristatin E (MMAE, a synthetic analog of the tubulin polymerization inhibitor dolastatin 10) through a peptide valine-citrulline peptide linker.Citation64 Trastuzumab emtansine (T-DM1), composed of trastuzumab linked to the cytotoxin maytansinoid DM1 (DM1) via a thioether linker, is undergoing evaluation in multiple Phase 3 trials for HER2-positive metastatic breast cancer and a marketing application for T-DM1 was submitted to the Food and Drug Administration in August 2012.Citation70 Preliminary results from a Phase 3, randomized, multicenter, international, two-arm, open-label clinical trial comparing the safety and efficacy of T-DM1 and the current standard of care capecitabine + lapatinib (XL) in patients harboring HER2-positive advanced metastatic breast cancer was recently published.Citation71 The primary analysis of the data from the study showed that T-DM1 treatment is well tolerated and can provide a progression free survival of 9.4 mo compared with the 5.8 mo achieved with XL treatment.Citation71 Gemtuzumab ozogamicin (Mylotarg®) was approved by the FDA in 2000 for use in patients over 60 y of age suffering from relapsed acute myelocytic leukemia.Citation72 Gemtuzumab ozogamicin consists of a humanized anti-CD33 antibody linked to N-acetyl-γ-calicheamicin (an enediyne antibiotic that causes double-strand breaks of DNA resulting in apoptosis) through a bifunctional, hydrazone-based linker which is conjugated to lysine residues on the CD33 antibody.Citation73 In June 2010, gemtuzumab ozogamicin was voluntarily withdrawn from the US market due to safety and efficacy concerns that developed during a randomized Phase 3 comparative clinical trial performed after accelerated approval.Citation74 This outcome highlights the need for a better understanding of all facets involved in ADC development.
The efficacy of an ADC relies, in part, on the interactions of the antibody component with elements of the extracellular environment. It is clear that antigen binding affinity and molecular size can affect tumor penetration, and a balance between molecular size and binding affinity is crucial to maximizing efficacy. Furthermore, selection of a proper tumor antigen should involve analyses of not only cell specificity and copy number, but also the internalization kinetics of the receptor. However, we still lack the fundamental knowledge of how molecular size, binding affinity and internalization kinetics of the receptor correlate to differences in overall ADC cell kill potency. There is thus a need for additional functional readouts for correlation analyses such as tumor cell toxicity studies, analyses of changes in cell surface expression of the target receptor and analysis of how downstream signaling cascades of the target receptor are modulated by the antibody. This information will provide a more comprehensive understanding of how to properly balance the selection of the target antigen with the molecular size and binding affinity of the ADC.
The linker-payload choice is also a critical element in determining successful delivery of the payload to the cellular cytosol of a target cell. We now know that different linker types can themselves lead to differences in both the efficacy and the level of off-target toxicity observed for a given ADC. These facts highlight the importance of linker selection and provide the basis for future studies aimed at defining the precise behavior of these different linkers in the intracellular and extracellular environment. Studies aimed at assessing quantitatively the sub-cellular compartment in which the linker is liberated from the antibody and the kinetics of that liberation will be critical to furthering our understanding of how linker choice influences efficacy in different situations. Quantitative data on how stable different linkers are in the extracellular space and how this stability impacts off-target toxicity is also needed.
Progress in the development of ADCs depends additionally on a better understanding of how the antibody component of an ADC interacts and traffics within the intracellular space of a target cell. Recent studies have shown that antibody engineering can be used to create antibodies that exploit the endosomal pathway and this may have a substantial impact in the field of ADCs in the future. Numerous cellular contexts will need to be considered, e.g., whether histidines within the binding interface of the receptor changes the antibody affinity in different pH environments; whether the target is a recycling receptor or a lysosomal-targeted receptor; whether the cell expresses FcRn and whether this will effect trafficking of an IgG back to the cell surface. To effectively exploit the endo/lysosomal pathways to the fullest extent for antibody-based therapy, we need to understand why antibodies follow a particular intracellular trafficking route. Therefore, identifying the precise molecular mechanisms mediating endosomal sorting should remain a priority. Importantly, we need to resolve the precise molecular mechanisms mediating how endosomes distinguish whether a receptor undergoes recycling vs. endo-lysosomal degradation. This should also be assessed under different cellular contexts, including in different cell types, at different stages of the cell cycle and during differential ligand activation of the same receptor. As experimental assays improve and enable understanding of intracellular processes in greater detail, the results may resolve current discrepancies and increase our ability to efficiently select novel and appropriate targets for ADCs.
| Abbreviations: | ||
| ADC | = | antibody drug conjugate |
| EE | = | early endosome |
| RE | = | recycling endosome |
| LE | = | late endosome |
| MVB | = | multivesicular bodies |
| ILV | = | intraluminal vesicle |
| FcRn | = | neonatal Fc Receptor |
| IgG | = | Immunoglobulin G |
| TCZ | = | tocilizumab |
| IL6-R | = | interleukin 6 receptor |
| PCSK9 | = | proprotein convertase subtilisin kexin type 9 |
| LDL | = | low density lipoprotein |
| CDR | = | complementarity-determining region |
| PRL | = | human prolactin protein |
| PRLR | = | human prolactin protein receptor |
| HER2 | = | human epidermal growth factor receptor 2 |
| EGFR | = | epidermal growth factor receptor |
| ScFv | = | single chain variable fragment |
| CEA | = | carcinoembryonic antigen |
| Dyn | = | dynmin |
References
- Adams GP, Weiner LM. Monoclonal antibody therapy of cancer. Nat Biotechnol 2005; 23:1147 - 57; http://dx.doi.org/10.1038/nbt1137; PMID: 16151408
- Beck A, Haeuw JF, Wurch T, Goetsch L, Bailly C, Corvaïa N. The next generation of antibody-drug conjugates comes of age. Discov Med 2010; 10:329 - 39; PMID: 21034674
- Mayor S, Pagano RE. Pathways of clathrin-independent endocytosis. Nat Rev Mol Cell Biol 2007; 8:603 - 12; http://dx.doi.org/10.1038/nrm2216; PMID: 17609668
- Le Roy C, Wrana JL. Clathrin- and non-clathrin-mediated endocytic regulation of cell signalling. Nat Rev Mol Cell Biol 2005; 6:112 - 26; http://dx.doi.org/10.1038/nrm1571; PMID: 15687999
- Höning S, Ricotta D, Krauss M, Späte K, Spolaore B, Motley A, et al. Phosphatidylinositol-(4,5)-bisphosphate regulates sorting signal recognition by the clathrin-associated adaptor complex AP2. Mol Cell 2005; 18:519 - 31; http://dx.doi.org/10.1016/j.molcel.2005.04.019; PMID: 15916959
- Owen DJ, Evans PR. A structural explanation for the recognition of tyrosine-based endocytotic signals. Science 1998; 282:1327 - 32; http://dx.doi.org/10.1126/science.282.5392.1327; PMID: 9812899
- McMahon HT, Boucrot E. Molecular mechanism and physiological functions of clathrin-mediated endocytosis. Nat Rev Mol Cell Biol 2011; 12:517 - 33; http://dx.doi.org/10.1038/nrm3151; PMID: 21779028
- Wigge P, Köhler K, Vallis Y, Doyle CA, Owen D, Hunt SP, et al. Amphiphysin heterodimers: potential role in clathrin-mediated endocytosis. Mol Biol Cell 1997; 8:2003 - 15; PMID: 9348539
- Ferguson SM, Raimondi A, Paradise S, Shen H, Mesaki K, Ferguson A, et al. Coordinated actions of actin and BAR proteins upstream of dynamin at endocytic clathrin-coated pits. Dev Cell 2009; 17:811 - 22; http://dx.doi.org/10.1016/j.devcel.2009.11.005; PMID: 20059951
- Sundborger A, Soderblom C, Vorontsova O, Evergren E, Hinshaw JE, Shupliakov O. An endophilin-dynamin complex promotes budding of clathrin-coated vesicles during synaptic vesicle recycling. J Cell Sci 2011; 124:133 - 43; http://dx.doi.org/10.1242/jcs.072686; PMID: 21172823
- Roux A, Uyhazi K, Frost A, De Camilli P. GTP-dependent twisting of dynamin implicates constriction and tension in membrane fission. Nature 2006; 441:528 - 31; http://dx.doi.org/10.1038/nature04718; PMID: 16648839
- Stowell MH, Marks B, Wigge P, McMahon HT. Nucleotide-dependent conformational changes in dynamin: evidence for a mechanochemical molecular spring. Nat Cell Biol 1999; 1:27 - 32; http://dx.doi.org/10.1038/8997; PMID: 10559860
- Sweitzer SM, Hinshaw JE. Dynamin undergoes a GTP-dependent conformational change causing vesiculation. Cell 1998; 93:1021 - 9; http://dx.doi.org/10.1016/S0092-8674(00)81207-6; PMID: 9635431
- Stenmark H. Rab GTPases as coordinators of vesicle traffic. Nat Rev Mol Cell Biol 2009; 10:513 - 25; http://dx.doi.org/10.1038/nrm2728; PMID: 19603039
- Helenius A, Marsh M. Endocytosis of enveloped animal viruses. Ciba Found Symp 1982; •••:59 - 76; PMID: 6129957
- van Weering JR, Verkade P, Cullen PJ. SNX-BAR-mediated endosome tubulation is co-ordinated with endosome maturation. Traffic 2012; 13:94 - 107; http://dx.doi.org/10.1111/j.1600-0854.2011.01297.x; PMID: 21973056
- Yamashiro DJ, Maxfield FR. Acidification of morphologically distinct endosomes in mutant and wild-type Chinese hamster ovary cells. J Cell Biol 1987; 105:2723 - 33; http://dx.doi.org/10.1083/jcb.105.6.2723; PMID: 2447098
- Maxfield FR, McGraw TE. Endocytic recycling. Nat Rev Mol Cell Biol 2004; 5:121 - 32; http://dx.doi.org/10.1038/nrm1315; PMID: 15040445
- Choudhury A, Sharma DK, Marks DL, Pagano RE. Elevated endosomal cholesterol levels in Niemann-Pick cells inhibit rab4 and perturb membrane recycling. Mol Biol Cell 2004; 15:4500 - 11; http://dx.doi.org/10.1091/mbc.E04-05-0432; PMID: 15292453
- Radhakrishna H, Donaldson JG. ADP-ribosylation factor 6 regulates a novel plasma membrane recycling pathway. J Cell Biol 1997; 139:49 - 61; http://dx.doi.org/10.1083/jcb.139.1.49; PMID: 9314528
- Naslavsky N, Weigert R, Donaldson JG. Characterization of a nonclathrin endocytic pathway: membrane cargo and lipid requirements. Mol Biol Cell 2004; 15:3542 - 52; http://dx.doi.org/10.1091/mbc.E04-02-0151; PMID: 15146059
- Naslavsky N, Weigert R, Donaldson JG. Convergence of non-clathrin- and clathrin-derived endosomes involves Arf6 inactivation and changes in phosphoinositides. Mol Biol Cell 2003; 14:417 - 31; http://dx.doi.org/10.1091/mbc.02-04-0053; PMID: 12589044
- Huotari J, Helenius A. Endosome maturation. EMBO J 2011; 30:3481 - 500; http://dx.doi.org/10.1038/emboj.2011.286; PMID: 21878991
- Roederer M, Bowser R, Murphy RF. Kinetics and temperature dependence of exposure of endocytosed material to proteolytic enzymes and low pH: evidence for a maturation model for the formation of lysosomes. J Cell Physiol 1987; 131:200 - 9; http://dx.doi.org/10.1002/jcp.1041310209; PMID: 2438291
- Russell MR, Nickerson DP, Odorizzi G. Molecular mechanisms of late endosome morphology, identity and sorting. Curr Opin Cell Biol 2006; 18:422 - 8; http://dx.doi.org/10.1016/j.ceb.2006.06.002; PMID: 16781134
- Karin M, Mintz B. Receptor-mediated endocytosis of transferrin in developmentally totipotent mouse teratocarcinoma stem cells. J Biol Chem 1981; 256:3245 - 52; PMID: 6259157
- Dautry-Varsat A, Ciechanover A, Lodish HF. pH and the recycling of transferrin during receptor-mediated endocytosis. Proc Natl Acad Sci U S A 1983; 80:2258 - 62; http://dx.doi.org/10.1073/pnas.80.8.2258; PMID: 6300903
- Trivedi RR, Bhattacharyya S. Constitutive internalization and recycling of metabotropic glutamate receptor 5 (mGluR5). Biochem Biophys Res Commun 2012; 427:185 - 90; http://dx.doi.org/10.1016/j.bbrc.2012.09.040; PMID: 22995293
- Pellinen T, Ivaska J. Integrin traffic. J Cell Sci 2006; 119:3723 - 31; http://dx.doi.org/10.1242/jcs.03216; PMID: 16959902
- Harari D, Yarden Y. Molecular mechanisms underlying ErbB2/HER2 action in breast cancer. Oncogene 2000; 19:6102 - 14; http://dx.doi.org/10.1038/sj.onc.1203973; PMID: 11156523
- Harford J, Wolkoff AW, Ashwell G, Klausner RD. Monensin inhibits intracellular dissociation of asialoglycoproteins from their receptor. J Cell Biol 1983; 96:1824 - 8; http://dx.doi.org/10.1083/jcb.96.6.1824; PMID: 6304116
- Harford J, Bridges K, Ashwell G, Klausner RD. Intracellular dissociation of receptor-bound asialoglycoproteins in cultured hepatocytes. A pH-mediated nonlysosomal event. J Biol Chem 1983; 258:3191 - 7; PMID: 6298227
- Roepstorff K, Grandal MV, Henriksen L, Knudsen SL, Lerdrup M, Grøvdal L, et al. Differential effects of EGFR ligands on endocytic sorting of the receptor. Traffic 2009; 10:1115 - 27; http://dx.doi.org/10.1111/j.1600-0854.2009.00943.x; PMID: 19531065
- Ellinger I, Schwab M, Stefanescu A, Hunziker W, Fuchs R. IgG transport across trophoblast-derived BeWo cells: a model system to study IgG transport in the placenta. Eur J Immunol 1999; 29:733 - 44; http://dx.doi.org/10.1002/(SICI)1521-4141(199903)29:03<733::AID-IMMU733>3.0.CO;2-C; PMID: 10092075
- Rocca A, Lamaze C, Subtil A, Dautry-Varsat A. Involvement of the ubiquitin/proteasome system in sorting of the interleukin 2 receptor beta chain to late endocytic compartments. Mol Biol Cell 2001; 12:1293 - 301; PMID: 11359922
- Jones SM, Foreman SK, Shank BB, Kurten RC. EGF receptor downregulation depends on a trafficking motif in the distal tyrosine kinase domain. Am J Physiol Cell Physiol 2002; 282:C420 - 33; PMID: 11832327
- Shenoy SK, McDonald PH, Kohout TA, Lefkowitz RJ. Regulation of receptor fate by ubiquitination of activated beta 2-adrenergic receptor and beta-arrestin. Science 2001; 294:1307 - 13; http://dx.doi.org/10.1126/science.1063866; PMID: 11588219
- Fujita Y, Krause G, Scheffner M, Zechner D, Leddy HE, Behrens J, et al. Hakai, a c-Cbl-like protein, ubiquitinates and induces endocytosis of the E-cadherin complex. Nat Cell Biol 2002; 4:222 - 31; http://dx.doi.org/10.1038/ncb758; PMID: 11836526
- Ober RJ, Martinez C, Lai X, Zhou J, Ward ES. Exocytosis of IgG as mediated by the receptor, FcRn: an analysis at the single-molecule level. Proc Natl Acad Sci U S A 2004; 101:11076 - 81; http://dx.doi.org/10.1073/pnas.0402970101; PMID: 15258288
- Mircic M, Kavanaugh A. The clinical efficacy of tocilizumab in rheumatoid arthritis. Drugs Today (Barc) 2009; 45:189 - 97; http://dx.doi.org/10.1358/dot.2009.45.3.1343794; PMID: 19436841
- Igawa T, Ishii S, Tachibana T, Maeda A, Higuchi Y, Shimaoka S, et al. Antibody recycling by engineered pH-dependent antigen binding improves the duration of antigen neutralization. Nat Biotechnol 2010; 28:1203 - 7; http://dx.doi.org/10.1038/nbt.1691; PMID: 20953198
- Chaparro-Riggers J, Liang H, DeVay RM, Bai L, Sutton JE, Chen W, et al. Increasing serum half-life and extending cholesterol lowering in vivo by engineering antibody with pH-sensitive binding to PCSK9. J Biol Chem 2012; 287:11090 - 7; http://dx.doi.org/10.1074/jbc.M111.319764; PMID: 22294692
- Tettamanzi MC, Keeler C, Meshack S, Hodsdon ME. Analysis of site-specific histidine protonation in human prolactin. Biochemistry 2008; 47:8638 - 47; http://dx.doi.org/10.1021/bi800444t; PMID: 18652486
- Keeler C, Jablonski EM, Albert YB, Taylor BD, Myszka DG, Clevenger CV, et al. The kinetics of binding human prolactin, but not growth hormone, to the prolactin receptor vary over a physiologic pH range. Biochemistry 2007; 46:2398 - 410; http://dx.doi.org/10.1021/bi061958v; PMID: 17279774
- Kulkarni MV, Tettamanzi MC, Murphy JW, Keeler C, Myszka DG, Chayen NE, et al. Two independent histidines, one in human prolactin and one in its receptor, are critical for pH-dependent receptor recognition and activation. J Biol Chem 2010; 285:38524 - 33; http://dx.doi.org/10.1074/jbc.M110.172072; PMID: 20889499
- Rudnick SI, Lou J, Shaller CC, Tang Y, Klein-Szanto AJ, Weiner LM, et al. Influence of affinity and antigen internalization on the uptake and penetration of Anti-HER2 antibodies in solid tumors. Cancer Res 2011; 71:2250 - 9; http://dx.doi.org/10.1158/0008-5472.CAN-10-2277; PMID: 21406401
- Fujimori K, Covell DG, Fletcher JE, Weinstein JN. Modeling analysis of the global and microscopic distribution of immunoglobulin G, F(ab’)2, and Fab in tumors. Cancer Res 1989; 49:5656 - 63; PMID: 2790783
- Weinstein JN, Eger RR, Covell DG, Black CD, Mulshine J, Carrasquillo JA, et al. The pharmacology of monoclonal antibodies. Ann N Y Acad Sci 1987; 507:199 - 210; http://dx.doi.org/10.1111/j.1749-6632.1987.tb45802.x; PMID: 3327413
- Juweid M, Neumann R, Paik C, Perez-Bacete MJ, Sato J, van Osdol W, et al. Micropharmacology of monoclonal antibodies in solid tumors: direct experimental evidence for a binding site barrier. Cancer Res 1992; 52:5144 - 53; PMID: 1327501
- Eger RR, Covell DG, Carrasquillo JA, Abrams PG, Foon KA, Reynolds JC, et al. Kinetic model for the biodistribution of an 111In-labeled monoclonal antibody in humans. Cancer Res 1987; 47:3328 - 36; PMID: 3581071
- Ingle GS, Chan P, Elliott JM, Chang WS, Koeppen H, Stephan JP, et al. High CD21 expression inhibits internalization of anti-CD19 antibodies and cytotoxicity of an anti-CD19-drug conjugate. Br J Haematol 2008; 140:46 - 58; PMID: 17991300
- Ackerman ME, Pawlowski D, Wittrup KD. Effect of antigen turnover rate and expression level on antibody penetration into tumor spheroids. Mol Cancer Ther 2008; 7:2233 - 40; http://dx.doi.org/10.1158/1535-7163.MCT-08-0067; PMID: 18645032
- Thurber GM, Schmidt MM, Wittrup KD. Antibody tumor penetration: transport opposed by systemic and antigen-mediated clearance. Adv Drug Deliv Rev 2008; 60:1421 - 34; http://dx.doi.org/10.1016/j.addr.2008.04.012; PMID: 18541331
- Reddy S, Shaller CC, Doss M, Shchaveleva I, Marks JD, Yu JQ, et al. Evaluation of the anti-HER2 C6.5 diabody as a PET radiotracer to monitor HER2 status and predict response to trastuzumab treatment. Clin Cancer Res 2011; 17:1509 - 20; http://dx.doi.org/10.1158/1078-0432.CCR-10-1654; PMID: 21177408
- Schier R, Bye J, Apell G, McCall A, Adams GP, Malmqvist M, et al. Isolation of high-affinity monomeric human anti-c-erbB-2 single chain Fv using affinity-driven selection. J Mol Biol 1996; 255:28 - 43; http://dx.doi.org/10.1006/jmbi.1996.0004; PMID: 8568873
- Schier R, McCall A, Adams GP, Marshall KW, Merritt H, Yim M, et al. Isolation of picomolar affinity anti-c-erbB-2 single-chain Fv by molecular evolution of the complementarity determining regions in the center of the antibody binding site. J Mol Biol 1996; 263:551 - 67; http://dx.doi.org/10.1006/jmbi.1996.0598; PMID: 8918938
- Adams GP, Schier R, McCall AM, Simmons HH, Horak EM, Alpaugh RK, et al. High affinity restricts the localization and tumor penetration of single-chain fv antibody molecules. Cancer Res 2001; 61:4750 - 5; PMID: 11406547
- Zahnd C, Kawe M, Stumpp MT, de Pasquale C, Tamaskovic R, Nagy-Davidescu G, et al. Efficient tumor targeting with high-affinity designed ankyrin repeat proteins: effects of affinity and molecular size. Cancer Res 2010; 70:1595 - 605; http://dx.doi.org/10.1158/0008-5472.CAN-09-2724; PMID: 20124480
- Schmidt MM, Wittrup KD. A modeling analysis of the effects of molecular size and binding affinity on tumor targeting. Mol Cancer Ther 2009; 8:2861 - 71; http://dx.doi.org/10.1158/1535-7163.MCT-09-0195; PMID: 19825804
- Polson AG, Calemine-Fenaux J, Chan P, Chang W, Christensen E, Clark S, et al. Antibody-drug conjugates for the treatment of non-Hodgkin’s lymphoma: target and linker-drug selection. Cancer Res 2009; 69:2358 - 64; http://dx.doi.org/10.1158/0008-5472.CAN-08-2250; PMID: 19258515
- Doronina SO, Toki BE, Torgov MY, Mendelsohn BA, Cerveny CG, Chace DF, et al. Development of potent monoclonal antibody auristatin conjugates for cancer therapy. Nat Biotechnol 2003; 21:778 - 84; http://dx.doi.org/10.1038/nbt832; PMID: 12778055
- Kovtun YV, Audette CA, Ye Y, Xie H, Ruberti MF, Phinney SJ, et al. Antibody-drug conjugates designed to eradicate tumors with homogeneous and heterogeneous expression of the target antigen. Cancer Res 2006; 66:3214 - 21; http://dx.doi.org/10.1158/0008-5472.CAN-05-3973; PMID: 16540673
- Erickson HK, Park PU, Widdison WC, Kovtun YV, Garrett LM, Hoffman K, et al. Antibody-maytansinoid conjugates are activated in targeted cancer cells by lysosomal degradation and linker-dependent intracellular processing. Cancer Res 2006; 66:4426 - 33; http://dx.doi.org/10.1158/0008-5472.CAN-05-4489; PMID: 16618769
- Tanaka M, Kano Y, Akutsu M, Tsunoda S, Izumi T, Yazawa Y, et al. The cytotoxic effects of gemtuzumab ozogamicin (mylotarg) in combination with conventional antileukemic agents by isobologram analysis in vitro. Anticancer Res 2009; 29:4589 - 96; PMID: 20032408
- Lewis Phillips GD, Li G, Dugger DL, Crocker LM, Parsons KL, Mai E, et al. Targeting HER2-positive breast cancer with trastuzumab-DM1, an antibody-cytotoxic drug conjugate. Cancer Res 2008; 68:9280 - 90; http://dx.doi.org/10.1158/0008-5472.CAN-08-1776; PMID: 19010901
- Erickson HK, Lewis Phillips GD, Leipold DD, Provenzano CA, Mai E, Johnson HA, et al. The effect of different linkers on target cell catabolism and pharmacokinetics/pharmacodynamics of trastuzumab maytansinoid conjugates. Mol Cancer Ther 2012; 11:1133 - 42; http://dx.doi.org/10.1158/1535-7163.MCT-11-0727; PMID: 22408268
- Sievers EL, Senter PD. Antibody-Drug Conjugates in Cancer Therapy. [Epub ahead of print] Annu Rev Med 2012; http://dx.doi.org/10.1146/annurev-med-050311-201823; PMID: 23043493
- Lambert JM. Drug-conjugated monoclonal antibodies for the treatment of cancer. Curr Opin Pharmacol 2005; 5:543 - 9; http://dx.doi.org/10.1016/j.coph.2005.04.017; PMID: 16087399
- Haeuw JF, Caussanel V, Beck A. [Immunoconjugates, drug-armed antibodies to fight against cancer]. Med Sci (Paris) 2009; 25:1046 - 52; http://dx.doi.org/10.1051/medsci/200925121046; PMID: 20035677
- LoRusso PM, Weiss D, Guardino E, Girish S, Sliwkowski MX. Trastuzumab emtansine: a unique antibody-drug conjugate in development for human epidermal growth factor receptor 2-positive cancer. Clin Cancer Res 2011; 17:6437 - 47; http://dx.doi.org/10.1158/1078-0432.CCR-11-0762; PMID: 22003071
- Verma S, Miles D, Gianni L, Krop IE, Welslau M, Baselga J, et al, EMILIA Study Group. Trastuzumab emtansine for HER2-positive advanced breast cancer. [Epub ahead of print] N Engl J Med 2012; 367:1783 - 91; http://dx.doi.org/10.1056/NEJMoa1209124; PMID: 23020162
- Bross PF, Beitz J, Chen G, Chen XH, Duffy E, Kieffer L, et al. Approval summary: gemtuzumab ozogamicin in relapsed acute myeloid leukemia. Clin Cancer Res 2001; 7:1490 - 6; PMID: 11410481
- Hamann PR, Hinman LM, Beyer CF, Lindh D, Upeslacis J, Flowers DA, et al. An anti-CD33 antibody-calicheamicin conjugate for treatment of acute myeloid leukemia. Choice of linker. Bioconjug Chem 2002; 13:40 - 6; http://dx.doi.org/10.1021/bc0100206; PMID: 11792177
- Löwenberg B, Beck J, Graux C, van Putten W, Schouten HC, Verdonck LF, et al, Dutch-Belgian Hemato-Oncology Cooperative Group (HOVON), German Austrian AML Study Group (AMLSG), Swiss Group for Clinical Cancer Research Collaborative Group (SAKK). Gemtuzumab ozogamicin as postremission treatment in AML at 60 years of age or more: results of a multicenter phase 3 study. Blood 2010; 115:2586 - 91; http://dx.doi.org/10.1182/blood-2009-10-246470; PMID: 20103782