Abstract
The single-chain variable fragment, scFv-h3D6, has been shown to prevent in vitro toxicity induced by the amyloid β (Aβ) peptide in neuroblastoma cell cultures by withdrawing Aβ oligomers from the amyloid pathway. Present study examined the in vivo effects of scFv-h3D6 in the triple-transgenic 3xTg-AD mouse model of Alzheimer disease. Prior to the treatment, five-month-old female animals, corresponding to early stages of the disease, showed the first behavioral and psychological symptoms of dementia -like behaviors. Cognitive deficits included long- and short-term learning and memory deficits and high swimming navigation speed. After a single intraperitoneal dose of scFv-h3D6, the swimming speed was reversed to normal levels and the learning and memory deficits were ameliorated. Brain tissues of these animals revealed a global decrease of Aβ oligomers in the cortex and olfactory bulb after treatment, but this was not seen in the hippocampus and cerebellum. In the untreated 3xTg-AD animals, we observed an increase of both apoJ and apoE concentrations in the cortex, as well as an increase of apoE in the hippocampus. Treatment significantly recovered the non-pathological levels of these apolipoproteins. Our results suggest that the benefit of scFv-h3D6 occurs at both behavioral and molecular levels.
Introduction
Amyloid β (Aβ) immunotherapy has shown promise in the treatment of Alzheimer disease (AD), as indicated by the implementation of ongoing clinical trials.Citation1 The effectiveness of active immunization with Aβ-peptide in removing amyloid plaques, was clearly shown in the noted AN-1792 clinical trial.Citation2 Although the occurrence of neuroinflammatory complications halted this trial,Citation3 follow-up of the immunized patients revealed slowing down of cognitive decline, even one year after administration of the last doses.Citation4 The main drawback of active immunotherapy is the inability to intervene once the immune system is activated; this makes passive immunotherapy (i.e., administration of Aβ-directed antibodies) an attractive alternative.
Humanized monoclonal antibody AAB-001 (mAb-h3D6), or bapineuzumab, which targets the N-terminal 1–5 residues of the Aβ peptide, is being tested in several clinical trials.Citation5 In Phase 2 trials, besides vasogenic edema, side effects of bapineuzumab were mostly mild and transient. The observed edema was thought to be dose-related and more likely to occur in APOE4 carriers. In subsequent Phase 3 trials, which avoided high doses of bapineuzumab and recruited only APOE4 non-carriers, results obtained were still disappointing, and the trial was stopped in August 2012.Citation6
To avoid meningoencephalitis, vasogenic edema and micro-cerebral hemorrhages that can occur with the use of complete antibodies, the use of single-chain variable fragments (scFv), which are antibodies devoid of an Fc portion and incapable of directly activating microglia, is becoming an attractive therapeutic strategy.Citation7-Citation9 We previously described preparation of recombinant scFv-h3D6, a single chain variable fragment derived from mAb-h3D6,Citation5,Citation10,Citation11 which precludes cytotoxicity of Aβ1–42-oligomers in SH-SY5Y neuroblastoma cell culture, by withdrawing them from the amyloid pathway.Citation12 Since evidence suggests that therapeutic interventions that reduce Aβ fibrils at the cost of augmenting non-fibrillar Aβ assemblies, including Aβ oligomers, could be harmfulCitation13 targeting Aβ-oligomers, rather than amyloid plaques, is highly recommended.Citation14
Our results from cell culture studies of scFv-h3D6 treatment led us to investigate its disease prevention potential in an in vivo model of AD. The triple-transgenic mouse for Alzheimer disease (3xTg-AD) harboring APPSwe, PS1M146V and tauP301L transgenes, represents a unique animal model that mimics both amyloid and tau AD neuropathologies in an age-dependent manner and in disease-relevant brain regions.Citation15 At early stages of the disease, immunohistochemical presence of intraneuronal Aβ can be detected in the cortex, hippocampus and amygdale with this demonstrable at 4 mo of age.Citation15-Citation17 At these early stages of the disease, the model mimics the first behavioral and psychological symptoms of dementia (BPSD), which are followed by impairment of cognition.Citation18-Citation21 By six months of age, studies have reported the presence of deficits at the electrophysiological, synaptic and cholinergic levels as well.
The present work aimed to study the effects of intraperitoneal (i.p.) administration of scFv-h3D6 in 3xTg-AD females on some behavioral and molecular aspects at early stages of Alzheimer disease.
Results
BPSD-like symptoms before treatment
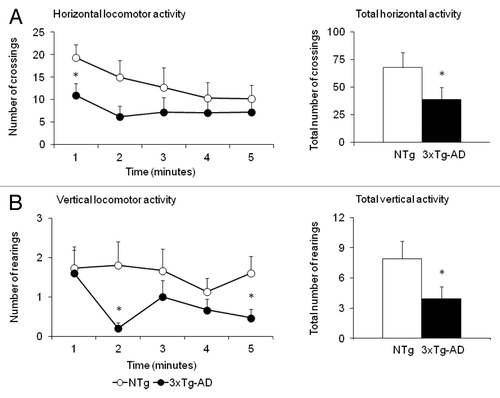
As detailed in , the corner and the open-field tests evidenced that, at 5 mo of age, the first BPSD-like symptoms were already apparent in the 3xTg-AD mice. Thus, in the corner test, these mice exhibited an anxious-like flight behavior as measured by increased number of corner visits (Student t-test, p < 0.05). In the open-field test, the same animals exhibited freezing behavior (latency of initial movement) and impaired thigmotaxis (latency to leave the center and to reach the periphery). As illustrated in , reduced exploratory activity was observed at different times of the test and in the total accumulated counts in both horizontal and vertical locomotor activities (, respectively). No differences were found in the other variables.
Table 1. BPSD-like behaviors in female 3xTg-AD mice at 5 mo of age. Statistics: Student t-test, *p < 0.05, ***p < 0.001 vs NTg mice
Figure 1. Triple-transgenic 3xTg-AD mice exhibited anxious-like behavior in the open field test before the treatment. Results are expressed as means ± SEM. The time course (left panels) of horizontal locomotor activity (A) and vertical locomotor activity (B) in the open field test shows that the 3xTg-AD mice exhibited reduced locomotor activity from the beginning to the end of test. The accumulated counts (right panels) indicated that at 5 mo of age, the 3xTg-AD presented BPSD-like symptoms, in this case anxiety to a new open and illuminated environment, which implied a reduction of about 50% in their total horizontal (A) and vertical activity (B) compared with the NTg animals. Statistics: Student’s t-test * Indicates significant differences between 3xTg-AD (dark circles or bars, n = 15) and NTg animals (light circles or bars, n = 15), p < 0.05.
Assessment of behavioral effects of the treatment
illustrates the results obtained “day by day” and “trial by trial” in the different tasks performed in the Morris water maze (MWM) after the treatment. Escape latency (panel A) shows that all the animals spent the same time to reach the visual platform [V1: G, T and GxT, all F(1,26) > 0.395, n.s.] with a clear improvement through the trials [V11 to V14: t, RMA, F(1,26) = 8.506, p < 0.001]. On the next day, namely PT1, the platform was hidden and changed to a reversal position that was maintained until the end of the task. There, a genotype effect [PT1: G, F(1,26) = 4,023, p < 0.05] indicates a difference in long-term memory in the 3xTg-AD groups compared with non-transgenic (NTg) animals, which is mostly seen between the untreated 3xTg-AD and the treated NTg animals (post-hoc Duncan’s test, p < 0.05). The detailed “trial by trial” analysis indicated that scFvh3D6 was also able to improve the performance of the NTg animals on PT11, the first trial of the place learning task (post-hoc Duncan’s test, p < 0.05) since the animals insisted on looking for the platform in the prior location (Pearson correlation, r = 0.905**, p < 0.01). In contrast, the untreated NTg animals and the two 3xTg-AD groups spent the same amount of time as that in their first experience in the maze. Still, a generalized progress with time was seen in this [PT1: t, F(2,52) = 6.875, p < 0.01] and the following days [PT2: t, F(2,52) = 3.090, p < 0.05; PT3: t, F(2,52) = 7.422, p < 0.001]. On the last day and, overall, during the three days of the place learning “time × genotype × treatment” [PT3: txGxT, F(2,52) = 3.309, p < 0.05; PT1 to PT3: txGxT, RMA, F(2,52) = 3.624, p < 0.05] and “time × genotype” [PT1 to PT3: txG, F(2,52) = 3.190, p < 0.05] interaction effects were observed, which indicate genetic differences in the progress of the short and long-term learning and memory processes and that the effects of the treatment were dependent on the genotype.
Figure 2. Effects of scFv-h3D6 on learning and memory in the cue learning and the place task in the Morris water maze. Results are expressed as means ± SEM. The left panels illustrated the day-by-day long-term memory results obtained as mean latency (A) to reach the platform, the mean distance covered (B) and the swimming speed (C) during the cue learning (V) and the place task (PT). The right panels depict the results obtained in each trial of the test which involve long- and short-term learning and memory. As shown by the statistics, time (t), genotype (G) and treatment (T) effects were found in both long and short-term learning and memory results ***, p < 0.001, ** p < 0.01, * p < 0.05, Post-hoc Duncan’s test, * p < 0.05 non-treated 3xTg-AD mice vs all the other groups, a p < 0.05 vs the non-treated animals of the same genotype, b p < 0.05 vs the other genotype with the same treatment conditions, c p < 0.05, vs the non-treated 3xTg-AD mice.
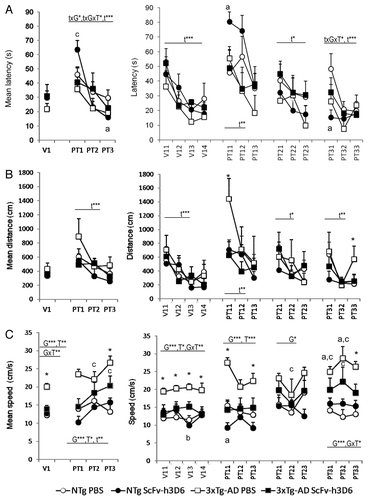
When the trajectories covered during the tasks were analyzed, consistent genotype differences were found in the navigation speed in all the tasks recorded. The results are presented as distance covered to reach the platform () and swimming speed ().The “day by day” analysis [V, PT1, PT2, PT3 and PT1 to PT3, G, all F’s(1,26) > 5.270, p < 0.05] indicated an overall higher swimming speed in the 3xTg-AD animals compared with the NTg genotype. In the place learning, a time effect [t, F(2,52) = 10.212, p < 0.01] was also found. Most importantly, treatment effects were found in the “day by day” analysis of both MWM tasks [V: T, F(1,26) = 7.397, p < 0.01; PT1 to PT3: F(1,26) = 5.280, p < 0.05; PT1: F(1,26) = 13.794, p < 0.001] with a reduction of the speed in scFv-h3D6 treated animals. Moreover, treatment x genotype interaction effects on cue learning [V: GxT, F(1,26) = 9.820, p < 0.01] and the last day of place task [PT3: GxT, F(1,26) = 4.638, p < 0.05] indicated selective treatment effects on the 3xTg-AD genotype, where scFv-h3D6 reduced the high swimming speed to the normal values shown by NTg animals. In most of these cases, the post-hoc Duncan’s test revealed that the untreated 3xTg-AD mice differed from the other three experimental groups of mice (p < 0.05). The distance revealed that the main difference was found on the first day of place learning [PT11: F(3,26) = 4.079, p < 0.05, all the other trials, F’s (3,26) > 0.091, n.s.] where the untreated 3xTg-AD mice covered a significant higher distance to find the platform compared with all the other groups of mice (post-hoc Duncan’s test, p < 0.05). The results also showed that on the last trial of the place task, untreated 3xTg-AD mice also differed from the untreated NTg mice [PT33: F(3,26) = 0.132, p < 0.05; post-hoc Duncan’s test, p < 0.05] and that this genotype difference was lost after treatment with scFv-h3D6.
On the probe trial (), preference for the trained quadrant vs. the other ones was measured. The computerized analysis of the trajectories indicated that the untreated 3xTg-AD mice showed an increased swimming speed () compared with untreated NTg mice (Student t-test, t = -2,936, p < 0.05), and this was reversed to normal levels in the scFv-h3D6-treated 3xTg-AD animals (Student t-test, t = 1.984, p < 0.05 vs 3xTg-AD and n.s. vs both NTg groups). Due to the differences in the speed, the standard measure of permanence () was complemented by the measure of annulus crossings () and distance covered (). All the groups showed a similar number of entries to the exact location of the platform in the previous test. The NTg group slightly discriminated the trained quadrant, albeit the result did not reach statistical significance. The factorial analysis, however, showed genotype x treatment interaction effects on both the percentage of time spent and the distance covered in the opposite quadrant [Distance in the O: F(1,26) = 4.220, p < 0.05; Time spent in the O: F(1,26) = 8.052, p < 0.01; all the others, F’s (1,26) < 2.934, n.s.]. The results indicated differences between the untreated 3xTg-AD and NTg groups of mice, with 3xTg-AD mice spending a higher time and covering a longer distance in the opposite quadrant compared with the NTg counterparts (post-hoc Duncan’s test, p < 0.05 vs NTg mice, in both cases). The within subjects paired t-test analysis indicated that the opposed quadrant was preferred in both 3xTg-AD groups instead of the trained one, but treatment with scFv-h3D6 reduced the significance of this preference for the wrong quadrant (the statistical significance was reduced from p < 0.001 to p < 0.05). These results were in agreement with the search strategies shown ( and ). That is, “scanning” and “random swimming” were the most frequent strategies in all the groups while “wall hugging” and “flotation” were only observed in the untreated NTg and 3xTg-AD mice, respectively. “Direct swims” and “focal searching” were more characteristic of NTg animals and scarcely seen in the groups of 3xTg-AD mice. The treatment improved the search strategies in both genotypes, in the case of 3xTg-AD by reducing the non-goal directed “chaining”, while in the NTg mice the benefit was obtained by increasing “direct swims”.
Figure 3. Effects of scFv-h3D6 in the probe trial in the Morris water maze. Results are expressed as means ± SEM. The swimming speed (A) was significantly increased in 3xTg-AD compared with NTg mice and the treatment with scFv-h3D6 reversed this effect to normal swimming speed. The animals did not differ in the number of annulus crossings (B). Genotype per treatment (GxT) effects were found in the preferences shown in the different quadrants of the maze: P (trained quadrant were the platform was previously located), AL (adjacent left), O (opposed) and AR (adjacent right) during the free swim trial in both the time (C) and the distance covered (D). A preference for the opposed quadrant was found in the 3xTg-AD mice but the treatment reduced its statistical significance D. Annulus crossings (number). Statistics: Between groups, ANOVA, Post-hoc Duncan’s or Student t-tests, * p < 0.05 vs the non-treated NTg mice; a p < 0.05 vs the non-treated 3xTg-AD mice. Within groups, paired-t-test, bbb, p < 0.001 and b p < 0.05 O vs P; c p < 0.05 O vs AL.
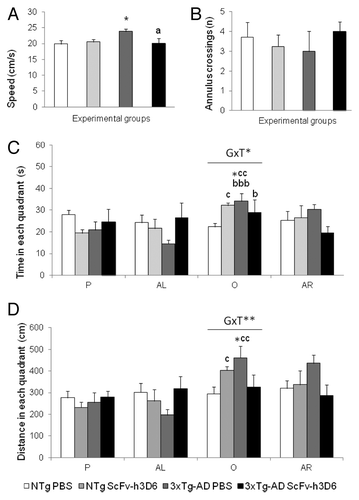
Figure 4. Trajectories of the animals during the probe trial. The figure illustrates the navigation trajectories of all the animals during the probe trial which are thereafter (see ) used to quantify the search strategy according to Lang et al.Citation51
Table 2. Searching strategies in the probe trial. Incidence of animals exhibiting a certain search strategy expressed as percentage
Effect of scFv-h3D6 in the Aβ-aggregation profile in vivo
Mice subjected to the behavioral studies were subsequently sacrificed and several brain subregions, hippocampus, cortex, olfactory bulb and cerebellum, were dissected to prepare protein extracts of each group as described in the Materials and Methods Section. depicts an illustrative immunoblotting analysis with 6E10 antibody of the soluble Aβ-amyloid oligomers from extracellular extracts (TBS fraction). Medium-molecular-mass assemblies (> 10kDa) that might represent Aβ oligomers were observed. This species pattern were unaltered when the initial SDS-PAGE did not contain 8 M urea (not shown), suggesting that these species are held together by urea-resistant hydrogen bonding. In agreement with other studies,Citation9,Citation22,Citation23 multiples of trimeric Aβ oligomers were detected, i.e., dodecamers, nonamers, hexamers and trimers. Additionally, sAPPα (secreted form of APP by α-secretase cleavage) was also detected.
Figure 5. Immunoblotting analysis of the soluble Aβ-amyloid oligomers from extracellular extracts of 5 mo-old NTg and 3xTg-AD mice. (A) Extracellular extracts from several brain subregions (HC, hippocampus; CX, cortex; OB, olfactory bulb and CR, cerebellum), from non-transgenic (NTg) and triple transgenic (3xTg-AD) mice i.p. treated with 85 μg of scFv-h3D6 (+) and i.p. treated with PBS (-), were analyzed. Profiles for NTg mice did not change upon scFv-h3D6 treatment. The profiles for 3xTg-AD mice of extracellular soluble Aβ oligomers in CX and OB showed a clear decrease of the dodecameric, nonameric, hexameric and trimeric Aβ-species upon treatment (squared), while in HC and CR remained the same. Arrows indicate respective migration position of monomers (1-mer), trimers (3-mer), hexamers (6-mer), nonamers (9-mer), dodecamers (12-mer) and sAPPα (secreted form of APP that has been cleaved by α-secretase). Synthetic human Aβ1–42 peptide (hAβ42) was used as a positive control (left lanes). Total protein applied to each lane was 45 μg. Blots were normalized by β-actin concentration. (B) Bar diagram showing the mean ± SD of the quantification of the bands from three experiments.
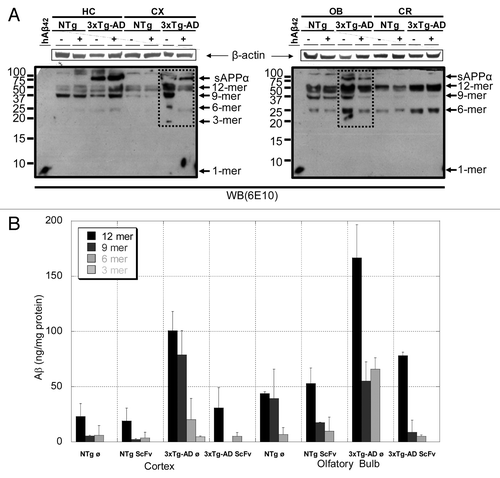
Aβ-aggregation profiles for NTg mice did not change upon scFv-h3D6 treatment. Mouse sAPPα, dodecamers, nonamers and hexamers of Aβ-peptide were detected in all of the samples, this being the profile characteristic of the studied brain subregion. It is essential to note that other studies have also detected these mouse species with 6E10 antibody.Citation22 Similar species were seen in the 3xTg-AD mice, although human sAPPα has higher mobility than mouse sAPPα and the relative abundance of each oligomer was dramatically increased. In fact, since mouse oligomers are being detected in the NTg mice, this evident increase is due to the oligomers being processed from human sAPPα.
As expected, human sAPPα was not detected in the cerebellum, and scFv-h3D6 treatment did not change the profile pattern of the oligomeric species in this area. In the hippocampus, human sAPPα was clearly detected, although the profile pattern of the oligomeric species upon scFv-h3D6 treatment did not change. In contrast, the treatment changed the aggregation profile of the Aβ-peptide in the cortex and in the olfactory bulb. Both cortex and olfactory bulb showed a dramatic decrease in dodecamers, nonamers and hexamers levels (). It was also likely that the trimer detected in the untreated cortex disappeared upon scFv-h3D6 treatment.
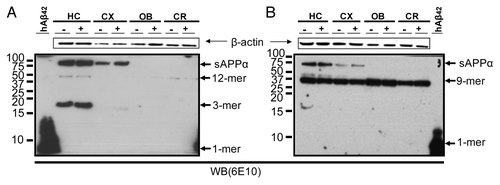
Since the intracellular Aβ has been proposed to be responsible for the first behavioral symptoms observed in 3xTg-AD transgenic mice,Citation15,Citation18,Citation19,Citation24 the intracellular protein (1% Triton fraction) was analyzed by immunoblotting. No significant change in the profiles was found between treated and non-treated groups (). Mainly α-APP and trimeric Aβ were detected in the hippocampus, whereas the band for dodecamers was rather tiny and those for nonamers and hexamers were not detected. In the cortex, only α-APP was detected, whereas in the olfactory bulb and cortex any band was observed. Similarly, in the membrane-associated protein (2% SDS fraction) only a faint band for α-APP and a clear band for the nonamer were detected in the hippocampus, and in the rest of the areas just the nonamer was present (data not shown). Finally, the formic acid extracts did not show any result (data not shown). On the other hand, NTg groups rendered similar results with both the intracellular and membrane-associated protein extracts (data not shown).
Figure 6. Immunoblotting analysis of the soluble Aβ-amyloid oligomers from intracellular and membrane-associated protein extracts of 5 mo-old 3xTg-AD mice. Intracellular protein fractions (A) and membrane-associated protein fractions (B), from several brain subregions (HC, hippocampus; CX, cortex; OB, olfactory bulb and CR, cerebellum), from triple transgenic (3xTg-AD) mice i.p. treated with 85 μg of scFv-h3D6 (+) and i.p. treated with PBS (-), were analyzed. Only sAPPα, dodecamer and trimer Aβ-species in the intracellular fraction and sAPPα and nonamer Aβ-species in the membrane-associated fractions were detected, but the profile pattern of the oligomeric species upon scFv-h3D6 treatment did not change. NTg groups rendered similar results (not shown). Arrows indicate respective migration position of monomers (1-mer), trimers (3-mer), nonamers (9-mer), dodecamers (12-mer) and sAPPα (secreted form of APP that has been cleaved by α-secretase). Synthetic human Aβ1–42 peptide (hAβ42) was used as a positive control (left lanes). Total protein applied to each lane was 45 μg.
Effect of scFv-h3D6 in recruiting clusterin (APOJ) and APOE
Because there is evidence of the in vivo involvement of clusterin (apoJ) and apoE on Aβ amyloid fibril formation,Citation25,Citation26 we quantified their content in the extracellular TBS fraction by ELISA (). Clusterin concentration was strongly increased in the cortex of 3xTg-AD compared with NTg animals (, 2.8 fold). ScFv-h3D6 treatment of 3xTg-AD mice significantly decreased this level and there were no significant difference between the NTg groups (treated or untreated) and the treated 3xTg-AD, indicating that the treatment restored the non-pathological level of clusterin in the cortex. The levels of clusterin in hippocampus, olfactory bulb and cerebellum did not significantly differ upon treatment.
Figure 7. Clusterin (A) and apoE (B) concentrations in TBS extracts determined by ELISA. Mean ± SD, n = 5, Mann-Whitney U test, *p < 0.05 vs untreated NTg mice. #p < 0.05 vs untreated 3xTg-AD mice.
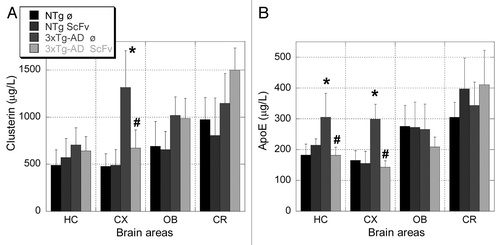
ApoE concentration was increased in hippocampus (1.7 folds) and cortex (1.8 folds) of 3xTg-AD mice compared with NTg animals, and scFv treatment clearly restored the non-pathological levels in both areas (). The level of apoE in olfactory bulb and cerebellum did not show significant differences.
Discussion
We recently showed that an antibody fragment, the single-chain variable fragment scFv-h3D6, has the ability to prevent the toxicity induced by the Aβ peptide in human neuroblastoma cell cultures.Citation12 In the present work, the scFv-h3D6 in vivo effects were studied in the triple-transgenic 3xTg-AD mouse model of Alzheimer disease at 5 mo of age, which corresponds to early stages of the disease.Citation15 The therapeutic potential of a single i.p. dose of 85 µg scFv-h3D6 was assessed in females because (1) this route of administration enables an easy translation to humans and (2) females have been reported to better exhibit the cognitive and BPSD-like symptoms of the disease.Citation16,Citation19-Citation21
ScFv-h3D6 treatment improved cognition and reversed BPSD-like symptoms
Before treatment, we confirmed the presence of BPSD-like symptomsCitation19,Citation27 and determined the level of behavioral disturbances of 3xTg-AD animals at early stages of the disease compared with NTg mice. In agreement with our previous reports,Citation19-Citation21,Citation27 anxious-like behavior was observed in the corner test, where the novelty of the home-cage induced an increased number of corner visits and rearings as a fight-or-flight coping with stress strategy. With the progression of the disease or under more anxiogenic experimental conditions, like those of the open-field test, this neophobia is expressed as freezing and reduced activity.Citation19 Thus, in the subsequent behavioral test, neophobia to the white and illuminated open arena was expressed as an initial freezing behavior, which was, among all, the most sensitive variable to show the genetic differences between 3xTg-AD and the NTg mice. The initial fear was followed by an extra delay in all the sequence of behavioral events that usually occur in this test: the exit of the center to reach the periphery, the start of vertical exploratory activity and the self-grooming behavior that starts as soon as the main exploratory activity slows down. A clear reduction of horizontal and vertical components of locomotor activity was also observed. Thereafter, these results, which confirmed the presence of behavioral disturbances, were also used as criteria to allocate, for each genotype, the mice in the two treatment groups in a counterbalanced manner.Citation28
To assess the effects of scFv-h3D6 on cognition and, indirectly, on some BPSD-like symptoms, animals were submitted to a series of paradigms in the MWM.Citation29 The tests started with a process of acquisition in the cue learning task, which is useful as a first experience in the maze in fearful animals.Citation19 The performance was optimal in all the groups, with the intramaze cue facilitating the localization of the platform. The lack of genotype differences in this task allowed us to discard the possible confounds of motivational or sensoriomotor differences. All the animals were able to achieve the same level of post-habituation baseline at V14, which emphasized the subsequent results. In the first trial of the place task, the hidden platform and the unexpected change in the usual platform position introduced a certain degree of difficulty to the test. This is shown by the animal’s insistence on looking for the platform in the previous location, and thus needing more time and covering more distance, before finding the new position compared with the previous sessions. Therefore, the genotype effect found in this first trial of the place task was indicative of differences in long–term memory and worse behavioral flexibility in the 3xTg-AD mice. The results were reinforced by the longer distance covered to find the platform, both at the beginning and the end of the place learning task, only shown by the untreated 3xTg-AD mice while the 3xTg-AD animals treated with scFv-h3D6 showed a normal behavior.
Another finding at the behavioral level was the ability of the scFv-h3D6 treatment to reverse the increased swimming speed shown by 3xTg-AD animals into a normalized pattern. This hyperactive swimming pattern was previously characterized,Citation20 and it is considered a common BPSD-like symptom in this animal model because these animals also show hyperactivity in non-anxiogenic conditions,Citation19 including increased voluntary running activity in wheels.Citation21 Among all, the effectiveness of scFv-h3D6 on swimming speed provided the most striking finding because it was a consistent phenomenon throughout the different trials of the test. Moreover, this is the first time that a treatment modality completely reversed this behavioral hallmark of 3xTg-AD mice, unlike other cases where treatment success modifying swimming speed was not as clearly consistent and effective.Citation20,Citation21 It is also interesting to note that the benefits of scFv-h3D6 were extended to the NTg mice, which may suggest the putative benefits of scFv-h3D6 in the normal population as well. In this sense, AD-purposed treatments targeting Aβ processing have also been shown to act as cognitive enhancers in NTg mice.Citation28 The time effect in the latency and the distance in both the cue and place learning tasks are mostly related to short- and long-term hippocampal-dependent learning processes. In contrast, the time effect in the navigation speed could be understood as a result of a more goal-directed navigation ability, thanks to the prior experiences.Citation20 The increased swimming speed and the search strategies observed in the 3xTg-AD mice in the removal test confirmed the observations obtained in the previous paradigms. Altogether, these results reinforce the fact that the behavioral pattern of 3xTg-AD at five months of age is already disrupted, as shown by the presence of BPSD-like symptoms and poorest cognitive performance. This included observed increased swimming speed and cognitive impairments mostly affecting long-term memory, although some short-term alterations could be observed. The present results suggest that scFv-h3D6 can be a promising therapeutic strategy and further studies are needed to extent these data.
ScFv-h3D6 treatment eliminated Aβ-oligomers
It is now widely accepted that soluble Aβ-oligomers are the cause of loss of synapses and the neuronal injury, that are characteristic of Alzheimer disease.Citation14,Citation30 This hypothesis explains the lack of correlation between cognitive decline and the presence of amyloid plaques.Citation31 Therefore, it makes sense to develop strategies to promote their elimination, a fact that is supported by the finding that therapeutic interventions that reduce Aβ fibrils at the cost of augmenting Aβ oligomers could be harmful.Citation13 ScFv-h3D6 derives from mAb-h3D6,Citation12 also known as bapineuzumab, and specifically recognizes Aβ oligomers.Citation10
To compare the aggregation extent of the Aβ peptide in different brain areas of treated and non-treated animals, protein extracts from hippocampus, cortex, olfactory bulb and cerebellum were analyzed by western-blot analysis with the 6E10 antibody. Twelve, nine and six-mer oligomers of the Aβ peptide were globally decreased in cortex and olfactory bulb upon treatment, but this effect was observed neither in the hippocampus nor in the cerebellum. The different sensitivity observed in the cortex and olfactory bulb is in agreement with the neuroanatomical progression of the affectation of the brain in the human patient, starting at the enthorrinal cortex. The olfactory bulb, which is mostly cholinergic, is also one of the most sensitive areas. Similar results have been also obtained in our colony of animals, with cortex being the best area of study at early stages of the disease.Citation32
Despite the fact that the 6E10 antibody should theoretically detect all forms Aβ, we only detected α-APP and multiples of trimeric Aβ oligomers (dodecamers, nonamers, hexamers and trimers). This could be due to the presence of low molecular weight species (mainly monomers and dimers) being outside the lower limit of detection of our methodology or to their total absence. Interestingly, the oligomeric species detected in the extracellular extracts, which were decreased upon treatment as mentioned above, are those that have been related to the pathology of the disease in the literature. Dodecamers and nonamers promoted cognitive decline when intracranially injected in the Tg2576 mouse model of AD,Citation22 whereas hexamers and trimers were decreased upon DNA vaccination of the 3xTg-AD mouse model.Citation23 Therefore, scFv-h3D6 is effective in eliminating the most toxic species of Aβ-oligomers and constitutes a promising therapy.
Apart from the controversy raised with the report claiming that the intraneuronal material share epitopes with full-length APP, but not free Aβ,Citation33 there is abundant evidence documenting the occurrence of intraneuronal Aβ in the normal and diseased human brain.Citation34 In any case, it is not clear whether the impairment of neural function is due to the extracellular fraction or intracellular fraction or to both. LaFerla et al. correlated synapses and memory loss with the amount of intracellular Aβ.Citation35 Precisely in the 3xTg-AD model, removal of extracellular Aβ plaques is immediately followed by the clearance of intraneuronal Aβ, indicating that a dynamic equilibrium exists between the two pools.Citation36 The fact that in the present work the effect of immunotherapy was observed on the extracellular pool and not in the intracellular one has been also reported in other cases where fractionation with detergents is applied.Citation9,Citation22,Citation34 Moreover, there are contradictory results showing a decrease in the intracellular pool concomitant to a increase in the extracellular one upon immunotherapy.Citation9 Also, some groups state that intracellular Aβ is not an indicator of cognitive decline neither in AD nor DS because its level is maintained during the progression of both diseases.Citation37 In any case, AD dementia strongly correlates with soluble Aβ level,Citation31 which is clearly diminished in the cortex and olfactory bulb of five-month-old 3xTg-AD females upon scFv-h3D6 treatment.
ScFv-h3D6 treatment restored clusterin and apoE levels
In this work, we found increased levels of clusterin and apoE in the cortex of 3xTg-AD animals, together with an increased level of apoE in hippocampus, and, more interestingly, the restoration to non-pathological levels upon scFv-h3D6 treatment. The different sensitivity observed in the cortex and hippocampus is in agreement with the neuroanatomical progression of the affectation of the brain in the 3xTG-AD mouse.Citation38
Apart from being a lipid-transporter, clusterin (encoded by the CLU gene), also known as apolipoprotein J (apoJ), is an ATP-independent extracellular chaperone, mainly secreted by astrocytes, that has been found in association with all extracellular deposits of protein aggregates associated with diseases that have been studied so far,Citation39 including those produced in AD.Citation25,Citation26 It is known that clusterin inhibits the formation of aggregates by binding to their surface hydrophobic-residuesCitation40 and that β oligomers of different sizes (from dimers to 50-mers) interact with clusterin to form stable complexes.Citation26 These complexes are removed by interaction with megalin (LRP-2)Citation41,Citation42 and other receptors of the LDLR family.Citation43 Since exposition of hydrophobic residues of the Aβ:scFv-h3D6 complex has been reported,Citation12 it is tempting to propose that clusterin could have internalized the complex and, as a consequence, both clusterin and Aβ-oligomers would be decreased in the extracellular fraction of treated animals.
There is strong evidence that clusterin and apoE are regulated in a cooperative manner.Citation44 Non-neuronal cell types, most notably astroglia and microglia, are the primary producers of the lipid transporter apoE, while neurons preferentially express apoE receptors. ApoE is found to co-localize with amyloid-β and plays a role in enhancing its clearance and degradation.Citation45,Citation46 In fact, the human APOE4 allele increases the risk of developing late-onset AD plausibly because it binds Aβ with approximately 20-fold lesser avidity than the more abundant allele APOE3. APOE4-specific changes in Aβ accumulation have also been recently described in EFAD-Tg mice.Citation47,Citation48 On the other hand, haploinsufficiency of human APOE reduces amyloid deposition in a mouse model of Aβ amyloidosis and results in significantly decreased amyloid plaque deposition and microglial activation,Citation49 suggesting a relationship between inflammation and apoE. From our results, it is likely that the opposite is also true, i.e., targeting Aβ-oligomers with scFv-h3D6 has diminished them and, as a consequence, apoE levels have been restored. This is of importance because, although further studies with scFv-h3D6 are required, neuroinflammation was not likely to occur as a side effect. This would be in agreement with other studies claiming that immunotherapy with scFv molecules is safer than that with the complete antibodies.Citation7-Citation9
The suggested benefits of scFv-h3D6 at the behavioral level, its capability for eliminating Aβ oligomers and to restore clusterin and apoE levels in the 3xTg-AD model of Alzheimer disease encourages us to further investigate its therapeutic potential.
Because scFvs are known to have short half-lives, the stability of scFv-h3D6-WT has been increased up to ~26% and the aggregation tendency decreased by ~4°C.Citation50 This rational design, together with further constructs using these stabilized mutants, i.e., in the form of diabodies, could help to translate this antibody fragment into a therapeutic for treating humans.
Materials and Methods
Animals
Triple-transgenic 3xTg-AD mice harboring PS1/M146V, APPSwe and tauP301L transgenes were genetically engineered at the University of California Irvine, as previously described.Citation15 Briefly, two independent transgenes (encoding human APPSwe and human tauP301L, both under control of the mouse Thy1.2 regulatory element) were co-injected into single-cell embryos harvested from homozygous mutant PS1M146V knock-in (PS1KI) mice. Since the PS1 knock-in mice were originally generated in a hybrid 129/C57BL6, this hybrid was used as the control strain.
Thirty 5-mo-old females animals from the Spanish colony of homozygous 3xTg-AD and wild-type non-transgenic (NTg) mice (n = 15 each group), established in the Medical Psychology Unit, Autonomous University of BarcelonaCitation27 were used in the present study. Three to five animals of the same genotype and sex were maintained (Makrolon, 35 × 35 × 25 cm) under standard laboratory conditions (food and water ad lib, 22 ± 2°C, 12 h light: dark cycle starting at 08:00).
Production of scFv-h3D6
ScFv-h3D6 was recombinantly expressed in E. coli and purified as previously described.Citation12 Lipopolysaccharides (LPS), the major endotoxins of gram-negative bacteria, were removed from the protein by using Detoxi-Gel Endotoxin Removing columns (Thermo Scientific).
Experimental design
Animals were assessed in the corner (CT) and open-field (OF) tests to determine the level of behavioral disturbances shown by 3xTg-AD mice before the treatment and compared with age- and gender-matched NTg mice.Citation19 Thereafter, mice were allocated in two treatment groups counterbalanced on the basis of the mean locomotor activity recorded in these tests.Citation28
Animals were treated with a single I.p. dose of 85 μg scFv-h3D6 or vehicle (PBS-buffer). Twenty-four hours after the administration, the animals were assessed during 4 consecutive days for cognition and BPSD-like behaviors in a series of MWM paradigms.Citation19 Behavior was evaluated by both direct observation and analysis of videotape-recorded images by an observer unaware of the animals’ genotype and treatment. Experiments were performed under dim white-light conditions (16–20 lx) from 10:00 to 13:00 and in accordance with the Spanish legislation on “Protection of Animals Used for Experimental and Other Scientific Purposes” and the European Communities Council Directive (86/609/EEC Council) on this subject.
Behavioral assessment prior to the treatment
Corner test
Neophobia to a new home-cage was evaluated in the corner test for 30 sec. Animals were individually placed in the center of a clean standard home-cage (Makrolon, 35 × 35 × 25 cm) filled with wood-shavings as bedding. The number of corner visits, latency to realize the first rearing, and the number of rearings were recorded.
Open-field test
Immediately after the corner test, mice were placed in the center of the apparatus (home-made, wooden, white, 55 × 55 × 25 cm high) and observed for 5 min. Horizontal (crossings of 5 × 5 cm squares) and vertical (rearings) locomotor activities were recorded for each minute of the test. We also recorded the latency of the sequence of the following behavioral events: initial freezing (latency of initial movement), thigmotaxis or discrimination of unprotected/protected areas in the test (latency of leaving the central 5 × 5 cm square and that of entering the peripheral ring 5 cm to the walls). Emotional behaviors such as self-grooming behavior (latency, number and duration of groomings), number of defecations and presence/absence of urine were also measured.
Assessment of behavioral effects of the treatment
Morris water maze tests
The effects of the treatment on the cognitive abilities of the animals were assessed in three paradigms for learning and memory in the Morris water maze (MWM)Citation29 consisting of one day of cue learning with a visual platform (V), three days of place task for long-term and short-term spatial reference memory with a hidden platform (PT) followed, after removal of the platform (RM), by one probe trial for short-term memory. The mice were trained to locate a platform (7 cm diameter) in a circular pool (Intex Recreation Corp., Calif., USA; 91 cm diameter, 40 cm height, 25°C opaque water) located in a black test room with distal cues. Animals failing to escape within 90 sec were manually guided to the platform. In the trials with platform, all animals stayed on it for 5–10 sec before being removed. Immediately after, the animals were dried and then placed in a holding cage with a heating pad to prevent hypothermia.
Day 1, cue learning with a visual platform
On the first day, animals were trained to criterion (90% escaping under 60 sec). This required four visible platform trials (V1‒V4) in a single day. The last visible platform trial of any animal is considered to be its post-habituation baseline, and was designated V4 (visible platform trial 4). Inter-trial interval was 30 min. The visible platform remained in the same place for all of the trials and was outlined by a black and white stripped flag with high contrast to the white background made by the addition of white, non-toxic tempera paint. Animals were delivered to either the west or east quadrant (the target quadrant being designated as north) in a random manner for each trial as these are equidistant from the target. High contrast visual cues were placed on the wall of the pool in each quadrant.
Day 2 to day 4, place learning with a hidden platform
Twenty-four hours after the last visible platform trial, the animals were tested in a series of three hidden platform trials (PT1–PT3). Animals were delivered to either the west or east quadrant and the target quadrant being designated as south, that is, reversal to the location in the previous paradigm. As before, the trials were staggered in time to ensure a stable inter-trial interval of about 30 min and each trial was a maximum of 90 sec long.
Day 4, removal
To measure the retention and level of accuracy of the precise location of the platform position achieved, the animals were allowed to swim in the probe trial or “removal” 2 h after the end of the last session of the place task. The procedure consisted of removing the platform from the maze and releasing the mouse from the north starting point and letting the animal navigate for 60 sec.
During the trials of the learning tasks, the escape latency was measured with a stop-watch. In all trials, the trajectories were recorded and analyzed using the SMART system 2.5.14 (Panlab, S.L.) to measure the total distance covered and to calculate the swimming speed. In the probe trial, the trajectories were analyzed to determine the number of annulus crossings, the time spent in each quadrant of the pool, as well as the incidence of non-goal directed (floating, wall hugging, chaining and random swimming) and goal-directed (scanning, focal searching and direct searching) search strategies.Citation51
Protein extraction
Animals were sacrificed at the end of the behavioral tests, which is 5 d after administration of scFv-h3D6. The extraction was prepared by sequential centrifugation of brain subregions homogenates as previously described.Citation22 Frozen tissues of hippocampus, cortex, olfactory bulb and cerebellum from 5-mo-old 3xTg-AD and NTg mice used in the experiment were weighted and mechanically homogenized in ice-cold TBS supplemented with protease inhibitors (Roche tablet 1836153) (8 μL TBS/mg tissue). In brief, samples were gently sonicated (1 cycle of 35 sec, at 35% duty cycle and output 4 in a Dynatech Sonic Dismembrator ARTEK 300 with smallest tip), centrifuged at 100,000 g for 1 h at 4°C, and the collected supernatant was labeled as extracellular protein TBS fraction. Subsequently, the insoluble pellet was dissolved to the same volume and rehomogenized in cold TBS-1% Triton X-100 solution supplemented with protease inhibitors and centrifuged again; the supernatant was labeled as intracellular protein 1% Triton fraction. The Triton X-100 insoluble pellet was dissolved then to the same volume and rehomogenized in 2% SDS solution supplemented with protease inhibitors and centrifuged again; the supernatant was labeled as membrane-associated protein 2% SDS fraction. Insoluble material was finally sonicated in 70% formic acid solution in water, centrifuged, and the supernatant was dried overnight using a vacuum concentrator (Savant SpeedVac® concentrator). The final protein extract was resuspended in 40 µL of DMSO. The total protein concentration of each soluble sample was determined by the BCA assay (Thermo Scientific, Rockford, IL). All of the fractions were aliquoted and stored at -80°C until its use.
Immunoblotting
Samples were heated in 2X Bicine loading buffer for 5 min at 95°C and resolved by Bicine/BisTris SDS-PAGE system under reducing conditions and in the presence of Urea 8 M.Citation52 Proteins were transferred to PVDF membranes (Immobilon Psq membrane, Millipore) boiled for 7 min in PBS and blocked with Tween®20-Phosphate-Buffered Saline (T-PBS) containing 5% non-fat milk for 1 h at 20°C. After overnight incubation at 4°C with primary antibody 6E10, a monoclonal antibody that recognizes all forms of Aβ, (Signet Labs) at a dilution of 1:350, blots were washed in T-PBS for 20 min and incubated for 1 h at 20°C with HRP-conjugated secondary antibody (Bio-Rad) at a dilution of 1:2500. Blots were developed with ECL detection system (Supersignal Pico Western system, Pierce). Quantification of each band was performed by densitometry and reference to linear standards of synthetic human Aβ1–42 peptide.
Clusterin and apoE ELISA
Murine ELISA kits were purchased from USCN Life Science Inc. and used as recommended by the supplier.
Statistics
Statistical analyses were performed using SPSS 12.0 software. In the behavioral studies, results are expressed as means ± SEM. The effects of the factors genotype (G), treatment (T) and time (t), as well as their interaction effects (GxT and txGxT), were analyzed with Repeated Measures ANOVA (RMA) and ANOVA with post-hoc Duncan or Student-t-test for independent samples. To analyze differences between two related samples, Paired t-test was used instead. The relationship between latencies and search strategies in the maze were investigated using Pearson correlation. In the molecular studies, results are expressed as mean ± SD and the Mann Whitney U test was used for assessing statistical differences. Statistical significance was always considered at p < 0.05.
| Abbreviations: | ||
| AD | = | Alzheimer disease, BPSD, behavioral and psychological symptoms of dementia |
| MWM | = | Morris water maze |
| scFV | = | single chain variable fragment |
Acknowledgements
We thank Laura Cervera (UAB, Spain) for her skillful technical assistance and Enrique Verdú (UdG, Spain) for valuable scientific discussion. The animals used in the present study come from the colony of homozygous 3xTg-AD and wild-type NTg mice established by Lydia Giménez-Llort at the Universitat Autònoma de Barcelona, Spain, from progenitors kindly provided by Frank M. LaFerla, Department of Neurobiology and Behavior, University of California Irvine, California, USA. This work was supported by FMM-2008; FISPI10–00975, -00265 and -00283; SGR2009–00761 and -42271. G.R-H is supported by a MAEC-AECI fellowship (Spanish government) and M.M-A by a PIF (UAB, Spain) fellowship.
Submitted
05/14/2013
Revised
06/14/2013
Accepted
06/15/2013
Note
APP nomenclature is misinterpreted. When referring to the upper band in , APP corresponds to sAPPα (generated by α-secretase proteolysis), and the band just below to sAPPβ (generated by β-secretase proteolysis). In , which variant of APP is present cannot be distinguished and in oligomers corresponds to 12-mer. This does not invalidate any of the conclusions of the work.
Disclosure of Potential Conflicts of Interest
No potential conflict of interest was disclosed.
Notes
† These authors contributed equally to this work.
References
- Panza F, Frisardi V, Solfrizzi V, Imbimbo BP, Logroscino G, Santamato A, et al. Immunotherapy for Alzheimer’s disease: from anti-β-amyloid to tau-based immunization strategies. Immunotherapy 2012; 4:213 - 38; http://dx.doi.org/10.2217/imt.11.170; PMID: 22339463
- Schenk D. Amyloid-beta immunotherapy for Alzheimer’s disease: the end of the beginning. Nat Rev Neurosci 2002; 3:824 - 8; http://dx.doi.org/10.1038/nrn938; PMID: 12360327
- Nicoll JA, Wilkinson D, Holmes C, Steart P, Markham H, Weller RO. Neuropathology of human Alzheimer disease after immunization with amyloid-beta peptide: a case report. Nat Med 2003; 9:448 - 52; http://dx.doi.org/10.1038/nm840; PMID: 12640446
- Hock C, Konietzko U, Streffer JR, Tracy J, Signorell A, Müller-Tillmanns B, et al. Antibodies against beta-amyloid slow cognitive decline in Alzheimer’s disease. Neuron 2003; 38:547 - 54; http://dx.doi.org/10.1016/S0896-6273(03)00294-0; PMID: 12765607
- Panza F, Frisardi V, Imbimbo BP, Seripa D, Paris F, Santamato A, et al. Anti-β-amyloid immunotherapy for Alzheimer’s disease: focus on bapineuzumab. Curr Alzheimer Res 2011; 8:808 - 17; http://dx.doi.org/10.2174/156720511798192718; PMID: 21592055
- Thomas K. Trials for Alzheimer’s drug halted after poor results. The New York Times 2012.
- Wang YJ, Pollard A, Zhong JH, Dong XY, Wu XB, Zhou HD, et al. Intramuscular delivery of a single chain antibody gene reduces brain Abeta burden in a mouse model of Alzheimer’s disease. Neurobiol Aging 2009; 30:364 - 76; http://dx.doi.org/10.1016/j.neurobiolaging.2007.06.013; PMID: 17686552
- Wang YJ, Gao CY, Yang M, Liu XH, Sun Y, Pollard A, et al. Intramuscular delivery of a single chain antibody gene prevents brain Aβ deposition and cognitive impairment in a mouse model of Alzheimer’s disease. Brain Behav Immun 2010; 24:1281 - 93; http://dx.doi.org/10.1016/j.bbi.2010.05.010; PMID: 20595065
- Cattepoel S, Hanenberg M, Kulic L, Nitsch RM. Chronic intranasal treatment with an anti-Aβ(30-42) scFv antibody ameliorates amyloid pathology in a transgenic mouse model of Alzheimer’s disease. PLoS One 2011; 6:e18296; http://dx.doi.org/10.1371/journal.pone.0018296; PMID: 21483675
- Jacobson JS. Antibodies specific for epitopes within amyloid β (Aβ), for use in improving cognition. PCT Int Appl (2006), WO 2006066171 A1 20060622.
- Wisniewski T, Konietzko U. Amyloid-beta immunisation for Alzheimer’s disease. Lancet Neurol 2008; 7:805 - 11; http://dx.doi.org/10.1016/S1474-4422(08)70170-4; PMID: 18667360
- Marín-Argany M, Rivera-Hernández G, Martí J, Villegas S. An anti-Aβ (amyloid β) single-chain variable fragment prevents amyloid fibril formation and cytotoxicity by withdrawing Aβ oligomers from the amyloid pathway. Biochem J 2011; 437:25 - 34; http://dx.doi.org/10.1042/BJ20101712; PMID: 21501114
- Cheng IH, Scearce-Levie K, Legleiter J, Palop JJ, Gerstein H, Bien-Ly N, et al. Accelerating amyloid-beta fibrillization reduces oligomer levels and functional deficits in Alzheimer disease mouse models. J Biol Chem 2007; 282:23818 - 28; http://dx.doi.org/10.1074/jbc.M701078200; PMID: 17548355
- Walsh DM, Selkoe DJ. A beta oligomers - a decade of discovery. J Neurochem 2007; 101:1172 - 84; http://dx.doi.org/10.1111/j.1471-4159.2006.04426.x; PMID: 17286590
- Oddo S, Caccamo A, Shepherd JD, Murphy MP, Golde TE, Kayed R, et al. Triple-transgenic model of Alzheimer’s disease with plaques and tangles: intracellular Abeta and synaptic dysfunction. Neuron 2003; 39:409 - 21; http://dx.doi.org/10.1016/S0896-6273(03)00434-3; PMID: 12895417
- Clinton LK, Billings LM, Green KN, Caccamo A, Ngo J, Oddo S, et al. Age-dependent sexual dimorphism in cognition and stress response in the 3xTg-AD mice. Neurobiol Dis 2007; 28:76 - 82; http://dx.doi.org/10.1016/j.nbd.2007.06.013; PMID: 17659878
- Mastrangelo MA, Bowers WJ. Detailed immunohistochemical characterization of temporal and spatial progression of Alzheimer’s disease-related pathologies in male triple-transgenic mice. BMC Neurosci 2008; 9:81; http://dx.doi.org/10.1186/1471-2202-9-81; PMID: 18700006
- Billings LM, Oddo S, Green KN, McGaugh JL, LaFerla FM. Intraneuronal Abeta causes the onset of early Alzheimer’s disease-related cognitive deficits in transgenic mice. Neuron 2005; 45:675 - 88; http://dx.doi.org/10.1016/j.neuron.2005.01.040; PMID: 15748844
- Giménez-Llort L, Blázquez G, Cañete T, Johansson B, Oddo S, Tobeña A, et al. Modeling behavioral and neuronal symptoms of Alzheimer’s disease in mice: a role for intraneuronal amyloid. Neurosci Biobehav Rev 2007; 31:125 - 47; http://dx.doi.org/10.1016/j.neubiorev.2006.07.007; PMID: 17055579
- Giménez-Llort L, García Y, Buccieri K, Revilla S, Suñol C, Cristofol R, et al. Gender-specific neuroimmunoendocrine response to treadmill exercise in 3xTg-AD mice. Int J Alzheimers Dis 2010; 2010:128354; http://dx.doi.org/10.4061/2010/128354; PMID: 20981262
- García-Mesa Y, López-Ramos JC, Giménez-Llort L, Revilla S, Guerra R, Gruart A, et al. Physical exercise protects against Alzheimer’s disease in 3xTg-AD mice. J Alzheimers Dis 2011; 24:421 - 54; PMID: 21297257
- Lesné S, Koh MT, Kotilinek L, Kayed R, Glabe CG, Yang A, et al. A specific amyloid-beta protein assembly in the brain impairs memory. Nature 2006; 440:352 - 7; http://dx.doi.org/10.1038/nature04533; PMID: 16541076
- Movsesyan N, Ghochikyan A, Mkrtichyan M, Petrushina I, Davtyan H, Olkhanud PB, et al. Reducing AD-like pathology in 3xTg-AD mouse model by DNA epitope vaccine - a novel immunotherapeutic strategy. PLoS One 2008; 3:e2124; http://dx.doi.org/10.1371/journal.pone.0002124; PMID: 18461171
- España J, Giménez-Llort L, Valero J, Miñano A, Rábano A, Rodriguez-Alvarez J, et al. Intraneuronal beta-amyloid accumulation in the amygdala enhances fear and anxiety in Alzheimer’s disease transgenic mice. Biol Psychiatry 2010; 67:513 - 21; http://dx.doi.org/10.1016/j.biopsych.2009.06.015; PMID: 19664757
- Calero M, Rostagno A, Matsubara E, Zlokovic B, Frangione B, Ghiso J. Apolipoprotein J (clusterin) and Alzheimer’s disease. Microsc Res Tech 2000; 50:305 - 15; http://dx.doi.org/10.1002/1097-0029(20000815)50:4<305::AID-JEMT10>3.0.CO;2-L; PMID: 10936885
- Narayan P, Orte A, Clarke RW, Bolognesi B, Hook S, Ganzinger KA, et al. The extracellular chaperone clusterin sequesters oligomeric forms of the amyloid-β(1-40) peptide. Nat Struct Mol Biol 2012; 19:79 - 83; http://dx.doi.org/10.1038/nsmb.2191; PMID: 22179788
- Giménez-Llort L, Blázquez G, Cañete T, Rosa R, Vivó M, Oddo S, et al. Modeling neuropsychiatric symptoms of Alzheimer’s disease dementia in 3xTg-AD mice. In: Alzheimer’s Disease: New Advances. Iqbal K, Winblad B, Avila J, eds. Pianoro (BO), Italy: Medimond SRL,eds., 2006:513-516.
- Ratia M, Giménez-Llort L, Camps P, Muñoz-Torrero D, Clos MV, Badia A. Behavioural effects and regulation of PKCalpha and MAPK by huprine X in middle aged mice. Pharmacol Biochem Behav 2010; 95:485 - 93; http://dx.doi.org/10.1016/j.pbb.2010.03.013; PMID: 20363245
- Morris R. Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods 1984; 11:47 - 60; http://dx.doi.org/10.1016/0165-0270(84)90007-4; PMID: 6471907
- Klein WL. Synaptotoxic amyloid-β oligomers: a molecular basis for the cause, diagnosis, and treatment of Alzheimer’s disease?. J Alzheimers Dis 2013; 33:Suppl 1 S49 - 65; PMID: 22785404
- McLean CA, Cherny RA, Fraser FW, Fuller SJ, Smith MJ, Beyreuther K, et al. Soluble pool of Abeta amyloid as a determinant of severity of neurodegeneration in Alzheimer’s disease. Ann Neurol 1999; 46:860 - 6; http://dx.doi.org/10.1002/1531-8249(199912)46:6<860::AID-ANA8>3.0.CO;2-M; PMID: 10589538
- Hedberg MM, Clos MV, Ratia M, Gonzalez D, Lithner CU, Camps P, et al. Effect of huprine X on β-amyloid, synaptophysin and α7 neuronal nicotinic acetylcholine receptors in the brain of 3xTg-AD and APPswe transgenic mice. Neurodegener Dis 2010; 7:379 - 88; http://dx.doi.org/10.1159/000287954; PMID: 20689242
- Winton MJ, Lee EB, Sun E, Wong MM, Leight S, Zhang B, et al. Intraneuronal APP, not free Aβ peptides in 3xTg-AD mice: implications for tau versus Aβ-mediated Alzheimer neurodegeneration. J Neurosci 2011; 31:7691 - 9; http://dx.doi.org/10.1523/JNEUROSCI.6637-10.2011; PMID: 21613482
- Gouras GK, Willén K, Tampellini D. Critical role of intraneuronal Aβ in Alzheimer’s disease: technical challenges in studying intracellular Aβ. Life Sci 2012; 91:1153 - 8; http://dx.doi.org/10.1016/j.lfs.2012.06.004; PMID: 22727791
- LaFerla FM, Green KN, Oddo S. Intracellular amyloid-beta in Alzheimer’s disease. Nat Rev Neurosci 2007; 8:499 - 509; http://dx.doi.org/10.1038/nrn2168; PMID: 17551515
- Oddo S, Billings L, Kesslak JP, Cribbs DH, LaFerla FM. Abeta immunotherapy leads to clearance of early, but not late, hyperphosphorylated tau aggregates via the proteasome. Neuron 2004; 43:321 - 32; http://dx.doi.org/10.1016/j.neuron.2004.07.003; PMID: 15294141
- Wegiel J, Kuchna I, Nowicki K, Frackowiak J, Mazur-Kolecka B, Imaki H, et al. Intraneuronal Abeta immunoreactivity is not a predictor of brain amyloidosis-beta or neurofibrillary degeneration. Acta Neuropathol 2007; 113:389 - 402; http://dx.doi.org/10.1007/s00401-006-0191-4; PMID: 17237937
- Oddo S, Caccamo A, Kitazawa M, Tseng BP, LaFerla FM. Amyloid deposition precedes tangle formation in a triple transgenic model of Alzheimer’s disease. Neurobiol Aging 2003; 24:1063 - 70; http://dx.doi.org/10.1016/j.neurobiolaging.2003.08.012; PMID: 14643377
- Yerbury JJ, Poon S, Meehan S, Thompson B, Kumita JR, Dobson CM, et al. The extracellular chaperone clusterin influences amyloid formation and toxicity by interacting with prefibrillar structures. FASEB J 2007; 21:2312 - 22; http://dx.doi.org/10.1096/fj.06-7986com; PMID: 17412999
- Humphreys DT, Carver JA, Easterbrook-Smith SB, Wilson MR. Clusterin has chaperone-like activity similar to that of small heat shock proteins. J Biol Chem 1999; 274:6875 - 81; http://dx.doi.org/10.1074/jbc.274.11.6875; PMID: 10066740
- Hammad SM, Ranganathan S, Loukinova E, Twal WO, Argraves WS. Interaction of apolipoprotein J-amyloid beta-peptide complex with low density lipoprotein receptor-related protein-2/megalin. A mechanism to prevent pathological accumulation of amyloid beta-peptide. J Biol Chem 1997; 272:18644 - 9; http://dx.doi.org/10.1074/jbc.272.30.18644; PMID: 9228033
- Nuutinen T, Suuronen T, Kauppinen A, Salminen A. Clusterin: a forgotten player in Alzheimer’s disease. Brain Res Rev 2009; 61:89 - 104; http://dx.doi.org/10.1016/j.brainresrev.2009.05.007; PMID: 19651157
- Mahon MG, Lindstedt KA, Hermann M, Nimpf J, Schneider WJ. Multiple involvement of clusterin in chicken ovarian follicle development. Binding to two oocyte-specific members of the low density lipoprotein receptor gene family. J Biol Chem 1999; 274:4036 - 44; http://dx.doi.org/10.1074/jbc.274.7.4036; PMID: 9933595
- DeMattos RB, Cirrito JR, Parsadanian M, May PC, O’Dell MA, Taylor JW, et al. ApoE and clusterin cooperatively suppress Abeta levels and deposition: evidence that ApoE regulates extracellular Abeta metabolism in vivo. Neuron 2004; 41:193 - 202; http://dx.doi.org/10.1016/S0896-6273(03)00850-X; PMID: 14741101
- Koistinaho M, Lin S, Wu X, Esterman M, Koger D, Hanson J, et al. Apolipoprotein E promotes astrocyte colocalization and degradation of deposited amyloid-beta peptides. Nat Med 2004; 10:719 - 26; http://dx.doi.org/10.1038/nm1058; PMID: 15195085
- Deane R, Sagare A, Hamm K, Parisi M, Lane S, Finn MB, et al. apoE isoform-specific disruption of amyloid beta peptide clearance from mouse brain. J Clin Invest 2008; 118:4002 - 13; http://dx.doi.org/10.1172/JCI36663; PMID: 19033669
- LaDu MJ, Falduto MT, Manelli AM, Reardon CA, Getz GS, Frail DE. Isoform-specific binding of apolipoprotein E to beta-amyloid. J Biol Chem 1994; 269:23403 - 6; PMID: 8089103
- Youmans KL, Tai LM, Nwabuisi-Heath E, Jungbauer L, Kanekiyo T, Gan M, et al. APOE4-specific changes in Aβ accumulation in a new transgenic mouse model of Alzheimer disease. J Biol Chem 2012; 287:41774 - 86; http://dx.doi.org/10.1074/jbc.M112.407957; PMID: 23060451
- Kim J, Jiang H, Park S, Eltorai AE, Stewart FR, Yoon H, et al. Haploinsufficiency of human APOE reduces amyloid deposition in a mouse model of amyloid-β amyloidosis. J Neurosci 2011; 31:18007 - 12; http://dx.doi.org/10.1523/JNEUROSCI.3773-11.2011; PMID: 22159114
- Rivera-Hernández G, Marín-Argany M, Blasco-Moreno B, Bonet J, Oliva B, Villegas S. Elongation of the C-terminal domain of an anti-amyloid b single-chain variable fragment increases its thermodynamic stability and decreases its aggregation tendency. MAbs 2013; 5 In press
- Lang UE, Lang F, Richter K, Vallon V, Lipp HP, Schnermann J, et al. Emotional instability but intact spatial cognition in adenosine receptor 1 knock out mice. Behav Brain Res 2003; 145:179 - 88; http://dx.doi.org/10.1016/S0166-4328(03)00108-6; PMID: 14529816
- Wiltfang J, Arold N, Neuhoff V. A new multiphasic buffer system for sodium dodecyl sulfate-polyacrylamide gel electrophoresis of proteins and peptides with molecular masses 100,000-1000, and their detection with picomolar sensitivity. Electrophoresis 1991; 12:352 - 66; http://dx.doi.org/10.1002/elps.1150120507; PMID: 1718736