Abstract
The in vivo modified forms of low-density lipoprotein (LDL) are important for the formation of foam cells and as mediators of the immuno-inflammatory process involved in the progression of atherosclerosis. Electronegative LDL, LDL(-), is a LDL subfraction with pro-inflammatory properties that is present in human blood. To investigate possible atheroprotective effects, an anti-LDL(-) single-chain variable fragment (scFv) was expressed in the methylotrophic yeast Pichia pastoris and its activity was evaluated in vitro against macrophages and in experimental atherosclerosis in Ldlr-/- mice. The recombinant 2C7 scFv was produced in a yield of 9.5 mg of protein/L. The specificity and affinity of purified 2C7 scFv against LDL(-) was confirmed by ELISA. To assess the activity of 2C7 scFv on foam cell formation, RAW 264.7 macrophages were exposed to LDL(-) in the presence or absence of 2C7 scFv. The 2C7 scFv inhibited the uptake of LDL(-) by macrophages in a dose-dependent manner, and internalization of LDL(-) by these cells was found to be mediated by the CD36 and CD14 receptor. In addition, compared with untreated cells, lipid accumulation in macrophages was decreased, and the expression of Cd36, Tlr-4 and Cox-2 was downregulated in macrophages treated with 2C7 scFv. Importantly, compared with untreated mice, the treatment of Ldlr-/- mice with 2C7 scFv decreased the atherosclerotic lesion area at the aortic sinus. In conclusion, our data show that 2C7 scFv inhibits foam cell formation and atherosclerotic plaque development by modulating the expression of genes relevant to atherogenesis. These results encourage further use of this antibody fragment in the development of new therapeutic strategies that neutralize the pro-atherogenic effects of LDL(-).
Introduction
Recombinant monoclonal antibodies (mAbs) are used as therapeutic agents to treat autoimmune and inflammatory diseases because of their high specificity and capacity to function as high-affinity targeting reagents.Citation1,Citation2 As of January 2013, 19 mAbs were in Phase 3 clinical trials for non-cancer purposes, including AMG145 and alirocumab for high cholesterol treatment, and an additional 10 mAbs were in Phase 3 studies as treatments for cancer.Citation3 Although widely used for numerous indications, full length mAb therapeutics have disadvantages due to their large size, pharmacokinetics and restricted access to some tissues. Molecular biology strategies thus have been used to generate monovalent antigen-binding (Fab) or single chain variable (scFv) fragments and divalent (e.g., Fab2´, diabodies, minibodies) antibody fragments that may also have clinical utility.Citation4
The scFv contains the smallest functional unit of the antibody. It is composed of the variable domains of antibody light and heavy chains joined by a hydrophilic and flexible spacer peptide that is 10 to 25 amino acid residues in length.Citation4 The antibody binding site is kept intact within the scFv, and there is usually no significant loss of specificity.Citation5 Pharmacokinetic properties, however, are changed; for example, scFv are rapidly cleared from the blood and have lower retention time in non-target tissues.Citation6 A potential advantage conferred by the small size of the scFv is access to hidden epitope regions where full-length mAbs cannot reach. In addition, the cytoxicity of scFv is reduced due to their faster removal from the circulation and better disposal of immune complexes that are formed.Citation1 Because they can be fused with proteins and peptides, the production of scFvs against virtually any important therapeutic target could provide biopharmaceuticals capable of neutralizing key soluble proteins involved in the initiation and progression of diseases such as chronic inflammation and cancer.Citation7 The size and simplicity of scFv allow these molecules to be produced in simple heterologous expression systems like Pichia pastoris, which is a methylotrophic yeast capable of metabolizing methanol as its sole carbon source that is widely used for high-yield recombinant protein expression.
LDL(-) is an endogenous, minimally modified LDL subfraction found in blood plasma.Citation8 Modified forms of LDL are immunogenic and activate both cell-mediated and humoral immune responses, which are pro-inflammatory and likely act in the progression of the chronic inflammatory reaction that is characteristic of atherosclerosis.Citation9 The concentration of LDL(-) is elevated in the plasma of patients at high risk for cardiovascular disease as a result of hypercholesterolemia,Citation10,Citation11 hypertriglyceridemia,Citation12 diabetesCitation13 or coronary artery disease.Citation14,Citation15 LDL(-) has demonstrated pro-inflammatory and pro-atherogenic properties that contribute to the development of atherosclerosis by inducing the recruitment of monocytes to the arterial wall, the secretion of pro-inflammatory mediators by macrophages and endothelial cells, and the induction of autoantibodies.Citation16 Macrophages retained in the vascular wall accumulate large amounts of modified LDL and become foam cells.Citation17 Moreover, macrophages produce pro-in〉ammatory cytokines and participate in functions that integrate the innate and adaptive immune responses during atherosclerosis, including expression of scavenger receptors, such as CD36, and Toll-like receptors (TLRs), such as TLR-4.Citation18
We previously reported that passive immunization using an anti-LDL(-) mAb in Ldlr−/− mice decreased both the cross-sectional area and the number of foam cells in atherosclerotic lesions.Citation19 In this study, we cloned and expressed an anti-LDL(-) 2C7 scFv in P. pastoris and determined its anti-atherogenic activity on 264.7 RAW macrophages and in LDL receptor gene knockout mice (Ldlr−/−). Our findings reinforce the potential of novel antibody-based immunotherapeutic approaches that can lead to therapies for complex diseases such as atherosclerosis.
Results
Obtention of the 2C7 scFv
The cDNAs that code for the VH and VL of 2C7 mAb were obtained by reverse transcription polymerase chain reaction using specific immunoglobulin primer libraries that recognize all VH and VL chain V regions from murine families. The analysis of the sequences in the GenBank and Kabat databanks showed that 2C7 mAb uses a VH segment from Vmu 3.2 (J558) and a Jh4 segment, while VL uses an 8.24/Jk5 segment. The 2C7 scFv was assembled based in the pIg16 vector, a vector for bacterial expression, and then it was subcloned into the P. pastoris expression vector pPIgLE, downstream of the AOX1 promoter (). The expression of 2C7 scFv by recombinant P. pastoris SMD1168 clone was induced by adding 1% methanol and 0.1 M PMSF every 24 h, at a temperature of 20°C. Under these conditions, we obtained a yield of 9.5 mg/L scFv. The protein was purified by nickel affinity chromatography and two bands were detected in the silver-stained polyacrylamide gels and with western blotting (). The apparent affinity of 2C7 scFv for LDL(-) was assayed by direct ELISA using nLDL as a negative control and 2C7 mAb as a positive control. The results showed that either recombinant 2C7 scFv or mAb were able to bind specifically to LDL(-) ().
Figure 1. Schematic representation of the 2C7 scFv expression cassette. The scFv expression is driven by the Pichia pastoris Alcohol Oxidase 1 promoter. The Saccharomyces cerevisiae α-mating type pre-pro-protein leader sequence (PS) is upstream of the 2C7 scFv coding region. The VH gene is flanked by XmaI (X) and Xba I (Xb) restrictions sites. After the linker peptide coding region (L), the VL coding sequence is found in between BglII (B) and Xho I (Xh) sites. A hexahistidine tag (H) is found at the 3′end of the gene followed by a stop codon just before the EcoRI (E) site.
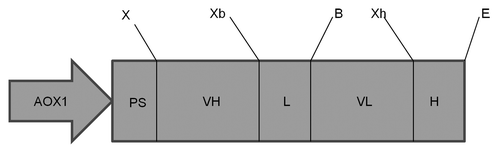
Figure 2. Recombinant protein purification. (A) SDS-PAGE analysis of the protein purified by affinity chromatography from the crude supernatant in line 2 and purified scFv protein from previously concentrated and dialyzed supernatant in line 3. Line 1 corresponds to molecular weight marker. (B) Western blotting analysis. Line 1: purified scFv protein from previously concentrated and dialyzed supernatant. Line 2: purification from the crude supernatant. Line 3: molecular weight marker.
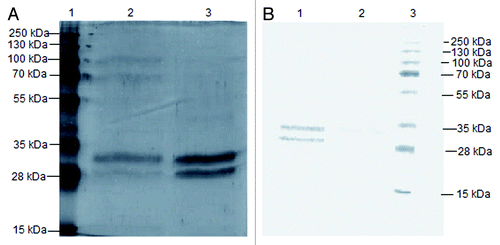
Figure 3. Evaluation of the specificity of 2C7 scFv to LDL(-) by ELISA. 2C7 scFv was added at a concentration of 20 µg/mL to ELISA microplate coated with 1 µg/mL of LDL(-) or nLDL. The microplate was incubated with an anti-His mouse IgG antibody and HRP-conjugated anti-mouse IgG. The absorbance was measured at 450 nm. The results of independent experiments, performed in triplicate, are expressed as the means ± SEM *p < 0.05; **p < 0.01 compared with control; ANOVA followed by the Tukey-Kramer test.
Analysis of glycosylation of the 2C7 scFv
The purified 2C7 scFv showed two bands in SDS-PAGE with apparent expected MWs of 30 and 28 kDa, respectively, that were immunoreactive with anti-His antibody. To investigate whether the two purified bands were produced due to hyperglycosylation, the protein was deglycosylated with Endo H. Only one putative N-glycosylation site at CDR-1 of 2C7 scFv light chain was predicted using the BioEdit software. The Endo H-treated material was analyzed by gel electrophoresis and western blotting. The results showed that the deglycosylation treatment of 2C7 scFv converted the two bands into a single band, confirming the predicted glycosylation ().
Figure 4. Recombinant protein glycosylation profile. The affinity-purified recombinant 2C7 scFv was treated with Endoglucanase H. The eletrophoretic profile was analyzed by SDS-PAGE (left) and western blotting (right) using anti-His IgG Mouse, anti-mouse IgG-HRP and detection with ECL substrate. A protein of one band is observed after endoglucanase treatment (line 2) and compared with the two bands shown in the untreated samples (line 1).

Detection of negatively charged LDL subfraction in blood plasma of Ldlr−/− mice
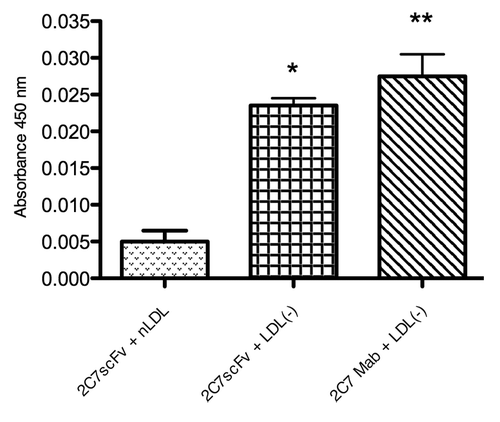
The anion exchange FLPC chromatography used to separate the LDL subfractions () showed three peaks where the first corresponds to the components of the antioxidant cocktail used to prevent oxidation of samples. A second peak corresponds to the native LDL subfraction, similar to the chromatogram of human LDL (). The third peak contains the LDL subfraction with the highest negative charge () with a retention time similar to the human LDL(-) subfraction. Thus, the peaks 2 and 3 detected in the fast protein liquid chromatography (FPLC) chromatogram correspond to mouse unmodified LDL(or nLDL) and to LDL(-), respectively. To confirm the identity of the mice LDL subfractions isolated by FPLC, ELISA assays were done with each of these LDL subfractions and compared with nLDL and LDL(-) separated from human LDL by using the 1A3 and 2C7 monoclonal antibodies and the 2C7 scFv, developed by our group. The reactivity profiles of both mouse and human LDL subfractions to the antibodies were similar (). The reactivity of the 1A3 mAb was lower to human and murine LDL(-) compared with the 2C7 mAb and the 2C7 scFv. Thus, the presence of LDL(-) in the LDL fraction of Ldlr−/−mice was confirmed by physical chemical and antigenic characteristics.
Figure 5. Isolation of LDL(-) from Ldlr−/− mice. FPLC chromatographic analysis of mice LDL (A) and human LDL (B), fractionated into peaks 1, 2 and 3. Mice LDL samples were fractionated by anion exchange liquid chromatography based on differences of superficial charges of LDL subfractions. The peak 1 contains components of the antioxidant cocktail used to avoid in vitro LDL oxidation. The reactivity of peaks 2 and 3 to 1A3 and 2C7 monoclonal antibodies and 2C7 scFv were tested by (C) ELISA assays with anti-his and HRP-conjugated anti-mouse antibodies. Absorbance was measured at 450 nm.
Macrophage viability
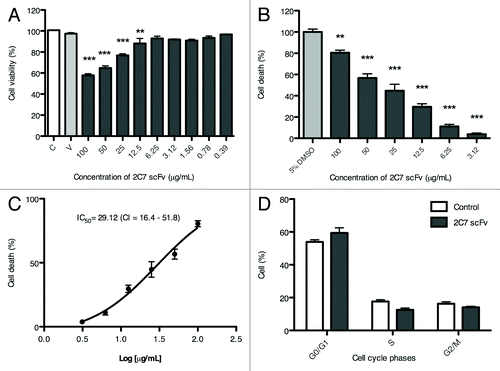
The MTT assay showed that cell viability was not affected in the presence of up to 6.25 µg/mL 2C7 scFv (). At the highest concentration tested (100 µg/mL 2C7 scFv), cell viability was approximately 60%. In the flow cytometry assays, only 2C7 scFv concentrations higher than 6.25 µg/mL induced death compared with non-treated macrophages (). The percentage of cell death relative to the log of the concentration of 2C7 scFv is shown in ; 50% of total cell death (apoptosis + necrosis) occurred at 29.12 µg/mL 2C7 scFv. At 6.25 µg/mL 2C7 scFv, no significant changes were observed in any stage of the cell cycle in relation to the control ().
Figure 6. Effect of 2C7 scFv on RAW macrophages. (A) Cell viability evaluated by MTT. (B) Relative cell death results normalized in relation to DMSO control (100%). (C) Percentage of cell death relative to the log of 2C7 scFv concentration. (D) Cell cycle data. The results of independent experiments, performed in triplicate, are expressed as the means ± SEM *p < 0.05; **p < 0.01 compared with control; ANOVA followed by the Tukey-Kramer test.
LDL(-) uptake by RAW macrophages
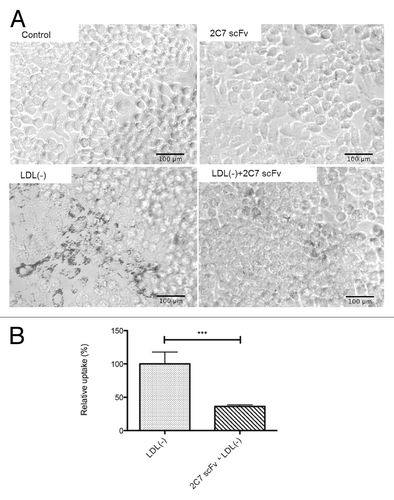
The effect of 2C7 scFv on the formation of foam cells by RAW 264.7 macrophages is shown in . The macrophages incubated with LDL(-) in the presence of 2C7 scFv showed a decrease in intracellular lipid droplets compared with the macrophages treated with LDL(-) in the absence of 2C7 scFv. The semi-quantification of foam cells showed lower LDL(-) uptake by the macrophages when treated with 2C7 scFv compared with untreated cells ().
Figure 7. LDL uptake by RAW macrophages. RAW macrophages (105 cells/well) were incubated in the presence of LDL(-) and 2C7 scFv for 16 h. (A) Representative images show macrophages stained with Oil Red O. Images were obtained using the Motic Images Plus version 2.0 program at a 20 × magnification. (B) Semi-quantification of lipid droplet accumulation in macrophages treated with 2C7 scFv and LDL(-) compared with macrophages treated only with LDL(-). Representative images are from three independent experiments.
Receptor binding studies
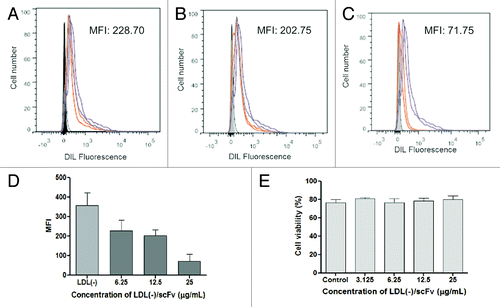
To investigate the binding of LDL(-) to RAW 264.7 macrophage receptors, studies were done by flow cytometry and measurement of fluorescence intensity of DIL-labeled LDL(-) to assess the uptake of LDL(-) by macrophages. show representative flow cytometry analyses with median fluorescence intensity (MFI) illustrating the autofluorescence of cells. The uptake of LDL(-)-DIL by macrophages (positive control) was inhibited by antibodies reacting with CD36 and CD14 (). MFI values in show that anti-CD36 and anti-CD14 antibodies were able to reduce the uptake of LDL(-) by macrophages compared with the control. As expected, the anti-TLR4 antibody did not decreased fluorescence intensity compared with control. In cells preincubated with sets of anti-CD36/CD14 and anti-CD14/TLR4 antibodies, there was greater reduction in LDL(-) uptake compared with the incubation of anti-CD36/TLR4 antibodies, which showed higher LDL(-) uptake compared with anti-CD14 antibody alone. The DIL-labeled LDL(-) uptake by RAW macrophages was decreased by 2C7 scFv in relation to the uptake of DIL-LDL(-) alone (). The higher the 2C7 scFv concentration, the lower the uptake of LDL(-), as shown in . Also, treatment of LDL(-) and 2C7 scFv induced low death in cells by apoptosis and necrosis assays, so the results with only viable cells were demonstrated ()
Figure 8. Representative images from flow cytometry analysis of the fluorescence intensity of LDL(-)-DIL taken up by RAW 264.7 macrophages blocked with the following antibodies: (A) anti-CD36, (B) anti-CD14, (C) anti-TLR4, (D) anti-CD36/CD14, (E) anti-CD36/TLR4, (F) anti-CD14/TLR4. (G) Graph showing the decrease of LDL(-)-DIL uptake with blocking antibodies specific to CD36, CD14, and TLR4 receptors. Data are represented as mean of MFI values.
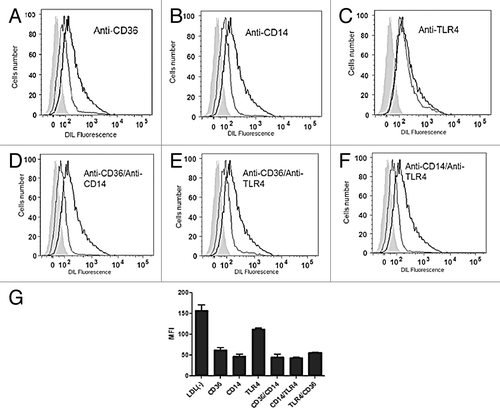
Table 1. Fluorescence intensity of LDL(-)-DIL taken up by RAW macrophages in the presence of anti-CD36, anti-CD14 and anti-TLR4 antibodies
Figure 9. Inhibition of LDL(-)-DIL uptake by different concentrations of 2C7 scFv. The concentrations (A) 6.25, (B) 12.5, and (C) 25 µg/mL were tested. (D) represents quantitative data of uptake inhibition, from the mean of MFI values and (E) cell viability with co-incubation of LDL(-) and 2C7 scFv measured by flow cytometry analysis.
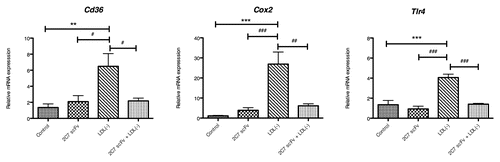
Expression of pro-atherogenic genes in macrophages
To understand the mechanisms of action of 2C7 scFv on RAW macrophages treated with LDL(-), the expression of several genes linked to the development of atherosclerosis was analyzed, and the results are shown in . The incubation of RAW macrophages with 6.25 µg/mL 2C7 scFv did not induce a significant effect on mRNA expression levels. In contrast, the incubation of macrophages with 37.5 µg/mL LDL(-) induced a statistically significant increase of Cd36, Cox-2 and Tlr-4 mRNA levels. When RAW macrophages were incubated with LDL(-) in the presence of 2C7 scFv, however, significant inhibition of the LDL(-) induced effects on the atherogenic gene mRNA levels was observed.
Figure 10. Effect of 2C7 scFv on the relative expression of Cd36, Cox-2 and Tlr- 4 mRNA. Cells were treated with 2C7 scFv (6.25 μg/mL), LDL(-) (37.5 μg/ml) or 2C7 scFv + LDL(-) for three hours. The results of independent experiments, performed in triplicate, are expressed as the means ± SEM *p < 0.05 vs. control; #p < 0.05 compared with treatment with LDL(-); ANOVA followed by the Tukey-Kramer test.
Effect of 2C7 scFv on experimental atherosclerosis
The atherosclerotic lesions at aortic sinus of Ldlr−/− mice treated with 2C7 scFv are shown in . The morphometric analysis of the atherosclerotic plaques demonstrated that the lesion area was significantly decreased (p < 0.05) following passive immunization of Ldlr−/− mice with 2C7 scFv compared with controls treated with the PBS vehicle (). The percentages of the atherosclerotic lesion areas of treated groups relative to the control group (vehicle) are represented in . The lipid profile data showed no significant changes of lipid levels among the studied groups ().
Figure 11. Effect of passive immunization of Ldlr−/− male mice with 2C7 scFv on the atherosclerotic lesion development at the aortic sinus. (A) Representative sections of the aortic sinus from the control, 2C7 scFv and positive control groups are shown. Images were obtained using the NIS-Elements AR(tm) version 3.10 at a 10 × magnification. (B) Mean ± SEM of atherosclerotic lesion area. (C) Percent of atherosclerotic lesion area in relation to the control. p < 0.05 compared with control; ANOVA followed by the Tukey-Kramer test.
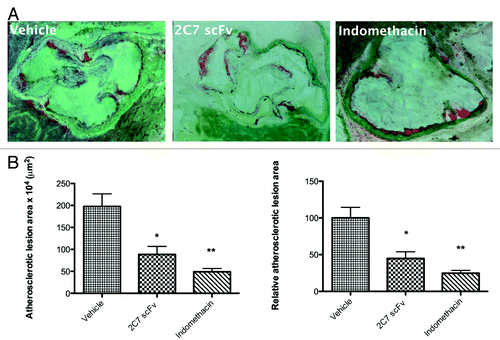
Table 2. Lipid profile of Ldlr−/− mice after passive immunization with 2C7 scFv
Discussion
In this study, we described the construction, expression and characterization of the recombinant 2C7 scFv antibody fragment and its effect on macrophages and atherosclerotic lesions. Recombinant antibodies, including scFv, are good alternatives for the treatment of various diseases because they are targeted therapeutics that generally show good pharmacokinetics and biodistribution. In addition, their production can be rapid and economical.Citation20
Our 2C7 scFv was expressed in P. pastoris, an eukaryotic organism capable of producing secretable soluble proteins with modifications such as disulfide bridges and glycosylation,Citation21 and showed an apparent affinity for LDL(-) only slightly lower than that of the parental 2C7 mAb. This result was expected because scFvs are monovalent, whereas the full length mAb harbors two binding sites for the antigen. Importantly, the 2C7 scFv maintained the same parental antibody specificity for LDL(-), and it is not reactive to native LDL.
Although a 28 kDa protein was detected in the culture supernatant, we observed the presence of two bands with molecular weights of ~28 and 30 kDa in polyacrylamide gels after purification with nickel affinity chromatography. Some studies also reported the expression of two bands of scFv in P. pastoris; however, they attributed this to degradationCitation22,Citation23 or to incomplete cleavage of the signal sequence.Citation24 Other studies indicate that the additional bands detected may be due to the glycosylation of recombinant proteins with the addition of mannose residues that increase the recombinant protein molecular weight.Citation25,Citation26 Yeast can perform glycosylation of the amide nitrogen of asparagine residues in the consensus sequence Asn-X-Thr/Ser, providing N-linked glycosylation. This sequence was found in the 2C7 scFv VL CDR1. The electrophoretic profile of the 2C7 scFv was modified after treatment with Endo H and showed one band. This suggests that the presence of two bands after nickel purification was a result of glycosylation, and not proteolytic degradation.
Wild-type mice contain a low level of cholesterol in the IDL/LDL fraction. Ldlr−/− mice, however, show marked increase in the IDL/LDL fraction with high LDL-cholesterol, accompanied by an increase in the amount of apoB-100 and apoE in the plasma.Citation27 In Ldlr−/− mice, there is also a reduction in LDL clearance (half-life of 5 h) compared with wild-type mice (half-life of 2 h).Citation27 This increase in the permanence of LDL in blood circulation, combined with the higher LDL level in this animal model, should contribute to the modification of the LDL particles, which allowed their recognition by the 2C7 mAb and scFv, as was observed in the ELISA assay.
The MTT assay showed that glycosylation did not affect the cell viability for 24 h, as the treatment with RAW macrophages was performed for 16 h. Experimental data suggest that glycosylation was not observed in the murine Fab portion derived from anti-LDL(-) mAb because only one band was visualized in polyacrylamide gel (unpublished results). Thus, glycosylation may be a result of the heterologous expression in P. pastoris; this did not interfere with scFv binding specificity to LDL(-) or with its in vitro biologic activity.
In a cytotoxicity assay using RAW 264.7 macrophages, flow cytometry data showed no induction of either apoptosis or necrosis at concentrations up to 6.25 µg/mL 2C7 scFv. Thus, this concentration was used for further experiments with the macrophages. We previously reported that LDL(-) stimulates the expression of Cd36, promoting the accumulation of lipid droplets in the cytoplasm of macrophages and transforming them into foam cells.Citation28 Here, it is clearly shown that 2C7 scFv inhibited LDL(-) uptake by macrophages and downregulated the mRNA expression of Cd36. These findings suggest a possible inhibitory action by this recombinant scFv on atherogenesis because it could prevent formation of foam cells in arterial intima. Moreover, 2C7 scFv inhibited the overexpression of pro-inflammatory genes that play an important role in the atherogenic process. We have shown here that LDL(-) induces an upregulation of Tlr-4 and Cox-2 mRNA expression in RAW 264.7 macrophages. In contrast, 2C7 scFv was able to inhibit these LDL(-) actions by blocking the increase of both Tlr-4 and Cox-2 mRNA expression. The inhibition of TLR-4 by 2C7 scFv is highly relevantCitation29,Citation30 because it has been shown that minimally modified LDL induces the pro-atherogenic activation of macrophages by a TLR-4-dependent mechanism, stimulating the expression of pro-inflammatory cytokines.Citation30 The COX-2 gene is expressed in the foam cell macrophages present in atherosclerotic lesions,Citation31 and its overexpression induces the formation of early atherosclerotic lesions in Ldlr−/−miceCitation32 and probably in human atherosclerotic lesions.Citation33 Therefore, the effect of 2C7 scFv on RAW 264.7 macrophages, which promotes the downregulation of Cox-2, Tlr-4 and Cd36 mRNA expression, indicates that this recombinant antibody fragment is able to block the pro-inflammatory and pro-atherogenic actions of LDL(-).
The receptor binding assays done in the present study showed that the entry of LDL(-) in RAW macrophages can occur through CD14 and CD36 receptors, which could be a route by which LDL(-) was able to induce proinflammatory effects on macrophages. In fact, a previous report showed that minimally modified LDL can bind to CD14, making it a likely candidate receptor for LDL(-).Citation29 Recently, a relationship has been established between the increase of CD14 and CD36 expression in circulating human monocytes and the risk of coronary artery disease in patients with cardiovascular disease.Citation34 CD14 is also able to induce the release of pro-inflammatory cytokines in monocytes and macrophages after stimulation by mmLDL.Citation35 We demonstrated that at 6.25 µg/mL 2C7 scFv reduced the uptake of LDL(-)-DIL by macrophages, and the reduction was greater at higher concentrations of 2C7 scFv. Although cell viability was decreased in the presence of 12.5 and 25 µg/mL 2C7 scFv, cell viability was unaffected by the co-incubation of LDL(-) and 2C7 scFv at all concentrations used in the flow cytometry analysis. Thus, a dose-dependent effect occurs for the inhibition of LDL(-) uptake by 2C7 scFv.
The atheroprotective action of the 2C7 scFv was confirmed by our studies with Ldlr−/−mice. The antibody fragment was able to decrease the atheroma area in the aortic sinus of these animals by approximately 44% with a single weekly dose. Moreover, the atheroprotective action of 2C7 scFv was unrelated to changes in lipid concentrations in blood plasma. Recombinant antibodies against peptides of MDA-modified apoB 100 have been shown to significantly decrease atherosclerosis.Citation36 As previously reported, scFv and Fab against in vitro oxidized LDL inhibited foam cell formation and the progression of atherosclerotic lesions by blocking the binding of oxLDL to macrophages and their subsequent internalization.Citation37 Moreover, passive immunization with anti-tumor necrosis factor and anti-platelet glycoprotein IIb/IIIa antibodies have been reported for the treatment of unstable angina and the prevention of restenosis, respectively, as reviewed elsewhere.Citation38
In conclusion, this study, which focused on the production and assessment of a recombinant antibody fragment that recognizes negatively charged LDL particles, showed that 2C7 scFv was able to inhibit the formation of macrophage-derived foam cells, the expression of pro-inflammatory factors and the progression of atherosclerosis in Ldlr−/− mice. Based on these data, the 2C7 scFv has potential value for future studies on the prevention or treatment of atherosclerosis.
Materials and Methods
Bacteria strains, yeast strains and plasmids
Escherichia coli DH5α was used for all plasmid manipulations. SMD1168 strain P. pastoris was purchased from Invitrogen Life Technologies (Cat# C17500). For the assembly of the expression cassette, pGEM-T Easy plasmid was purchased from Promega (Cat# A1360). The pIg16 and pPIG16 plasmids were previously described.Citation39,Citation40
Cloning of the 2C7 scFv
The hybridoma 2C7D5F10 (2C7)Citation41 was cultivated in bottles containing RPMI medium supplemented with 10% fetal bovine serum, 100 μg/mL streptomycin sulfate, 100 U/mL penicillin G sodium and 0.25 μg/mL amphotericin B. The bottles were incubated at 37°C in a 5% CO2 atmosphere at 95% relative humidity until 106 cells were obtained. To isolate the total RNA, the cells were treated with 1 mL of TRIzol (Cat# 15596–026, Invitrogen Life Technologies) according to the manufacturer's instructions. The cDNAs coding for the antibody variable heavy-chain gene (VH) and the variable light-chain gene (VL) were synthesized using 1 µM each of the primers κ18 (5′TACAGTTGGTGCAGCATC3′) and 1 (5′TGGACAGGGATCCAGAGTTCCAGGTCACT3′) to prepare C and C, respectively. For the amplification of the VH and VL region cDNA, we used a library of sense primers and the anti-sense primers that were previously described.Citation42-Citation44 Amplified VH and VL cDNAs were cloned in the pGEM-T Easy plasmid following the manufacturer's instructions. Five clones from each variable region were sequenced in both directions with the T7 (5′ TAATACGACTCATATAGGG 3′) and SP6 (5′ GATTTAGGTGACACTATAG 3′) primers using an automatic sequencer MegaBACE 1000 (GE Healthcare) and a DYEnamic ET Dye Terminator Kit (with Thermo Sequenase™ II DNA Polymerase, Cat# US81095, GE Healthcare). For the assembly of murine scFv, the sequences were analyzed by Electropherogram Quality Analysis (available at www.biomol.unb.br/phph/) using the GenBank and Kabat databanks (www.ncbi.nlm.nih.gov/BLAST/).
The murine scFvs genes were assembled using the pIg16 plasmid expression cassette framework.Citation45 This plasmid encodes the gene for Z22 scFv fused to the staphylococcal protein A domain (SpA).Citation46 The 2C7 VH and VL genes were reamplified using oligonucleotides that created specific restriction sites. The assembly was performed by replacing the Z22 VH and VL genes with the anti-LDL(-) VH and VL genes and by introducing a hexahistidine tag at the 3′ terminus of 2C7 VL. This final sequence was inserted into pPIgLE yeast expression vector, a plasmid modified from pPIg 16 vector.
Production of 2C7 scFv in Pichia pastoris
P. pastoris SMD1168 cells were electroporated with a BTX electroporator model ECM 830, in the presence of linearized plasmid DNA. His+ transformants were screened and cultured using the method previously described.Citation47 2C7 scFv was expressed in 200 mL of BMGY medium at 30°C at 200 rpm until an OD600 of 2–6 was reached. The cells were then centrifuged and resuspended in 200 mL of BMMY medium, with an addition of 1% methanol and 1 mM PMSF every 24 h, and were then incubated for 2 d at 20°C with agitation. The supernatant was harvested by centrifugation, and the cells were resuspended in another 200 mL of BMMY medium. The culture was incubated for an additional 2 d in the same conditions. The supernatant of the culture was harvested by centrifugation, filtered through a 0.45 μm filter, and 1 mM PMSF was added. The supernatants were added to 1 mL of Ni Sepharose 6 Fast Flow resin (Cat# 17–5318–01, GE Healthcare). The supernatant (flow through) was decanted, and the resin was poured into a 1.5 cm x 12 cm (20 mL) Econo-Pac Chromatography column (Cat# 732–1010, Bio-Rad Laboratories). 2C7 scFv was eluted with binding buffer containing 500 mM imidazole. The appropriate fractions were pooled, and the buffer was exchanged with PBS and concentrated using centrifugal filtration devices (Vivaspin MWCO 10,000, Cat# 28–9323–60, GE Healthcare). The purified proteins were separated by SDS-PAGE and then transferred to a Hybond ECL nitrocellulose membrane (GE Healthcare). The membrane was blocked for 16 h with 5% skim milk in PBS at 4°C and subsequently incubated with the following antibodies for 1 h at room temperature: anti-His mouse IgG (Cat# 27–4710–01, GE Healthcare) and anti-mouse IgG-HRP (Cat# A1055, Zymed). The target proteins were detected using an ECL Advance Western Blotting Detection Kit (Cat# RPN2135, GE Healthcare) according to the manufacturer's instructions.
Deglycosylation of 2C7 scFv
For enzymatic deglycosylation, 1 μg of purified 2C7 scFv was denatured with 0.5% SDS and 0.04 M dithiothreitol (DTT) and heated at 100°C for 10 min. It was then added to a reaction buffer (0.5% sodium citrate, pH 5.5) with 1000 units of endoglycosidase H (Cat# P0702S, Endo H, New England Biolabs), which hydrolyzes a single N-acetyl-d-glucosamine (GlcNAc) sugar residue, to cleave high-mannose glycans. The digestion was incubated at 37°C for 16 h and assayed by SDS-PAGE and western blotting as described above.
ELISA assay for 2C7 scFv affinity
The isolation of LDL(-) from human plasma was performed as previously reported.Citation41 ELISA assays were done according to a previous workCitation41 with minor modifications including the addition of anti-His mouse IgG (diluted 1:1,000 with 1% skim milk; GE Healthcare) to recognize 2C7 scFv. Specific binding was detected with tetramethyl benzidine (TMB) substrate for color development, and the absorbance was measured at 450 nm. All experiments were approved by the Research Ethics Committee of the Faculty of Pharmaceutical Sciences of the University of Sao Paulo.
Analysis of LDL subfractions from Ldlr−/− mice
A pool of blood samples was obtained from Ldlr−/− mice treated with hypercholesterolemic diet. Blood was collected with heparinized syringes and the blood plasma was separated by centrifugation. Then, the total LDL fraction was isolated from plasma by ultracentrifugation at 56,000 rpm for 7 h at 4°C. After removing the triglycride-rich fractions in the supernatant, the infranatant was submitted to a second ultracentrifugation to isolate the LDL fraction. The subfractions of LDL were then separated by FPLC according to the protocol previously described.Citation41
For the ELISA assay, a 96-well microplate was coated with 10 µg/mL of the following samples: 2 and 3 peaks of FPLC chromatogram of mice samples, human nLDL and LDL(-) for 16 h at 4°C in carbonate-bicarbonate buffer, pH 9.6. After blocking the microplate with 2% milk diluted in PBS, the samples were incubated with 10 µg/mL of 1A3 and 2C7 mAbs and 2C7 scFv for 1 h and 30 min at 37°C. Then, the microplate was incubated with anti-mouse-HRP antibody (diluted 1:1,000 in 1% milk, CAT#1706516, BioRad) for detection with 1A3 and 2C7 mAbs and anti-His (diluted 1: 1,000 with 1% milk, CAT#27471001, GE Healthcare) for detection with 2C7 scFv. The binding of samples to the antibodies was evaluated by using TMB as substrate and measuring the absorbance at 450 nm.
Cell culture conditions
Murine macrophages of the RAW264.7 cell line were obtained from the cell bank of the Federal University of Rio de Janeiro (Cat# 0212, UFRJ). RAW 264.7 macrophages were cultured in RPMI media containing 2 mM L-glutamine, 100 μg/mL streptomycin, 100 U/mL penicillin and 10% fetal bovine serum at 37°C in 5% CO2 in fully humidified air.
Cell viability, cell death and cell cycle assays
The MTT assay was performed as previously described.Citation48 For the apoptosis and necrosis assays (cell death), wells containing 1x105 RAW macrophages were treated with different concentrations (3.12 to 100 µg/mL of 2C7 scFv, 12.5 to 62.5 µg/mL of LDL(-) and 37.5 µg/mL of LDL(-) with 3.125 to 25 µg/mL of 2C7 scFv). The cell death and cell cycle assays were performed by flow cytometry. Following 24 h of treatment, the cells were resuspended in the reaction buffer supplied with the kit for the detection of apoptosis and necrosis (APOAF, Cat# A9210, Sigma-Aldrich); 0.625 µg of annexin V - FITC and 2.0 µg of propidium iodide (Cat# P2667, Sigma-Aldrich) were added to the cells according to the manufacturer's instructions. The cells were incubated for 10 min at room temperature, protected from light, and analyzed with a FACSCanto flow cytometer (BD Biosciences, San Jose, CA, USA). Dimethyl sulfoxide (5%, DMSO, Cat# D8418, Sigma-Aldrich) was used as the positive control for cell death and 10,000 events were observed. For the cell cycle analysis, 2 × 105 cells per well of RAW macrophage were incubated under the same conditions mentioned previously, but the wells were only treated with a concentration of 6.25 µg/mL 2C7 scFv. The cells were lysed with 0.1% sodium citrate and 0.1% Triton, treated with 10 mg/mL RNase A (Cat# 12091–039, Invitrogen Life Technologies) and stained with 1 mg/mL propidium iodide for 30 min, with protection from light, before taking measurements. Data analysis was performed using FlowJo version 9.5.1 software (TreeStar).
LDL uptake assay
The LDL(-) uptake assay for RAW 264.7 macrophages was performed according to previous reports.Citation49 Macrophages were exposed to the following treatments: 37.5 μg/mL native LDL (nLDL), 37.5 μg/mL LDL(-) and 37.5 μg/mL LDL(-) plus 6.25 μg/mL 2C7 scFv. Untreated cells were used as the control. The cells were treated for 16 h and evaluated for their level of LDL uptake. The cells were fixed in PBS containing 10% formaldehyde for 30 min at room temperature. Subsequently, the intracellular lipid droplets were stained with Oil Red O (Cat# O0625, Sigma-Aldrich) for 1 h, and their images were obtained with Motic Images Plus 2.0 software (Micro-Optics) for semi-quantification of the foam cells.
Gene expression analysis by qRT-PCR
The LDL uptake assay was used for gene expression analysis. RNA from the treated cells was isolated with TRIzol according to the manufacturer's recommendations. The cDNA was synthesized from 2 μg of total RNA using oligo-dT 12–18 and Superscript III (Cat# 12574–018, Invitrogen Life Technologies). For the real time-PCR reactions, 20 ng of cDNA and specific primers were used. The reactions were performed according to the SYBR Green Master Mix (Cat# 4364346, Applied Biosystems) instructions. The following primers were used: CD36 scavenger receptor (Cd36) gene: sense primer, 5′-TTTCCTCTGACATTTGCAGGTCTA-3′, and anti-sense primer, 5′-AAAGGCATTGGCTGGAAGAA-3′; toll-like receptor-4 (Tlr-4): sense primer, 5′-TCATGGCACTGTTCTTCTCCT-3′ and anti-sense primer, 5′-CATCAGGGACTTTGCTGAGTT-3′; cyclooxygenase-2 (Cox-2) enzyme: sense primer, 5′-TGGTGCCTGGTCTGATGATG-3′ and anti-sense primer, 5′-GTGGTAACCGCTCAGGTGTTG-3′ and 18S rRNA: sense primer, 5′-GTAACCCGTTGAACCCCATT-3′ and anti-sense primer, 5′-CCATCCAATCGGTAGTAGCG-3′. The expression levels of mRNA were evaluated by the ΔΔCt method.Citation50
1,1’-diotadecyl-3–3-3′-3′-tetramethylindocarbocyanine perchlorate(DIL) labeling of LDL(-)
One mg of LDL(-) was incubated with 150 µg of DIL (CAT#D282, Life Technologies) diluted in 2 mL of lipoprotein deficient serumCitation51 and this mixture was incubated at 37°C for 8 h. After incubation, the mixture was separated by ultracentrifugation at 56,000 rpm for 7 h at 4°C to separate the LDL(-) from the excess of free DIL. LDL(-)-DIL was dialyzed against PBS and quantified by BCA method (CAT #23225, Thermo Scientific).
Receptors binding studies in macrophages
For binding studies, 10 × 105 macrophage cells were plated per well and 21 h later the cells were pre-incubated with 10 µg/mL of blocking antibodies against CD36 (CAT#Ab78054, Abcam), CD14 (CAT#Ab78313, Abcam) and TLR-4 (CAT#Ab47093, Abcam) receptors. After 3 h, 37.5 µg/mL LDL(-)-DIL was added to the cells and maintained for 16 h as mentioned for cell culture conditions described in the Materials and Methods section. To measure the inhibition of LDL(-)-DIL uptake, RAW macrophages were treated with a predetermined concentration of 37.5 µg/mL LDL(-) and varying concentrations of 2C7 scFv (6.25, 12.5 and 25 µg/mL) for 16 h. The medium was then removed and cells were detached from the plate using cold PBS and centrifuged at 1500 rpm for 5 min. The cells were washed 2 times with PBS. Finally, cells were resuspended in 200 µL of PBS and the fluorescence of LDL(-)-DIL was determined by flow cytometry. The signals from DIL were shown in a logarithmic fluorescence intensity, expressed as the difference in the MFI captured from cells treated with blocking antibodies or 2C7 scFv compared with cells treated only with LDL(-)-DIL.
Animals, chow, and experimental design
Male C57BL/6J homozygous LDL receptor-deficient mice (Ldlr−/−) were purchased from Jackson Laboratory (Bar Harbor). The animals were maintained in individual cages at 22°C on a 12 h light–dark cycle. A total of 24 Ldlr−/−mice (n = 8 per group, 12 weeks old) were divided into three groups and were intravenously administered a single dose per week of one of the following: vehicle (PBS), 2C7 scFv (5 mg/kg of body weight) and anti-inflammatory positive control (indomethacin, 1 mg/kg of body weight). The experiments were performed using an initial atherosclerotic lesion protocol as previously described.Citation19 All mice were fed a semisynthetic chow that was based on a Western-type diet containing 20% fat, 0.5% (w/w) cholesterol (Sigma-Aldrich), 0.5% (w/w) colic acid (Sigma-Aldrich), 16.5% casein, vitamins and minerals according to the recommendations of American Institute of Nutrition (AIN)-93.Citation52 All procedures were approved by the Ethics Committee for Animal Studies of the Faculty of Pharmaceutical Sciences, University of Sao Paulo in agreement with the guidelines of the Brazilian College for Animal Experimentation.
Biochemical assessment of serum lipid profile
After treatment, mice were anesthetized with xylazine hydrochloride (2.0 g/100 ml; Vetbrands) and ketamine hydrochloride (1.0 g/10 ml; Vetnil) at doses of 5 mg/Kg and 10 mg/kg, respectively, and blood was collected by cardiac puncture. The blood samples were then centrifuged at 1500 x g for 15 min at 4°C to obtain serum. The mice serum was utilized for determination of lipid profile [total cholesterol, triglyceride, cholesterol high-density lipoprotein (HDL-C), cholesterol low-density lipoprotein (LDL-C) and cholesterol very low-density lipoprotein (VLDL-C)]. All determinations were done with commercial kits from Labtest Diagnóstica, by direct methods without previous treatment of the samples. The results of the lipid profile were expressed in mg/dL.
Preparation of histological sections and measurement of atherosclerotic lesion area
The preparation of histological sections and the measurement of atherosclerotic lesion area were performed as previously reported.Citation53 The inclusion of the tissue for slicing was performed in three solutions of different concentrations of gelatin: initially 5% solution of gelatin for three hours, then a 10% solution for three hours and finally a 25% solution for 16 h, with all steps occurring in a water bath (temperature between 40–50°C). The ventricles were sectioned from the apex and base in a plane parallel to a line defined by the edges of the lateral atria. Consecutive cuts with 6 mm thick were collected between the aortic sinus and the aortic onset with an average length of 250–300 microns.Citation54 The slides were stained with Oil Red-O (Sigma) and the sections were analyzed by Nikon optical microscope coupled to a camera for image capture program performed by the NIS-Elements AR (tm) version 3.10. To obtain the area of the lesions and quantify atherosclerotic lesions close to the aortic valve in the aortic root, Axio Vision ® was used for image acquisition, processing and analyses of all histological sections. All analyses were double-blind and were performed independently by two observers.
Statistical analysis
Results were expressed as the means ± SEM. The mRNA expression data were analyzed using ANOVA and Student t-tests. Tukey's test was used for the analysis of paired comparisons. All calculations were performed using GraphPad Prism software. A value of p < 0.05 was considered statistically significant.
| Abbreviations: | ||
| scFv | = | single chain variable fragment |
| nLDL | = | native LDL |
| LDL(-) | = | electronegative LDL |
| Cd36 | = | cluster of differentiation 36 |
| Tlr-4 | = | toll like receptor 4 |
| Cox-2 | = | cyclooxygenase 2 |
Acknowledgments
The authors wish to thank M.Sc. Alejandro Esteban Cuevas Villegas for adjustment of the images, M.Sc. Elaine Moura Augusto for the isolation of LDL(-) and M.Sc. Renata Albuquerque for collaboration with flow cytometry. This research was supported by FAPESP (Fundação de Amparo à Pesquisa de São Paulo) and INCT_if/CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico). SMK and MFC received scholarships from FAPESP (Fundação de Amparo à Pesquisa do Estado de São Paulo).
Disclosure of Potential Conflicts of Interest
No potential conflict of interest was disclosed.
References
- Kipriyanov SM, Le Gall F. Generation and production of engineered antibodies. Mol Biotechnol 2004; 26:39 - 60; http://dx.doi.org/10.1385/MB:26:1:39; PMID: 14734823
- Chan AC, Carter PJ. Therapeutic antibodies for autoimmunity and inflammation. Nat Rev Immunol 2010; 10:301 - 16; http://dx.doi.org/10.1038/nri2761; PMID: 20414204
- Reichert JM. Which are the antibodies to watch in 2013?. MAbs 2013; 5:1 - 4; http://dx.doi.org/10.4161/mabs.22976; PMID: 23254906
- Holliger P, Hudson PJ. Engineered antibody fragments and the rise of single domains. Nat Biotechnol 2005; 23:1126 - 36; http://dx.doi.org/10.1038/nbt1142; PMID: 16151406
- Shen Z, Yan H, Zhang Y, Mernaugh RL, Zeng X. Engineering peptide linkers for scFv immunosensors. Anal Chem 2008; 80:1910 - 7; http://dx.doi.org/10.1021/ac7018624; PMID: 18290668
- Ahmad ZA, Yeap SK, Ali AM, Ho WY, Alitheen NBM, Hamid M. scFv antibody: principles and clinical application. Clin Dev Immunol 2012; 2012:980250; http://dx.doi.org/10.1155/2012/980250; PMID: 22474489
- Quiroz FG, Sinclair SM. Engineering antibody fragments: replicating the immune system and beyond. Revista Ingenieria Biomedica 2010; 4:39 - 51
- Avogaro P, Bon GB, Cazzolato G. Presence of a modified low density lipoprotein in humans. Arteriosclerosis 1988; 8:79 - 87; http://dx.doi.org/10.1161/01.ATV.8.1.79; PMID: 3341993
- Hulsmans M, Holvoet P. The vicious circle between oxidative stress and inflammation in atherosclerosis. J Cell Mol Med 2010; 14:70 - 8; http://dx.doi.org/10.1111/j.1582-4934.2009.00978.x; PMID: 19968738
- Bittolo-Bon G, Cazzolato G, Avogaro P. Probucol protects low-density lipoproteins from in vitro and in vivo oxidation. Pharmacol Res 1994; 29:337 - 44; http://dx.doi.org/10.1016/1043-6618(94)80055-3; PMID: 7971685
- Sánchez-Quesada JL, Otal-Entraigas C, Franco M, Jorba O, González-Sastre F, Blanco-Vaca F, et al. Effect of simvastatin treatment on the electronegative low-density lipoprotein present in patients with heterozygous familial hypercholesterolemia. Am J Cardiol 1999; 84:655 - 9; http://dx.doi.org/10.1016/S0002-9149(99)00411-7; PMID: 10498134
- Sánchez-Quesada JL, Benítez S, Otal C, Franco M, Blanco-Vaca F, Ordóñez-Llanos J. Density distribution of electronegative LDL in normolipemic and hyperlipemic subjects. J Lipid Res 2002; 43:699 - 705; PMID: 11971940
- Sánchez-Quesada JL, Pérez A, Caixàs A, Rigla M, Payés A, Benítez S, et al. Effect of glycemic optimization on electronegative low-density lipoprotein in diabetes: relation to nonenzymatic glycosylation and oxidative modification. J Clin Endocrinol Metab 2001; 86:3243 - 9; http://dx.doi.org/10.1210/jc.86.7.3243; PMID: 11443196
- Mello AP, da Silva IT, Abdalla DS, Damasceno NR. Electronegative low-density lipoprotein: origin and impact on health and disease. Atherosclerosis 2011; 215:257 - 65; http://dx.doi.org/10.1016/j.atherosclerosis.2010.12.028; PMID: 21292266
- Niccoli G, Bacà M, De Spirito M, Parasassi T, Cosentino N, Greco G, et al. Impact of electronegative low-density lipoprotein on angiographic coronary atherosclerotic burden. Atherosclerosis 2012; 223:166 - 70; http://dx.doi.org/10.1016/j.atherosclerosis.2012.04.005; PMID: 22640815
- Faulin TES, Cavalcante MF, Abdalla DSP. Role of electronegative LDL and its associated antibodies in the pathogenesis of atherosclerosis. Clin Lipidol 2010; 5:719 - 29; http://dx.doi.org/10.2217/clp.10.52
- Tiwari RL, Singh V, Barthwal MK. Macrophages: an elusive yet emerging therapeutic target of atherosclerosis. Med Res Rev 2008; 28:483 - 544; http://dx.doi.org/10.1002/med.20118; PMID: 18000963
- Galkina E, Ley K. Immune and inflammatory mechanisms of atherosclerosis (*). Annu Rev Immunol 2009; 27:165 - 97; http://dx.doi.org/10.1146/annurev.immunol.021908.132620; PMID: 19302038
- Grosso DM, Ferderbar S, Wanschel ACBA, Krieger MH, Higushi ML, Abdalla DSP. Antibodies against electronegative LDL inhibit atherosclerosis in LDLr-/- mice. Braz J Med Biol Res 2008; 41:1086 - 92; http://dx.doi.org/10.1590/S0100-879X2008001200007; PMID: 19148370
- Peterson E, Owens SM, Henry RL. Monoclonal antibody form and function: manufacturing the right antibodies for treating drug abuse. AAPS J 2006; 8:E383 - 90; PMID: 16796389
- Macauley-Patrick S, Fazenda ML, McNeil B, Harvey LM. Heterologous protein production using the Pichia pastoris expression system. Yeast 2005; 22:249 - 70; http://dx.doi.org/10.1002/yea.1208; PMID: 15704221
- Tanfous NGB, Kallel H, Jarboui MA, Fathallah DM. Expression in Pichia pastoris of a recombinant scFv form of MAb 107, an anti human CD11b integrin antibody. Enzyme Microb Technol 2006; 38:636 - 42; http://dx.doi.org/10.1016/j.enzmictec.2005.07.014
- Shi X, Karkut T, Chamankhah M, Alting-Mees M, Hemmingsen SM, Hegedus D. Optimal conditions for the expression of a single-chain antibody (scFv) gene in Pichia pastoris.. Protein Expr Purif 2003; 28:321 - 30; http://dx.doi.org/10.1016/S1046-5928(02)00706-4; PMID: 12699697
- Eldin P, Pauza ME, Hieda Y, Lin G, Murtaugh MP, Pentel PR, et al. High-level secretion of two antibody single chain Fv fragments by Pichia pastoris.. J Immunol Methods 1997; 201:67 - 75; http://dx.doi.org/10.1016/S0022-1759(96)00213-X; PMID: 9032410
- Heimo H, Palmu K, Suominen I. Human placental alkaline phosphatase: expression in Pichia pastoris, purification and characterization of the enzyme. Protein Expr Purif 1998; 12:85 - 92; http://dx.doi.org/10.1006/prep.1997.0808; PMID: 9473461
- Sainz-Pastor N, Tolner B, Huhalov A, Kogelberg H, Lee YC, Zhu D, et al. Deglycosylation to obtain stable and homogeneous Pichia pastoris-expressed N-A1 domains of carcinoembryonic antigen. Int J Biol Macromol 2006; 39:141 - 50; http://dx.doi.org/10.1016/j.ijbiomac.2006.03.022; PMID: 16678252
- Ishibashi S, Brown MS, Goldstein JL, Gerard RD, Hammer RE, Herz J. Hypercholesterolemia in low density lipoprotein receptor knockout mice and its reversal by adenovirus-mediated gene delivery. J Clin Invest 1993; 92:883 - 93; http://dx.doi.org/10.1172/JCI116663; PMID: 8349823
- Pedrosa AMC, Faine LA, Grosso DM, de Las Heras B, Boscá L, Abdalla DSP. Electronegative LDL induction of apoptosis in macrophages: involvement of Nrf2. Biochim Biophys Acta 2010; 1801:430 - 7; http://dx.doi.org/10.1016/j.bbalip.2009.12.001; PMID: 20005974
- Miller YI, Viriyakosol S, Binder CJ, Feramisco JR, Kirkland TN, Witztum JL. Minimally modified LDL binds to CD14, induces macrophage spreading via TLR4/MD-2, and inhibits phagocytosis of apoptotic cells. J Biol Chem 2003; 278:1561 - 8; http://dx.doi.org/10.1074/jbc.M209634200; PMID: 12424240
- Bae YS, Lee JH, Choi SH, Kim S, Almazan F, Witztum JL, et al. Macrophages generate reactive oxygen species in response to minimally oxidized low-density lipoprotein: toll-like receptor 4- and spleen tyrosine kinase-dependent activation of NADPH oxidase 2. Circ Res 2009; 104:210 - 8, 21p, 218; http://dx.doi.org/10.1161/CIRCRESAHA.108.181040; PMID: 19096031
- Li AC, Glass CK. The macrophage foam cell as a target for therapeutic intervention. Nat Med 2002; 8:1235 - 42; http://dx.doi.org/10.1038/nm1102-1235; PMID: 12411950
- Burleigh ME, Babaev VR, Oates JA, Harris RC, Gautam S, Riendeau D, et al. Cyclooxygenase-2 promotes early atherosclerotic lesion formation in LDL receptor-deficient mice. Circulation 2002; 105:1816 - 23; http://dx.doi.org/10.1161/01.CIR.0000014927.74465.7F; PMID: 11956125
- Schönbeck U, Sukhova GK, Graber P, Coulter S, Libby P. Augmented expression of cyclooxygenase-2 in human atherosclerotic lesions. Am J Pathol 1999; 155:1281 - 91; http://dx.doi.org/10.1016/S0002-9440(10)65230-3; PMID: 10514410
- Maiwald S, Zwetsloot PP, Sivapalaratnam S, Dallinga-Thie GM. Monocyte gene expression and coronary artery disease. Curr Opin Clin Nutr Metab Care 2013; 16:411 - 7; PMID: 23739627
- Chávez-Sánchez L, Chávez-Rueda K, Legorreta-Haquet MV, Zenteno E, Ledesma-Soto Y, Montoya-Díaz E, et al. The activation of CD14, TLR4, and TLR2 by mmLDL induces IL-1β, IL-6, and IL-10 secretion in human monocytes and macrophages. Lipids Health Dis 2010; 9:117; http://dx.doi.org/10.1186/1476-511X-9-117; PMID: 20946675
- Schiopu A, Bengtsson J, Söderberg I, Janciauskiene S, Lindgren S, Ares MP, et al. Recombinant human antibodies against aldehyde-modified apolipoprotein B-100 peptide sequences inhibit atherosclerosis. Circulation 2004; 110:2047 - 52; http://dx.doi.org/10.1161/01.CIR.0000143162.56057.B5; PMID: 15451805
- Tsimikas S, Miyanohara A, Hartvigsen K, Merki E, Shaw PX, Chou MY, et al. Human oxidation-specific antibodies reduce foam cell formation and atherosclerosis progression. J Am Coll Cardiol 2011; 58:1715 - 27; http://dx.doi.org/10.1016/j.jacc.2011.07.017; PMID: 21982317
- Nilsson J, Hansson GK, Shah PK. Immunomodulation of atherosclerosis: implications for vaccine development. Arterioscler Thromb Vasc Biol 2005; 25:18 - 28; PMID: 15514204
- Brígido MM, Polymenis M, Stollar BD. Role of mouse VH10 and VL gene segments in the specific binding of antibody to Z-DNA, analyzed with recombinant single chain Fv molecules. J Immunol 1993; 150:469 - 79; PMID: 8419479
- Andrade EV, Albuquerque FC, Moraes LMP, Brígido MM, Santos-Silva MA. Single-chain Fv with Fc fragment of the human IgG1 tag: construction, Pichia pastoris expression and antigen binding characterization. J Biochem 2000; 128:891 - 5; http://dx.doi.org/10.1093/oxfordjournals.jbchem.a022838; PMID: 11098129
- Santo Faulin TdoE, de Sena KC, Rodrigues Telles AE, de Mattos Grosso D, Bernardi Faulin EJ, Parra Abdalla DS. Validation of a novel ELISA for measurement of electronegative low-density lipoprotein. Clin Chem Lab Med 2008; 46:1769 - 75; http://dx.doi.org/10.1515/CCLM.2008.333; PMID: 19055454
- Coloma MJ, Larrick JW, Ayala M, Gavilondo-Cowley JV. Primer design for the cloning of immunoglobulin heavy-chain leader-variable regions from mouse hybridoma cells using the PCR. Biotechniques 1991; 11:152 - 4, 156; PMID: 1931008
- Zhou H, Fisher RJ, Papas TS. Optimization of primer sequences for mouse scFv repertoire display library construction. Nucleic Acids Res 1994; 22:888 - 9; http://dx.doi.org/10.1093/nar/22.5.888; PMID: 8139934
- Caldas C, Coelho VP, Rigden DJ, Neschich G, Moro AM, Brígido MM. Design and synthesis of germline-based hemi-humanized single-chain Fv against the CD18 surface antigen. Protein Eng 2000; 13:353 - 60; http://dx.doi.org/10.1093/protein/13.5.353; PMID: 10835109
- Brígido MM, Polymenis M, Stollar BD. Role of mouse VH10 and VL gene segments in the specific binding of antibody to Z-DNA, analyzed with recombinant single chain Fv molecules. J Immunol 1993; 150:469 - 79; PMID: 8419479
- Andrade EV, Albuquerque FC, Moraes LMP, Brígido MM, Santos-Silva MA. Single-chain Fv with Fc fragment of the human IgG1 tag: construction, Pichia pastoris expression and antigen binding characterization. J Biochem 2000; 128:891 - 5; http://dx.doi.org/10.1093/oxfordjournals.jbchem.a022838; PMID: 11098129
- Wung JL, Gascoigne NR. Antibody screening for secreted proteins expressed in Pichia pastoris.. Biotechniques 1996; 21:808 - , 810, 812; PMID: 8922617
- Denizot F, Lang R. Rapid colorimetric assay for cell growth and survival. Modifications to the tetrazolium dye procedure giving improved sensitivity and reliability. J Immunol Methods 1986; 89:271 - 7; http://dx.doi.org/10.1016/0022-1759(86)90368-6; PMID: 3486233
- Terra X, Fernández-Larrea J, Pujadas G, Ardèvol A, Bladé C, Salvadó J, et al. Inhibitory effects of grape seed procyanidins on foam cell formation in vitro. J Agric Food Chem 2009; 57:2588 - 94; http://dx.doi.org/10.1021/jf803450a; PMID: 19292475
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001; 25:402 - 8; http://dx.doi.org/10.1006/meth.2001.1262; PMID: 11846609
- Via DP, Smith LC. Fluorescent labeling of lipoproteins. Methods Enzymol 1986; 129:848 - 57; http://dx.doi.org/10.1016/0076-6879(86)29108-9; PMID: 3724556
- Reeves PG, Nielsen FH, Fahey GC Jr.. AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J Nutr 1993; 123:1939 - 51; PMID: 8229312
- Daleprane JB, Freitas VdaS, Pacheco A, Rudnicki M, Faine LA, Dörr FA, et al. Anti-atherogenic and anti-angiogenic activities of polyphenols from propolis. J Nutr Biochem 2012; 23:557 - 66; http://dx.doi.org/10.1016/j.jnutbio.2011.02.012; PMID: 21764281
- Paigen B, Morrow A, Holmes PA, Mitchell D, Williams RA. Quantitative assessment of atherosclerotic lesions in mice. Atherosclerosis 1987; 68:231 - 40; http://dx.doi.org/10.1016/0021-9150(87)90202-4; PMID: 3426656