Abstract
This study describes the development of the first neutralizing antibodies against Western equine encephalitis virus (WEEV), a member of the genus Alphavirus. WEEV is transmitted by mosquitoes and can spread to the human central nervous system, causing symptoms ranging from mild febrile reactions to life-threatening encephalitis. WEEV has been classified as a biological warfare agent by the US Centers for Disease Control and Prevention. No anti-WEEV drugs are currently commercially available. Neutralizing antibodies are useful for the pre- and post-exposure treatment of WEEV infections. In this study, two immune antibody gene libraries were constructed from two macaques immunized with inactivated WEEV. Four antibodies were selected from these libraries and recloned as scFv-Fc, with a human Fc part. These antibodies bound WEEV specifically in ELISA with little or no cross-reaction with other alphaviruses. They were further analyzed by immunohistochemistry. All binders were suitable for the intracellular detection of WEEV particles. Neutralizing activity was determined in vitro. Three of the four antibodies were found to be neutralizing; about 1 ng/mL of the best antibody (ToR69–3A2) neutralized 50% of 5x104 TCID50/mL. Due to its human-like nature with a germinality index of 89% (VH) and 91% (VL), the ToR69–3A2 antibody is a promising candidate for future passive vaccine development.
Background
Western equine encephalitis virus (WEEV) belongs to the genus Alphavirus within the Togaviridae family. This virus is originated by recombination of a new world Eastern equine encephalitis virus (EEEV) and the old world Sindbis virus (SINV).Citation1-Citation3 WEEV has a positive-strand RNA genome consisting of about 11,700 nucleotides.Citation1,Citation4 WEEV, EEEV and the related Venezuelan equine encephalitis virus (VEEV) were first isolated in the 1930s.Citation2,Citation5-Citation8 The enzootic transmission cycle of WEEV includes the vertebrate hosts, birds and mosquitoes.Citation9 The main mosquito vector of WEEV is Culex tarsalis.10 These alphaviruses can spread to the human central nervous system (CNS), causing symptoms ranging from mild febrile reactions to encephalitis, often resulting in permanent, fatal neurological damage. The severity of the illness depends on the virus strain, the age of the patient, the dose and the route of infection.Citation9,Citation11-Citation20 WEEV/EEEV and VEEV have caused epidemics in North, Central and South America. An outbreak in Canada in 1941 led to more than 1000 human infections,Citation21 and a fatal case of human WEEV infection was reported in Uruguay in 2009.Citation22 For humans, the WEEV case-fatality rate has been estimated at 3 to 7%, and 15 to 30% of convalescent patients develop secondary neurological damage.Citation9,Citation23 Mortality rates due to WEEV infection range from 0% to 100% in mice, and the time to death differs between strains.Citation16,Citation24,Citation25
Alphaviruses can be produced in large quantities, are moderately easy to disseminate and are highly infectious as aerosols.Citation26,Citation27 VEEV, WEEV and EEEV are therefore considered to be potential biological weaponsCitation28-Citation30 and are classified as category B bioterrorism agents by the US Centers for Disease Control and Prevention (http://www.bt.cdc.gov/agent/agentlist-category.asp).
Various active vaccination strategies, based on live-attenuated strains of WEEV,Citation31 the envelope protein E1,Citation21 an adenovirus-vectored WEEV vaccine,Citation32,Citation33 DNA vaccinesCitation34 and recombinant E1/E2 proteinsCitation35have been evaluated, as well as alternative therapies, such as cationic liposome-DNA complexes (CLDCs)Citation36 and CLDCs combined with the recombinant WEE E1 protein.Citation37 Due to the vast geographic distribution of the virus and the limited amount of infections, however, an active vaccination campaign is not realistic. Therefore, a post-exposure therapy is needed. Since “there are no commercial vaccines or anti-WEEV drugs available for humans.”Citation21, the development of human or human-like antibodies for passive vaccination is advised. Two antibody fragments (scFv) have been generated from the murine IgGs 11D2Citation38,Citation39 and 10B5 E7E2Citation40 for diagnostic purposes. To our knowledge, no neutralizing human or murine anti-WEEV antibodies have been described to date. Non-human primates (NHP) antibody gene libraries and phage display are highly suitable tools for the generation of human-like antibodies for diagnostic and therapeutic purposes.Citation41,Citation42 Phage display has been successfully used for the generation of murine,Citation43 humanCitation44,Citation45 and human-likeCitation46 antibodies against VEEV. This study concerns the generation and characterization of the first human-like neutralizing antibodies against WEEV.
Results
Animal immunization
Two macaques were immunized with β-PL-inactivated WEEV. The first macaque was immunized twice with a commercial veterinary vaccine (Fluvac Innovator Triple EFT plus EHV, 3EEV) against VEEV, EEEV and WEEV, and then three-times with β-PL-inactivated WEEV, with a final boost 5.5 mo after the initial immunization (referred to as “macaque 1”). The second macaque was immunized with 3EEV and VEEV,Citation46 then boosted once with WEEV 4 mo after the last VEEV immunization (referred to as “macaque 2”). The immunization scheme is given in . Anti-WEEV antibody titers were evaluated by ELISA. The titration ELISA for “macaque 1” is shown in . The titer reached 1/40960 before the final boost with WEEV, and 1/10249 for the second macaque (data not shown). The immunizaton strategy for “macaque 1” yielded higher anti-WEEV titers than immunzation strategy for “macaque 2” with a series of VEEV immunization followed by one WEEV boost.
Figure 1. Immunization scheme for macaque 1 and 2. *timepoint for WEEV library construction. +, timepoint for VEEV library construction.Citation46
Antibody phage display library construction
For library cloning, total RNA was isolated from bone marrow lymphocytes after the final boost and reverse-transcribed to generate cDNA. The genes encoding VH and VL (kappa) were cloned into the phagemid pHAL14.Citation47 Library quality was controlled by colony PCR, which showed over 90% full size inserts in both libraries. The final libraries consisted of 1.3x107 independent clones for the library derived from “macaque 1,” and 2.4x107 independent clones for the second library “macaque 2.” Both libraries were packaged with HyperphageCitation48,Citation49 and satisfactory antibody presentation on phage was confirmed by total phage SDS-PAGE followed by western-blot and anti-pIII immunostaining (data not shown).
Antibody selection and binder gene analysis
Antibody selection (panning) was performed on antibody-captured active WEEV material. Three rounds of panning were performed and 96 soluble scFv antibody clones from the “macaque 1” library (ToR68 antibodies), and 48 antibody clones from the “macaque 2” library (ToR69 antibodies), were produced and analyzed by antigen ELISA. Four binders were identified in the ToR68 panning, and 42 binders were identified in the ToR69 panning. DNA sequencing revealed four unique antibodies from the “macaque 1” library and two unique antibodies from the “macaque 2” library. Two of the six unique binders displayed no specific antigen binding in more stringent assays and were not evaluated further. The corresponding human germline sequences, according to IMGT (www.imgt.org), and the germinality indices of the four validated anti-WEEV antibodies are given in . For comparison, the corresponding human germline sequences, the murine germline sequences and the germinality index of the murine anti-WEEV scFv 10B5Citation40 are given.
Table 1. Antibodies selected against WEEV. The most similar human germline genes were identified by IMGT (www.IMGT.org)
The selected scFv were recloned into the mammalian expression vector pCMX2.5-hIgG1-Fc-XPCitation50 to produce the bivalent IgG-like scFv-Fc format. Chinese hamster ovary (CHO) cell lines were established and antibodies were produced in a bioreactor, purified and biotinylated.
Binding characteristics
The scFv-Fc versions of the antibodies were analyzed by titration ELISA on active WEEV particles, with a Semliki forest virus (SFV) monoclonal antibody 12/2 51 used as a capture antibody and the scFv-Fc antibodies or SFV12/2 as detection antibodies (). ToR68–2E9 and ToR68–3G2 showed the most efficient antigen binding, while some background binding was seen for ToR68–2E9 and ToR68–2C3. The determined detection limit was about 6x103 TCID50/mL when using ToR68–2E9.
Figure 3. Anti-WEEV scFv-Fc antibody sandwich ELISA. Plates coated with a dilution series of WEEV suspensions captured by anti-WEEV mAb SFV12/2 (3 µg/mL). Three titration ELISAs were made in parallel. Staining was performed by incubation with 1 µg/mL biotinylated anti-WEEV antibodies, followed by streptavidin-HRP conjugate (1:4,000). As a negative control, we assessed antibody binding to Vero cell culture material.
ELISA on active Alphavirus particles was used to analyze cross-reactions. Alphaviruses were captured in the range of 4x105 - 8x106 TCID50/mL, depending on the individual virus preparation used, and detected with the anti-WEEV antibodies in the scFv-Fc format (). Due to the different capture antibodies, this assay did not allow a truly quantitative comparison of antigen binding. All four antibodies bound to WEEV. Three of the antibodies were specific for WEEV, but ToR69–3A2 also showed weak binding to the Chikungunya.
Figure 4. Analysis of cross-reactivity with other alphaviruses by a qualitative sandwich ELISA. The monoclonal SFV12/2, mAb Pix c/t 6/2 or VEE-WIS1 (3 µg/mL) were coated for capturing and different alphaviruses were applied (TCID50/mL WEEV: 4x105; EEEV: 6x105; VEEV: 8x106; SINV: 8x105; SFV: 2.5x106; CHIKV: 7x105; PIXV: 8x105). Staining was performed with 2 µg/mL biotinylated anti-WEEV scFv-Fc antibodies (200 ng/mL for ToR68–3G2), followed by streptavidin-HRP conjugate (1:4,000). Three ELISA experiments were performed in parallel, with the exception of CKIKV and PIXV for ToR68–3G2. Here, only two ELISA experiments were performed. As negative control, antibody binding to Vero cell culture material was tested.
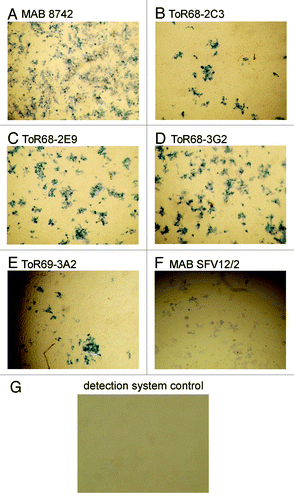
All antibodies allowed the detection of WEEV-infected Vero cells by immunohistochemistry ().
Figure 5. IHC analysis of anti-WEEV scFv-Fc antibody binding to VEEV infected Vero cells. Vero cells were infected with WEEV and fixed in formalin, for staining with a 1:5000 dilution of the antibodies ( = 200 ng/mL for the recombinant antibodies), followed by incubation with streptavidin-HRP conjugate (1:6,000). As positive controls, the anti-WEEV antibody MAB8742 and the anti-alphavirus antibody SFV12/2 were used. As negative control, the streptavidin-HRP conjugate was used without an anti-WEEV antibody.
WEEV neutralization
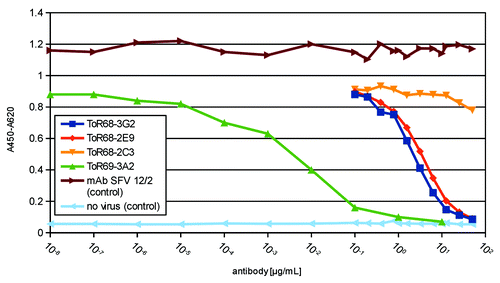
Inhibition of WEEV infection of Vero cells by the antibodies was analyzed in vitro using the neutralizing peroxidase-linked antibody (NPLA) assay. 5x104 TCID50/mL WEEV were incubated with a dilution series of the antibodies and, two hours later, 2x105 Vero cells were infected. The non-neutralizing mAb SFV12/2 was used as a control (). While ToR68–2C3 did not show neutralizing activity, ToR68–2E9 and ToR68–3G2 antibodies achieved half-maximal neutralization at about 1–10 µg/mL antibody. Very efficient neutralization was observed with ToR69–3A2 antibody where about 1 ng/mL antibody was sufficient for the half-maximal neutralization of 5x104 TCID50/mL.
Figure 6. Inhibition of WEEV infection by anti-WEEV scFv-Fc antibodies. Neutralization was analyzed in NPLA assays. Antibody dilution series were incubated with 5x104 TCID50/mL before 2x105 Vero cells were infected. As controls, the non-neutralizing mAb SFV12/2 was used or the cells were not infected. The infection of cells was demonstrated by the specific immunostaining of viral antigens by incubation with the biotinylated mAb SFV 12/2 (1:5,000) followed by streptavidin-HRP conjugate (1:4,000).
Discussion
WEEV belongs to the new world Alphavirus genus. This virus is transmitted by mosquitoes and can spread to the human CNS, causing symptoms ranging from mild febrile reactions to encephalitis.Citation2,Citation4,Citation9,Citation16
The post-exposure treatment of WEEV infections using interferon α protects mice only in a short time window of 6 h with limited efficacy.Citation52 While the protection of mice against WEEV by hyperimmune serum from rabbits has been reported,Citation53 no neutralizing or protective murine, human or human-like anti-WEEV or anti-EEEV monoclonal antibodies have yet been developed.Citation54 The situation is different for other Alphaviruses. For passive immunization, humanCitation55 and murineCitation56 antibody candidates for further therapeutic development have been described for Chikungunya. A panel of neutralizing murine,Citation26,Citation57 humanized,Citation58-Citation61 human-like monoclonal antibodies from non-human primatesCitation46 and one human antibodyCitation44 have been described for VEEV.
The immune antibody phage display libraries employed here were derived from immunized NHP, a technology that has been successfully used for the generation of neutralizing antibodies against other potential biological warfare agents, such as VEEV,Citation46 anthrax lethal factor,Citation62 botulinum toxinsCitation63 and ricin.Citation64 The results of this study confirm that this approach, combined with the conversion to IgG equivalent scFv-Fc antibodies,Citation50 provides a robust and quick solution for the generation of potential passive vaccination candidates. These phage display immune libraries are an alternative to the use of naive antibody phage display libraries for antibody generation for situations in which serum samples from patients are available or NHP immunization can be justified ethically.
The V-gene sequences of the four validated anti-WEEV NHP antibodies were very similar to the corresponding human V-genes. The germinality index (GI), a value proposed for the initial assessment of immunogenicity in patients, describes the degree of identity of the corresponding FRs to the most similar human germline FR sequences.Citation65 The GI values of the four scFv ranged from 83.5% to 93.4% for VH and from 73% to 91% for VL. Notably, at 73%, the GI of the light chain of ToR68–2C3 is very low. VBASE2 (www.vbase2.org) identifies the germline gene humIGKV085 as the most identical human gene, a gene that has not yet been assigned to any human kappa subfamily. The GI for this VL would be 91%. All macaque antibodies showed a higher identity to their human counterparts as the murine antibody 10B5Citation40 with a GI of 65.9%, respectively 68.5%. For comparison, the mean GI values of 100 average functional human scFv antibodies from a naïve scFv library (HAL4/7/8)Citation47 is 96.6% for VH and 94.8% for VL (unpublished data). Germline humanization of macaque antibodies has been successfully applied to improve the GI. The macaque antibody fragment (35PA83) neutralizing anthrax lethal toxinCitation65 has been subjected to germline humanization resulting in an increase of its GI value from 87.6% to 97.8%.Citation66
All antibodies allowed the detection of WEEV in ELISA and IHC after conversion to the scFv-Fc format. This format is functionally equivalent to IgG and may therefore be useful for the diagnostics of WEEV. In this work, two different immunization strategies were employed, with the secondary goal to generate cross reactive antibodies. While one macaque was immunized with the commercial horse vaccine 3EEV first, followed by WEEV only immunizations, the second macaque was immunized first with 3EEV, followed by VEEV immunizationsCitation46 and a final WEEV boost. However, this strategy failed to deliver an antibody with strong cross reactivity to VEEV within the limited number of analyzed clones.
No treatment is currently available for the post-exposure treatment of WEEV infections. The neutralizing antibody ToR69–3A2 against WEEV is a potential candidate for further development into the first passive anti-WEEV vaccine. Its human-like character should minimize potential immunogenicity and thus side effects of the treatment.
Material and Methods
Ethics statement and animal care
All animal studies presented here were specifically approved by the Institut de Recherche Biomédicale des Armées ethics committee (“Comité d'éthique de l'Institut de Recherche Biomédicale du Service de Santé des Armées“) under authorization no. 2008/03.0 and were performed in accordance with all relevant French laws and ethics guidelines, including, in particular (1) “partie règlementaire du livre II du code rural (titer I, chapitre IV, section 5, sous section 3: expérimentation sur l’animal),” (2) “décret 87-848 du 19-10/1987 relatif aux expériences pratiquées sur les animaux vertébrés modifié par le décret 2001/464 du 29/05/2001,” (3) “arrêté du 29 octobre 1990 relatif aux conditions de l’expérimentation animale pour le Ministère de la Défense” and (4) “instruction 844/DEF/DCSSA/AST/VET du 9 avril 1991 relative aux conditions de réalisation de l’expérimentation animale.”
Animal care procedures complied with the regulations detailed in the Animal Welfare ActCitation67 and the Guide for the Care and Use of Laboratory Animals.Citation68 Animals were kept at constant temperature (22 °C ± -2 °C) and relative humidity (50%), with 12 h of artificial lighting per day. They were housed in individual cages (6 per room) and each had a perch. Animals were fed twice daily, once with dried food and once with fresh fruits and vegetables, and water was provided with the food. Animal technicians observed food intake and general behavior during feeding, and were able to contact a veterinary surgeon if necessary. The veterinary surgeon performed systematic visits to each NHP-room twice weekly. Our environmental enrichment program for non-human primates is limited to games with animal care staff and access to approved toys. The well-being of the animals was monitored by the attending veterinary surgeon. Animals were anaesthetised by an intramuscular injection of 10 mg/kg ketamine (Imalgene®, Merial) before the collection of blood or bone marrow samples. Analgesics were subsequently administered in the form of a single intramuscular injection of 5 mg/kg flunixin (Finadyne®, Schering Plough), if animal technicians suspected that the animal was in pain on the days following an intervention, on the basis of observations of its behavior. No non-human primates were sacrificed during this work.
Cell culture and virus production
All the viruses used in this study are Alphavirus species constituting models of biowarfare agents and belong to the strain collection of the Armed Forces Scientific Institute for Protection Technologies—NBC Protection (WIS). The WEEV strain H160/99 and EEEV strain H178/99 used in this study were received from the National Collection of Pathogenic Viruses (NCPV), UK.
Alphaviruses were propagated in baby hamster kidney or Vero-B4 (African green monkey kidney) cells, at 37 °C, under an atmosphere containing 4% CO2, in a biosafety 3 facility, according to standard procedures, as described elsewhere.Citation26,Citation61,Citation69,Citation70 The cell lines were obtained from the DSMZ-ACC 33 (Deutsche Sammlung von Mikroorganismen und Zellkulturen GmbH).
All viruses were harvested from infected cell monolayers, when 50 to 75% of the cells in the monolayer presented evidence of a viral cytopathic effect (CPE). Virus titers were determined by the 50% tissue culture infective dose (TCID50/mL) method, as described by Spearman and Kaerber, or by plaque purification.Citation71
Alphavirus purification
Viruses were purified from the supernatants of infected Vero cells by affinity chromatography on Matrex Cellufine Sulfate medium (Virus Recovery System, VRS; Chisso America Inc.) or by isopycnic density gradient centrifugation, as described below. Matrex Cellufine Sulfate medium (VRS) is a cellulose bead medium functionalized with a low concentration of sulfate esters, which operates like a cation-exchange resin and has a high affinity for enveloped viruses. It selectively adsorbs complete virus particles and virus coats, according to their charge. Briefly, 50 mL of resin was equilibrated with adsorption buffer (0.01 M phosphate buffer, pH 7.5). Up to 200 mL of virus-containing prefiltered cell culture supernatant was loaded onto the column, which was then was washed twice with 0.01 M phosphate buffer, pH 7.5. Virus particles were then eluted with 1 M NaCl.
Virus particles were purified in two steps. The first step involved ultracentrifugation on a sucrose cushion (20% sucrose), causing low levels of mechanical stress and making it possible to concentrate and collect morphologically intact particles by centrifugation at 112,000 × g for 2 to 3 h. The pellet was resuspended in 0.5 to 1 mL phosphate-buffered saline (PBS) and further purified by isopycnic density gradient centrifugation (20 to 60% sucrose) for 18 h at 217,500 × g. The virus-containing fraction was collected and stored at -80 °C for further analysis.
Alphavirus inactivation
All viral antigens were inactivated by incubation with a final concentration of 0.2% β-propionolactone (β-PL, Sigma-Aldrich). Immediately before use, a 10% β-PL solution was prepared and 0.1 mL of this dilution was incubated with 5 mL of virus-containing cell culture supernatant (pH 8 to 8.5) for 1 h at 4 °C and 4 h at 37 °C, with constant stirring. After 2 and 4 h, the pH of the cell culture supernatant was checked and adjusted if necessary. For complete hydrolysis of the β-PL remaining after inactivation, the supernatant was stored at 4 °C for another 12 to 18 h. The sample was centrifuged for 30 min at 10,006 × g, and viral inactivation was checked by inoculating Vero cells and monitoring for cytopathic effects (CPE) for three to five days.
Animal immunization
A male cynomolgus macaque (Macaca fascicularis) was immunized by intramuscular injection with 100 µL of the commercially available veterinary vaccine Fluvac Innovator Triple EFT plus EHV (Fort Dodge Animal Health). This broad-range vaccine (3EEV) is normally administered to horses to protect them from equine encephalitis caused by the EEE, WEE and VEE viruses. This vaccine also contains inactivated rhinovirus and influenza virus together with tetanus toxoid, to protect horses against equine rhinopneumonitis, influenza and tetanus. One month later, the animal was immunized a second time with this vaccine. The macaque then received an intramuscular injection of 100 µL of VRS-purified β-PL-inactivated WEEV with complete Freund’s adjuvant after one month. Two additional injections of 100 µL of antigen preparation were performed, the first with incomplete Freund’s adjuvant after one month and the second with complete Freund’s adjuvant after 2.5 mo. One week after the final antigen injection, the immune library was generated. A second macaque, previously immunized with 3EEV and VEEV,Citation46 was immunized with WEEV four months after the final boost with VEEV. Usually, the complete Freund's adjuvant is used for the initial injections then incomplete Freund's adjuvant is used for the subsequent boosts. Here, the final boost was also realized using complete Freund’s adjuvant to induce a strong immune response against the targeted antigen, just before the immune library construction. As already observed in other projects, this final boost resulted in high yields of antibody RNA for RT-PCR. The immune library was generated 10 d after WEEV immunization. After each immune boost, serum samples were collected, inactivated by incubation for 20 min at 56 °C and tested for WEEV-specific antibodies by ELISA. For this purpose, VRS-purified culture supernatants from WEEV-infected Vero cells or uninfected cells were immobilized on 96-well microtiter plates (MaxisorpTM, Nunc). For the determination of WEEV-specific antibody titers, serial dilutions of pre-immune serum and of the sera obtained after second, third and fourth immunizations were used. Specifically bound antibodies were detected by incubation with rabbit anti-monkey IgG conjugated to horseradish peroxidase (1:10,000, Sigma-Aldrich) for 30 min at room temperature. Staining was performed with 3–3′, 5–5′-tetramethylbenzidine (TMB, Serva) and the reaction stopped with 0.5 M sulfuric acid after 10 min. Absorbance was measured at 450 nm.
Library construction and packaging
The library construction was performed as previously described.Citation62,Citation64,Citation72 In brief, macaque bone marrow lymphocytes were sampled, RNA was isolated and reverse-transcribed into cDNA. The coding regions for the variable regions VLĸ and VH were amplified and PCR products were cloned into the pGemT vector (Promega) yielding two antibody gene sublibraries, one encoding heavy chains (Fd fragment) and the other kappa light chains. The pGemT cloned PCR products were reamplified with two macaque oligonucleotide primer sets to introduce restriction sites for two step library cloning. The VH PCR products were first cloned into pHAL14,Citation47 with the VL PCR fragments inserted in a second cloning step. The library was packaged with hyperphageCitation48,Citation49 as previously described.Citation73 The scFv presentation of the library on the surface of the phage was assessed by SDS-PAGE, western blotting and anti-pIII immunostaining, as previously described.Citation73
Selection of anti-WEEV antibodies
The selection of recombinant antibodies was performed according toCitation45 with modifications. Panning was performed in 96-well microtiter plates (MaxiSorp, Nunc). The plates were coated with 3 µg/mL of the capture antibody MAB8742 (anti-WEE antibody, clone 2A2C.3, Merck Millipore) in PBS pH 7.4Citation74 by incubation overnight at 4 °C. Affinity-purified WEEV (100 µL) was then added to the wells and the plates were incubated for two hours. Non-specific binding was eliminated by incubating antigen-coated wells and wells for the preincubation of the library with affinity-purified supernatant from non-infected Vero cells overnight. The wells for panning and for preincubation were blocked by incubation with 2% (w/v) skim milk powder in PBST (PBS + 0.1% Tween 20; 2% MPBST). Phage particles (2.6 × 1010 or 8 × 1010) from the anti-WEEV libraries were diluted in PBST containing 1% skim milk, 1% bovine serum albumin (BSA), a nonspecific murine IgG D1–4G2–4-15Citation75 and the mixture was incubated for 1 h. The supernatant, containing the depleted library, was incubated in the wells with the captured WEEV particles at room temperature for 2 h and the wells were then washed 10 times with PBST. The bound scFv phage particles were eluted with 200 µL trypsin solution (10 µg/mL trypsin in PBS) at 37 °C for 30 min. The supernatant containing eluted scFv phage particles was transferred to a new tube. Eluted scFv phage (10 µL) was used for titration, as previously described.Citation73 E. coli XL1-Blue MRF' (Agilent; 20 mL of culture in the exponential growth phase; OD600 = 0.4 - 0.5) was infected with the remaining scFv phage, by incubation at 37 °C for 30 min, without shaking. The infected cells were harvested by centrifugation for 10 min at 3220 × g and the pellet was resuspended in 250 µL of 2xTY mediumCitation74 supplemented with 100 mM glucose and 100 µg/mL ampicillin (2xTY-GA), plated on a 15 cm 2xTY agar plate supplemented with 100 mM glucose and 100 µg/mL ampicillin and incubated overnight at 37 °C. The resulting colonies were harvested in 5 mL of 2xTY-GA. The harvested colony suspension (100 µL) was mixed with 30 mL of 2xTY-GA and cultured to an OD600 of 0.4 to 0.5 at 37 °C, with shaking at 250 rpm. The bacterial suspension (5 mL, ~2.5 × 109 bacteria) was infected with 5 × 1010 M13K07 helper phage (Agilent), incubated at 37 °C for 30 min without shaking, and then for 30 min with shaking at 250 rpm. Infected cells were harvested by centrifugation for 10 min at 3220 × g and the pellet was resuspended in 30 mL of 2xTY supplemented with 100 µg/mL ampicillin and 50 µg/mL kanamycin (2xTY-AK). Antibody phage were produced by incubation for 16 h at 30 °C, with shaking at 250 rpm. Cells were harvested by centrifugation for 10 min at 3220 × g. The supernatant containing the antibody phages (~1 × 1012 cfu/mL) were used directly, for the next round of panning, or were stored at 4 °C for a few days.
Identification of monoclonal scFv by ELISA
Monoclonal scFv were produced as previously described.Citation73 Plates were coated with 3 µg/mL of the capture antibody MAB8742 (anti-WEE antibody, clone 2A2C.3, Merck Millipore) in PBS pH 7.4Citation74 by incubation overnight at 4 °C. The VRS-purified WEEV supernatant was then added and plates were blocked with 2%MPBST. For binder identification, supernatants containing monoclonal scFv were incubated in the antigen-coated plates for 1.5 h at room temperature and washed three times with PBST. Bound scFv were detected with the murine mAb Myc1–9E10, which recognizes the C-terminal c-myc tag, and a goat anti-mouse serum conjugated to horseradish peroxidase (HRP) (Sigma; 1:10,000). Visualization was performed with TMB substrate (BIORAD) and the staining reaction was stopped by adding 100 µL of 0.5 M sulfuric acid. Absorbance at 450 nm and scattered light at 620 nm were measured and the value obtained at 620 nm was subtracted from the value obtained at 450 nm, with a SUNRISE microtiter plate reader (Tecan).
DNA sequencing
Antibody V-genes were sequenced by GATC Inc. using the oligonucleotide primer MHLacZ-Pro_f (5′ ggctcgtatgttgtgtgg 3′). Bioinformatic analysis was performed using web based resources, including IMGT/V-Quest (www.imgt.org) and VBASE2 (www.vbase2.org).
Construction of stable eukaryotic CHO transfectants and production of scFv-Fc fusions
WEEV specific scFv gene fragments were subcloned from the immune library vector pHAL14 into the mammalian expression vector pCMX2.5-hIgG1-FcCitation50 using NcoI and NotI restriction sites.
For the stable production of WEEV-specific scFv-Fc fusion proteins, CHO-K1 from the American Type Culture Collection, (ATCC, No. CCL61) were transfected, in the presence of 80 µL Polyfect® (Qiagen GmbH), with 2 to 5 µg of plasmid DNA. Stable clones were selected for resistance to the aminoglycoside antibiotic geniticin (G418). CHO cells were cultured overnight, to 60% to 80% confluence, in 1000 mm2 petri dishes (Nunc) containing non-selective Dulbecco's Modified Eagle's Medium (DMEM/HAM’s F-12), a nutrient mixture supplemented with L-glutamine and sodium bicarbonate, 10% (v/v) fetal calf serum (FCS) and 1% (w/v) penicillin and 1% (w/v) streptomycin (PAA), at 37 °C, under an atmosphere containing 4% CO2. DNA-lipid complex formation was supported by the prior incubation of plasmid DNA and Polyfect® in serum- and antibiotic-free medium for 10 min at room temperature before transfection. During lipid-DNA complex formation, the CHO-K1 cells were washed with PBS. Medium (7 mL) supplemented with FCS and penicillin/streptomycin was then added to the complex. The mixture was then immediately mixed with the washed cells by gently swirling. Cells were incubated with the transfection complex for 3 h. The medium was then removed and replaced with fresh non-selective DMEM/HAM’s F-12 medium containing 10% (v/v) FCS and 1% (w/v) penicillin/streptomycin before incubation of the plates overnight at 37 °C, under an atmosphere containing 4% CO2. The medium was changed again one day after transfection. The transfected cells were detached by treatment with trypsin and dispensed at a dilution of 1:50 or 1:100 in new petri dishes, on selective medium also containing 700 mg/mL G418. The medium was changed every three to four days and the first stable G418-resistant clones were observed about three weeks after transfection. Four and five weeks after transfection, single clones were isolated and cultured on 24-well plates (Nunc). As productivity varies between clones, the production of WEEV-specific scFv-Fc fusions was assessed in the supernatants of 24 clones, by ELISA, as described above. Two of the CHO-K1-clones with the highest antibody production levels were then cultured in suspension, in DMEM/HAM’s F-12 medium containing 10% (v/v) (FCS), 1% (w/v) penicillin/streptomycin and 700 mg/mL G418, in a miniPerm bioreactor (Sarstedt), at 37 °C, under an atmosphere containing 4% CO2. The culture supernatant was harvested twice weekly and replaced with fresh medium. Recombinant fusion proteins were purified by immunoaffinity chromatography on goat anti-human (GAH) Sepharose.
Biotinylation of mAbs and scFc-Fv fusions
The mAb or scFc-Fv antibodies (1 to 2 mg) were dissolved in sodium bicarbonate buffer, pH 8.5, and incubated with an aliquot of the biotin N-hydroxysuccinimide ester (long arm, water-soluble; Vector laboratories, CA, USA) corresponding to 1/10 the weight of the protein to be labeled. The mixture was incubated for 2 h at room temperature. The reaction was stopped by the addition of 10 mg glycine and the unreacted biotin was removed by gel filtration with PD-10 desalting columns containing Sephadex G25 (GE Healthcare, USA), in accordance with the manufacturer’s protocol.
Crossreactivity ELISA
Plates were coated with 3 µg/mL capture antibody SFV12/2 (anti-WEEV, anti- Sindbis virus, anti-SFV and Chikungunya virus),Citation76 mAb Pix c/t 6/2 (anti-Pixuna virus) (Greiser-Wilke, unpublished) or mAb VEE WIS1 (anti-VEEV)Citation46 in PBS pH 7.4,Citation74 by incubation overnight at 4 °C. The VRS-purified virus supernatant was then added and plates were blocked by incubation with 2%MPBST. The Alphaviruses analyzed were WEEV (4 × 105 TCID50/mL), EEEV (6 × 105 TCID50/mL), VEEV (8 × 106 TCID50/mL), SINV (8 × 105 TCID50/mL), SFV (2.5 × 106 TCID50/mL), Chikungunya virus (CHIKV, 7 × 105 TCID50/mL) and Pixuna virus (PIXV, 8 × 105 TCID50/mL). Vero cell culture material was used as a control. The captured Alphaviruses were detected with 2 µg/mL (200 ng/mL for ToR68–3G2) biotinylated anti-WEEV antibodies, followed by incubation with streptavidin-horseradish peroxidase conjugate (PSA, GE Healthcare) (1:6000 in PBS) as described above.
Immunohistochemistry
Vero cells (2 × 105 cells/mL) were grown in 96-well microtiter plates for 24 h and infected with serial 10-fold dilutions of WEEV strain 160/99. Non-infected Vero cells were used as a negative control. WEEV-infected cells were stained specifically with biotinylated scFv-Fc ToR68–2C3, ToR68–2E9, ToR68–3G2, ToR69–3A2, mAb SFV12/2 or mAb 8742 (anti-WEEV antibody clone 2A2C.3, Merck Millipore) as a positive control. One day post infection (pi), the infected cells were fixed by incubation with 3% formalin in PBS for 3 h at 4 °C. The fixed samples were then incubated with their cognate antibodies in either a 1:5,000 or 1:10,000 dilution in PBSF-T (PBS plus 1% FCS plus 0.1% Tween 20) for 1 h in a humid chamber at 37 °C. The fixed cells were washed three times in PBS-T (PBS plus 0.01% Tween 20) and incubated for 30 min with streptavidin-horseradish peroxidase conjugate (PSA, GE Healthcare) diluted 1:6,000 in PBS-FT. The cells were thoroughly washed and the infected cells were visualized under a microscope following incubation with the precipitating colorimetric peroxidase substrate TMB for 10 min. The staining reaction was stopped by rinsing the plates with Millipore-purified water.
Neutralizing peroxidase linked antibody assay (NPLA)
The NPLA assay for scFv-Fc fusions and complete IgG antibodies was performed by a modified version of the procedure described by Jensen.Citation77 All assays were performed in 96-well microtiter plates, with Vero cells used as the host for infection. A dilution series (50 µL) of scFv-Fc fusions or mAbs were incubated with an equal volume of WEE virus strain 160/99, with a TCID50/mL of 5 × 104, for 2 h at 37 °C, in 96-well plates. Following this incubation, 100 µL of freshly trypsin-treated Vero cells, at a density of 2 × 105 cells/mL, was mixed with the antibody virus mixture. As a positive control for infection, virus samples, not previously incubated with antibody, and virus samples previously incubated with WEEV-specific antibodies with no neutralizing activity, such as mAb 8742 or mAb SFV12/2, were used. Non-infected Vero cells were used as a negative control. Cell monolayers were fixed 20 to 24 h post infection, by incubation with 3% formalin in PBS for 2 h at 4 °C. The monolayers were washed with PBS-T, overlaid with 100 µL of a 1:5,000 dilution of the WEEV-specific biotinylated mAb SFV 12/2 and incubated for 90 min at room temperature. Bound biotinylated mAbs were detected by incubation with streptavidin-HRP conjugate (GE Healthcare, 1:4,000) as described above. An Olympus CK2 inverted microscope (Olympus) was used for bright field microscopy.
Oviedo MVN, Romoser WS, James CB, Mahmood F, Reisen WK. Infection dynamics of western equine encephalomyelitis virus (Togaviridae: Alphavirus) in four strains of Culex tarsalis (Diptera: Culicidae): an immunocytochemical study. Res Rep Trop Med 2011; 2011:65 - 77; PMID: 22629118
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Luzie Voß for excellent technical assistance.
References
- Hahn CS, Lustig S, Strauss EG, Strauss JH. Western equine encephalitis virus is a recombinant virus. Proc Natl Acad Sci U S A 1988; 85:5997 - 6001; http://dx.doi.org/10.1073/pnas.85.16.5997; PMID: 3413072
- Powers AM, Brault AC, Shirako Y, Strauss EG, Kang W, Strauss JH, Weaver SC. Evolutionary relationships and systematics of the alphaviruses. J Virol 2001; 75:10118 - 31; http://dx.doi.org/10.1128/JVI.75.21.10118-10131.2001; PMID: 11581380
- Strauss JH, Strauss EG. The alphaviruses: gene expression, replication, and evolution. Microbiol Rev 1994; 58:491 - 562; PMID: 7968923
- Sherman MB, Weaver SC. Structure of the recombinant alphavirus Western equine encephalitis virus revealed by cryoelectron microscopy. J Virol 2010; 84:9775 - 82; http://dx.doi.org/10.1128/JVI.00876-10; PMID: 20631130
- Giltner LT, Shahan MS. The immunological relationship of eastern and western strains of equine encephalomyelitis virus. Science 1933; 78:587 - 8; http://dx.doi.org/10.1126/science.78.2034.587-a; PMID: 17801697
- King LS. Studies on eastern equine encephalomyelitis : III. intraocular infection with fixed virus in the guinea pig. J Exp Med 1939; 69:691 - 704; http://dx.doi.org/10.1084/jem.69.5.691; PMID: 19870871
- Kubes V, Ríos FA. The causative agent of infectious equine encephalomyelitis in venezuela. Science 1939; 90:20 - 1; http://dx.doi.org/10.1126/science.90.2323.20; PMID: 17818578
- Weaver SC, Ferro C, Barrera R, Boshell J, Navarro J-C. Venezuelan equine encephalitis. Annu Rev Entomol 2004; 49:141 - 74; http://dx.doi.org/10.1146/annurev.ento.49.061802.123422; PMID: 14651460
- Zacks MA, Paessler S. Encephalitic alphaviruses. Vet Microbiol 2010; 140:281 - 6; http://dx.doi.org/10.1016/j.vetmic.2009.08.023; PMID: 19775836
- Calisher CH. Medically important arboviruses of the United States and Canada. Clin Microbiol Rev 1994; 7:89 - 116; PMID: 8118792
- Deresiewicz RL, Thaler SJ, Hsu L, Zamani AA. Clinical and neuroradiographic manifestations of eastern equine encephalitis. N Engl J Med 1997; 336:1867 - 74; http://dx.doi.org/10.1056/NEJM199706263362604; PMID: 9197215
- Feemster RF. Outbreak of Encephalitis in Man Due to the Eastern Virus of Equine Encephalomyelitis. Am J Public Health Nations Health 1938; 28:1403 - 10; http://dx.doi.org/10.2105/AJPH.28.12.1403; PMID: 18014957
- Leitenberg M. Biological weapons in the twentieth century: a review and analysis. Crit Rev Microbiol 2001; 27:267 - 320; http://dx.doi.org/10.1080/20014091096774; PMID: 11791799
- Letson GW, Bailey RE, Pearson J, Tsai TF. Eastern equine encephalitis (EEE): a description of the 1989 outbreak, recent epidemiologic trends, and the association of rainfall with EEE occurrence. Am J Trop Med Hyg 1993; 49:677 - 85; PMID: 8279635
- Mossel EC, Ledermann JP, Phillips AT, Borland EM, Powers AM, Olson KE. Molecular determinants of mouse neurovirulence and mosquito infection for Western equine encephalitis virus. PLoS One 2013; 8:e60427; http://dx.doi.org/10.1371/journal.pone.0060427; PMID: 23544138
- Rivas F, Diaz LA, Cardenas VM, Daza E, Bruzon L, Alcala A, De la Hoz O, Caceres FM, Aristizabal G, Martinez JW, et al. Epidemic Venezuelan equine encephalitis in La Guajira, Colombia, 1995. J Infect Dis 1997; 175:828 - 32; http://dx.doi.org/10.1086/513978; PMID: 9086137
- Rozdilsky B, Robertson HE, Chorney J. Western encephalitis: report of eight fatal cases. Saskatchewan epidemic, 1965. Can Med Assoc J 1968; 98:79 - 86; PMID: 5688720
- Sanchez JL, Takafuji ET, Lednar WM, LeDuc JW, Macasaet FF, Mangiafico JA, Rosato RR, Driggers DP, Haecker JC. Venezuelan equine encephalomyelitis: report of an outbreak associated with jungle exposure. Mil Med 1984; 149:618 - 21; PMID: 6438552
- Sudia WD, McLean RG, Newhouse VF, Johnston JG, Miller DL, Trevino H, Bowen GS, Sather G. Epidemic Venezuelan equine encephalitis in North America in 1971: vertebrate field studies. Am J Epidemiol 1975; 101:36 - 50; PMID: 1119481
- Swayze RD, Bhogal HS, Barabé ND, McLaws LJ, Wu JQH. Envelope protein E1 as vaccine target for western equine encephalitis virus. Vaccine 2011; 29:813 - 20; http://dx.doi.org/10.1016/j.vaccine.2010.11.009; PMID: 21084062
- Delfraro A, Burgueño A, Morel N, González G, García A, Morelli J, Pérez W, Chiparelli H, Arbiza J. Fatal human case of Western equine encephalitis, Uruguay. Emerg Infect Dis 2011; 17:952 - 4; http://dx.doi.org/10.3201/eid1705.101068; PMID: 21529429
- Reeves WC, Hutson GA, Bellamy RE, Scrivani RP. Chronic latent infections of birds with Western equine encephalomyelitis virus. Proc Soc Exp Biol Med 1958; 97:733 - 6; http://dx.doi.org/10.3181/00379727-97-23862; PMID: 13554464
- Logue CH, Bosio CF, Welte T, Keene KM, Ledermann JP, Phillips A, Sheahan BJ, Pierro DJ, Marlenee N, Brault AC, et al. Virulence variation among isolates of western equine encephalitis virus in an outbred mouse model. J Gen Virol 2009; 90:1848 - 58; http://dx.doi.org/10.1099/vir.0.008656-0; PMID: 19403754
- Nagata LP, Hu W-G, Parker M, Chau D, Rayner GA, Schmaltz FL, Wong JP. Infectivity variation and genetic diversity among strains of Western equine encephalitis virus. J Gen Virol 2006; 87:2353 - 61; http://dx.doi.org/10.1099/vir.0.81815-0; PMID: 16847131
- Phillpotts RJ. Venezuelan equine encephalitis virus complex-specific monoclonal antibody provides broad protection, in murine models, against airborne challenge with viruses from serogroups I, II and III. Virus Res 2006; 120:107 - 12; http://dx.doi.org/10.1016/j.virusres.2006.02.003; PMID: 16621103
- Reed DS, Larsen T, Sullivan LJ, Lind CM, Lackemeyer MG, Pratt WD, Parker MD. Aerosol exposure to western equine encephalitis virus causes fever and encephalitis in cynomolgus macaques. J Infect Dis 2005; 192:1173 - 82; http://dx.doi.org/10.1086/444397; PMID: 16136459
- Hawley RJ, Eitzen EM Jr.. Biological weapons--a primer for microbiologists. Annu Rev Microbiol 2001; 55:235 - 53; http://dx.doi.org/10.1146/annurev.micro.55.1.235; PMID: 11544355
- Sidwell RW, Smee DF. Viruses of the Bunya- and Togaviridae families: potential as bioterrorism agents and means of control. Antiviral Res 2003; 57:101 - 11; http://dx.doi.org/10.1016/S0166-3542(02)00203-6; PMID: 12615306
- Nagata LP, Wong JP, Hu W-G, Wu JQ. Vaccines and therapeutics for the encephalitic alphaviruses. Future Virol 2013; 8:661 - 74; http://dx.doi.org/10.2217/fvl.13.42
- Atasheva S, Wang E, Adams AP, Plante KS, Ni S, Taylor K, Miller ME, Frolov I, Weaver SC. Chimeric alphavirus vaccine candidates protect mice from intranasal challenge with western equine encephalitis virus. Vaccine 2009; 27:4309 - 19; http://dx.doi.org/10.1016/j.vaccine.2009.05.011; PMID: 19446595
- Barabé ND, Rayner GA, Christopher ME, Nagata LP, Wu JQH. Single-dose, fast-acting vaccine candidate against western equine encephalitis virus completely protects mice from intranasal challenge with different strains of the virus. Vaccine 2007; 25:6271 - 6; http://dx.doi.org/10.1016/j.vaccine.2007.05.054; PMID: 17630056
- Wu JQH, Barabé ND, Chau D, Wong C, Rayner GR, Hu W-G, Nagata LP. Complete protection of mice against a lethal dose challenge of western equine encephalitis virus after immunization with an adenovirus-vectored vaccine. Vaccine 2007; 25:4368 - 75; http://dx.doi.org/10.1016/j.vaccine.2007.03.042; PMID: 17467858
- Nagata LP, Hu W-G, Masri SA, Rayner GA, Schmaltz FL, Das D, Wu J, Long MC, Chan C, Proll D, et al. Efficacy of DNA vaccination against western equine encephalitis virus infection. Vaccine 2005; 23:2280 - 3; http://dx.doi.org/10.1016/j.vaccine.2005.01.032; PMID: 15755611
- Dupuy LC, Locher CP, Paidhungat M, Richards MJ, Lind CM, Bakken R, Parker MD, Whalen RG, Schmaljohn CS. Directed molecular evolution improves the immunogenicity and protective efficacy of a Venezuelan equine encephalitis virus DNA vaccine. Vaccine 2009; 27:4152 - 60; http://dx.doi.org/10.1016/j.vaccine.2009.04.049; PMID: 19406186
- Logue CH, Phillips AT, Mossel EC, Ledermann JP, Welte T, Dow SW, Olson KE, Powers AM. Treatment with cationic liposome-DNA complexes (CLDCs) protects mice from lethal Western equine encephalitis virus (WEEV) challenge. Antiviral Res 2010; 87:195 - 203; http://dx.doi.org/10.1016/j.antiviral.2010.04.013; PMID: 20452378
- Phillips AT, Stauft CB, Aboellail TA, Toth AM, Jarvis DL, Powers AM, Olson KE. Bioluminescent imaging and histopathologic characterization of WEEV neuroinvasion in outbred CD-1 mice. PLoS One 2013; 8:e53462; http://dx.doi.org/10.1371/journal.pone.0053462; PMID: 23301074
- Das D, Kriangkum J, Nagata LP, Fulton RE, Suresh MR. Development of a biotin mimic tagged ScFv antibody against western equine encephalitis virus: bacterial expression and refolding. J Virol Methods 2004; 117:169 - 77; http://dx.doi.org/10.1016/j.jviromet.2004.01.008; PMID: 15041214
- Xu B, Kriangkum J, Nagata LP, Fulton RE, Suresh MR. A single chain Fv specific against Western equine encephalitis virus. Hybridoma 1999; 18:315 - 23; http://dx.doi.org/10.1089/hyb.1999.18.315; PMID: 10571261
- Long MC, Jager S, Mah DC, Jebailey L, Mah MA, Masri SA, Nagata LP. Construction and characterization of a novel recombinant single-chain variable fragment antibody against Western equine encephalitis virus. Hybridoma 2000; 19:1 - 13; http://dx.doi.org/10.1089/027245700315743; PMID: 10768836
- Avril A, Froude JW, Mathieu J, Pelat T, Thullier P. Isolation of antibodies from non-human primates for clinical use. Curr Drug Discov Technol 2014; 11:20 - 7; http://dx.doi.org/10.2174/15701638113109990030; PMID: 23410051
- Pelat T, Hust M, Thullier P. Obtention and engineering of non-human primate (NHP) antibodies for therapeutics. Mini Rev Med Chem 2009; 9:1633 - 8; http://dx.doi.org/10.2174/138955709791012283; PMID: 20105119
- Duggan JM, Coates DM, Ulaeto DO. Isolation of single-chain antibody fragments against Venezuelan equine encephalomyelitis virus from two different immune sources. Viral Immunol 2001; 14:263 - 73; http://dx.doi.org/10.1089/088282401753266774; PMID: 11572636
- Hunt AR, Frederickson S, Maruyama T, Roehrig JT, Blair CD. The first human epitope map of the alphaviral E1 and E2 proteins reveals a new E2 epitope with significant virus neutralizing activity. PLoS Negl Trop Dis 2010; 4:e739; http://dx.doi.org/10.1371/journal.pntd.0000739; PMID: 20644615
- Kirsch MI, Hülseweh B, Nacke C, Rülker T, Schirrmann T, Marschall H-J, Hust M, Dübel S. Development of human antibody fragments using antibody phage display for the detection and diagnosis of Venezuelan equine encephalitis virus (VEEV). BMC Biotechnol 2008; 8:66; http://dx.doi.org/10.1186/1472-6750-8-66; PMID: 18764933
- Rülker T, Voß L, Thullier P, O’ Brien LM, Pelat T, Perkins SD, Langermann C, Schirrmann T, Dübel S, Marschall H-J, et al. Isolation and characterisation of a human-like antibody fragment (scFv) that inactivates VEEV in vitro and in vivo. PLoS One 2012; 7:e37242; http://dx.doi.org/10.1371/journal.pone.0037242; PMID: 22666347
- Hust M, Meyer T, Voedisch B, Rülker T, Thie H, El-Ghezal A, Kirsch MI, Schütte M, Helmsing S, Meier D, et al. A human scFv antibody generation pipeline for proteome research. J Biotechnol 2011; 152:159 - 70; http://dx.doi.org/10.1016/j.jbiotec.2010.09.945; PMID: 20883731
- Rondot S, Koch J, Breitling F, Dübel S. A helper phage to improve single-chain antibody presentation in phage display. Nat Biotechnol 2001; 19:75 - 8; http://dx.doi.org/10.1038/83567; PMID: 11135557
- Soltes G, Hust M, Ng KKY, Bansal A, Field J, Stewart DIH, Dübel S, Cha S, Wiersma EJ. On the influence of vector design on antibody phage display. J Biotechnol 2007; 127:626 - 37; http://dx.doi.org/10.1016/j.jbiotec.2006.08.015; PMID: 16996161
- Jäger V, Büssow K, Wagner A, Weber S, Hust M, Frenzel A, Schirrmann T. High level transient production of recombinant antibodies and antibody fusion proteins in HEK293 cells. BMC Biotechnol 2013; 13:52; http://dx.doi.org/10.1186/1472-6750-13-52; PMID: 23802841
- Greiser-Wilke I, Moenning V, Kaaden OR, Figueiredo LT. Most alphaviruses share a conserved epitopic region on their nucleocapsid protein. J Gen Virol 1989; 70:743 - 8; http://dx.doi.org/10.1099/0022-1317-70-3-743; PMID: 2471798
- Wu JQH, Barabé ND, Huang Y-M, Rayner GA, Christopher ME, Schmaltz FL. Pre- and post-exposure protection against Western equine encephalitis virus after single inoculation with adenovirus vector expressing interferon alpha. Virology 2007; 369:206 - 13; http://dx.doi.org/10.1016/j.virol.2007.07.024; PMID: 17761207
- Sabin AB. Quantitative studies on prophylactic effectiveness of western equine encephalitis antiserum in mice. Proc Soc Exp Biol Med 1951; 78:655 - 8; http://dx.doi.org/10.3181/00379727-78-19172; PMID: 14911984
- Reichert E, Clase A, Bacetty A, Larsen J. Alphavirus antiviral drug development: scientific gap analysis and prospective research areas. Biosecur Bioterror 2009; 7:413 - 27; http://dx.doi.org/10.1089/bsp.2009.0032; PMID: 20028250
- Warter L, Lee CY, Thiagarajan R, Grandadam M, Lebecque S, Lin RTP, Bertin-Maghit S, Ng LFP, Abastado J-P, Desprès P, et al. Chikungunya virus envelope-specific human monoclonal antibodies with broad neutralization potency. J Immunol 2011; 186:3258 - 64; http://dx.doi.org/10.4049/jimmunol.1003139; PMID: 21278338
- Pal P, Dowd KA, Brien JD, Edeling MA, Gorlatov S, Johnson S, Lee I, Akahata W, Nabel GJ, Richter MKS, et al. Development of a highly protective combination monoclonal antibody therapy against Chikungunya virus. PLoS Pathog 2013; 9:e1003312; http://dx.doi.org/10.1371/journal.ppat.1003312; PMID: 23637602
- Roehrig JT, Day JW, Kinney RM. Antigenic analysis of the surface glycoproteins of a Venezuelan equine encephalomyelitis virus (TC-83) using monoclonal antibodies. Virology 1982; 118:269 - 78; http://dx.doi.org/10.1016/0042-6822(82)90346-4; PMID: 6178209
- Goodchild SA, O’Brien LM, Steven J, Muller MR, Lanning OJ, Logue CH, D’Elia RV, Phillpotts RJ, Perkins SD. A humanised murine monoclonal antibody with broad serogroup specificity protects mice from challenge with Venezuelan equine encephalitis virus. Antiviral Res 2011; 90:1 - 8; http://dx.doi.org/10.1016/j.antiviral.2011.01.010; PMID: 21310183
- Hunt AR, Frederickson S, Hinkel C, Bowdish KS, Roehrig JT. A humanized murine monoclonal antibody protects mice either before or after challenge with virulent Venezuelan equine encephalomyelitis virus. J Gen Virol 2006; 87:2467 - 76; http://dx.doi.org/10.1099/vir.0.81925-0; PMID: 16894184
- Hu W-G, Phelps AL, Jager S, Chau D, Hu CC, O’Brien LM, Perkins SD, Gates AJ, Phillpotts RJ, Nagata LP. A recombinant humanized monoclonal antibody completely protects mice against lethal challenge with Venezuelan equine encephalitis virus. Vaccine 2010; 28:5558 - 64; http://dx.doi.org/10.1016/j.vaccine.2010.06.038; PMID: 20600509
- O’Brien LM, Underwood-Fowler CD, Goodchild SA, Phelps AL, Phillpotts RJ. Development of a novel monoclonal antibody with reactivity to a wide range of Venezuelan equine encephalitis virus strains. Virol J 2009; 6:206; http://dx.doi.org/10.1186/1743-422X-6-206; PMID: 19925641
- Pelat T, Hust M, Laffly E, Condemine F, Bottex C, Vidal D, Lefranc M-P, Dübel S, Thullier P. High-affinity, human antibody-like antibody fragment (single-chain variable fragment) neutralizing the lethal factor (LF) of Bacillus anthracis by inhibiting protective antigen-LF complex formation. Antimicrob Agents Chemother 2007; 51:2758 - 64; http://dx.doi.org/10.1128/AAC.01528-06; PMID: 17517846
- Chahboun S, Hust M, Liu Y, Pelat T, Miethe S, Helmsing S, Jones RG, Sesardic D, Thullier P. Isolation of a nanomolar scFv inhibiting the endopeptidase activity of botulinum toxin A, by single-round panning of an immune phage-displayed library of macaque origin. BMC Biotechnol 2011; 11:113; http://dx.doi.org/10.1186/1472-6750-11-113; PMID: 22111995
- Pelat T, Hust M, Hale M, Lefranc M-P, Dübel S, Thullier P. Isolation of a human-like antibody fragment (scFv) that neutralizes ricin biological activity. BMC Biotechnol 2009; 9:60; http://dx.doi.org/10.1186/1472-6750-9-60; PMID: 19563687
- Laffly E, Danjou L, Condemine F, Vidal D, Drouet E, Lefranc M-P, Bottex C, Thullier P. Selection of a macaque Fab with framework regions like those in humans, high affinity, and ability to neutralize the protective antigen (PA) of Bacillus anthracis by binding to the segment of PA between residues 686 and 694. Antimicrob Agents Chemother 2005; 49:3414 - 20; http://dx.doi.org/10.1128/AAC.49.8.3414-3420.2005; PMID: 16048955
- Pelat T, Bedouelle H, Rees AR, Crennell SJ, Lefranc M-P, Thullier P. Germline humanization of a non-human primate antibody that neutralizes the anthrax toxin, by in vitro and in silico engineering. J Mol Biol 2008; 384:1400 - 7; http://dx.doi.org/10.1016/j.jmb.2008.10.033; PMID: 18976662
- USDA Animal Welfare Act (AWA). 7 U.S.C. 2131 et seq., as amended and Health Research Extension Act of 1985 (“‘Animals in Research’”). 1985.
- National Research Council. Guide for the Care and Use of Laboratory Animals. Eighth Edition. Washington: The National Academies Press; 2011.
- Huelseweh B, Ehricht R, Marschall H-J. A simple and rapid protein array based method for the simultaneous detection of biowarfare agents. Proteomics 2006; 6:2972 - 81; http://dx.doi.org/10.1002/pmic.200500721; PMID: 16622830
- Sagripanti J-L, Hülseweh B, Grote G, Voss L, Böhling K, Marschall H-J. Microbial inactivation for safe and rapid diagnostics of infectious samples. Appl Environ Microbiol 2011; 77:7289 - 95; http://dx.doi.org/10.1128/AEM.05553-11; PMID: 21856830
- Hamilton MA, Russo RC, Thurston RV. Trimmed Spearman-Karber method for estimating median lethal concentrations in toxicity bioassays. Enviromental Sci Technol 1977; 11:714 - 9; http://dx.doi.org/10.1021/es60130a004
- Schütte M, Thullier P, Pelat T, Wezler X, Rosenstock P, Hinz D, Kirsch MI, Hasenberg M, Frank R, Schirrmann T, et al. Identification of a putative Crf splice variant and generation of recombinant antibodies for the specific detection of Aspergillus fumigatus. PLoS One 2009; 4:e6625; http://dx.doi.org/10.1371/journal.pone.0006625; PMID: 19675673
- Frenzel A, Kügler J, Wilke S, Schirrmann T, Hust M. Construction of human antibody gene libraries and selection of antibodies by phage display. Methods Mol Biol 2014; 1060:215 - 43; http://dx.doi.org/10.1007/978-1-62703-586-6_12; PMID: 24037844
- Sambrook J, Russell D. Molecular cloning: a laboratory manual. 3nd ed. New York: Cold Spring Harbor Laboratory Press; 2001.
- Henchal EA, Gentry MK, McCown JM, Brandt WE. Dengue virus-specific and flavivirus group determinants identified with monoclonal antibodies by indirect immunofluorescence. Am J Trop Med Hyg 1982; 31:830 - 6; PMID: 6285749
- Greiser-Wilke IM, Moennig V, Kaaden OR, Shope RE. Detection of alphaviruses in a genus-specific antigen capture enzyme immunoassay using monoclonal antibodies. J Clin Microbiol 1991; 29:131 - 7; PMID: 1847149
- Jensen MH. Detection of antibodies against hog cholera virus and bovine viral diarrhea virus in porcine serum. A comparative examination using CF, PLA and NPLA assays. Acta Vet Scand 1981; 22:85 - 98; PMID: 6266240