Abstract
Antagonist anti-CD28 antibodies prevent T-cell costimulation and are functionally different from CTLA4Ig since they cannot block CTLA-4 and PDL-1 co-inhibitory signals. They demonstrated preclinical efficacy in suppressing effector T cells while enhancing immunoregulatory mechanisms. Because a severe cytokine release syndrome was observed during the Phase 1 study with the superagonist anti-CD28 TGN1412, development of other anti-CD28 antibodies requires careful preclinical evaluation to exclude any potential immunotoxicity side-effects. The failure to identify immunological toxicity of TGN1412 using macaques led us to investigate more relevant preclinical models.
We report here that contrary to macaques, and like in man, all baboon CD4-positive T lymphocytes express CD28 in their effector memory cells compartment, a lymphocyte subtype that is the most prone to releasing cytokines after reactivation. Baboon lymphocytes are able to release pro-inflammatory cytokines in vitro in response to agonist or superagonist anti-CD28 antibodies. Furthermore, we compared the reactivity of human and baboon lymphocytes after transfer into non obese diabetic/severe combined immunodeficiency (NOD/SCID) interleukin-2rγ knockout mice and confirmed that both cell types could release inflammatory cytokines in situ after injection of agonistic anti-CD28 antibodies. In contrast, FR104, a monovalent antagonistic anti-CD28 antibody, did not elicit T cell activation in these assays, even in the presence of anti-drug antibodies. Infusion to baboons also resulted in an absence of cytokine release.
In conclusion, the baboon represents a suitable species for preclinical immunotoxicity evaluation of anti-CD28 antibodies because their effector memory T cells do express CD28 and because cytokine release can be assessed in vitro and trans vivo.
Introduction
Immunotherapies with monoclonal antibodies (mAbs) or other recombinant proteins targeting receptors directly expressed on immune cells became a success story and a flourishing field of development to modulate immune responses in diverse indications such as oncology, inflammation, autoimmunity, transplantation, neuroscience and infectious diseases.Citation1,Citation2 Among these immune cells, T lymphocytes represent a major therapeutic target, especially the costimulatory molecules they express, which regulate differentiation into either pathogenic effector T cells (Teff) or anti-inflammatory regulatory T cells (Treg). The CD28-CD80/86-CTLA-4 costimulatory system functions like a molecular rheostat, where CD28-CD80/86 engagement induce activation, proliferation and survival of Teff, as well as dampen Treg function, while CTLA-4-CD80/86 interaction is essential for the suppressive function of Treg, delivers antiproliferative signals to Teff and confers a sub-immunogenic function to antigen-presenting cells (APC).3 This central immune checkpoint pathway was hence the subject of intense research and development. CD80/86 antagonists have proved immunosuppressive efficacy and were approved for marketing as treatments for rheumatoid arthritisCitation4(abatacept, Orencia®; Bristol-Myers Squibb) and renal transplantationCitation5(belatacept, Nulojix®; Bristol-Myers Squibb). However, because these compounds also inhibit CTLA-4 inhibitory signals, we and others have suggested that selectively targeting CD28 might present advantages over CD80/86 blockade as it would prevent engagement of CD80/86 with CD28, but not with CTLA4.Citation3,Citation6-Citation12 The theoretical advantage of selective CD28 blockade compared with CD80/86 blockade was further reinforced by two recent discoveries in the field of costimulation: 1) PD-L1 was identified as an additional ligand of CD80 capable of inhibiting T cell responses,Citation13,Citation14and 2) ICOSL (B7-H2) interacts with CD28 to induce T lymphocytes proliferation, cytokines secretion and survival signals.Citation15
The clinical translation of compounds aimed at CD28, however, has been hampered by the poor evaluation of their potential immunotoxicology at the pre-clinical level. Eight years ago, administration of TGN1412, a superagonist anti-CD28 mAb, to healthy volunteers caused a dramatic accident in a Phase 1 trial due to an acute and severe cytokine release syndrome (CRS), which had not been predicted by current preclinical animal models.Citation16 Indeed, the target epitope of TGN1412 was the C”D basolateral domain of CD28,Citation17 which, after antibody-mediated cross-linking, induces a non-physiological and antigen-independent polyclonal activation of T lymphocytes in rodent and human T cells. In addition, anti-CD28 mAbs in their IgG form present agonist properties even when binding outside the C”D loop, resulting from receptor cross-linking and T lymphocytes costimulation in synergy with T-cell receptor (TCR) signals.Citation10 Interaction with Fc receptors does not seems to be a dominant mechanism driving the agonist properties of anti-CD28 mAbs because ‘silenced’ (with a mutated Fc domain preventing interaction with Fc receptors) divalent anti-CD28 mAbs still costimulate T cells.Citation18 Therefore, to avoid any agonistic or superagonisticanti-CD28 mAbs activities, they must target an epitope other than C”D and must be monovalent.Citation19 One of the reasons preclinical assessment failed to predict that TGN1412 would induce cytokine release in man is most probably that in macaques, the species used for this assessment, the effector memory subset of T lymphocytes (TEM) have lost CD28 expression in the CD4+ compartment.Citation20-Citation22 Yet TEM cells are the most prone cell population to rapidly release inflammatory cytokines after activation and represent the likely source of pro-inflammatory cytokines released after TGN1412 infusion.
Here, we report that in baboons (Papio anubis), in contrast with macaques, CD28-CD4+ T lymphocytes are barely detectable in peripheral blood and that, in baboons, like in man, TEM cells are CD28+. We therefore postulated that the baboon might be a more relevant species for predicting potential immunotoxicity of mAbs targeting CD28 at the preclinical level.
We previously developed FR104, a humanized monovalent pegylated anti-CD28 Fab’ antibody that demonstrated specific inhibition of CD28 interaction with CD80/86 and ICOSL.Citation7 In accordance with the recommendations published after the TGN1412 accident,Citation23,Citation24 we evaluated FR104 in vitro on human peripheral blood mononuclear cells (PBMC), and trans vivo in a humanized mouse model,Citation7 and also measured the absence of immunotoxicity in vivo in baboons. The data presented here adds to the relevance of this model to evaluate the absence of immunotoxicity by showing that baboon memory CD4+ T lymphocytes express CD28 similarly to human and are able to proliferate and secrete pro-inflammatory cytokines in vitro and trans vivo in the presence of agonist or superagonist anti-CD28 mAbs.
Results
CD28 expression by memory T lymphocytes of different primate species
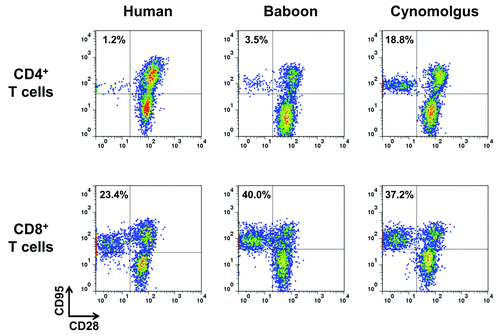
We first evaluated CD28 expression by peripheral blood CD4+ or CD8+ T lymphocytes from humans, baboons and cynomolgus macaques. We observed that the majority of baboon CD4+ T lymphocytes expressed CD28 (98.1 ± 1.2%, n = 7) similarly to human, whereas, as previously described,Citation20 a considerable frequency (ranging from 5 to 25%, n = 3) of CD4+ T lymphocytes did not express CD28 in cynomolgus (data not shown). We confirmed that these CD28-negative CD4+ T lymphocytes, as well as CD28-negative CD8+ T cells were of the memory phenotype since they expressed Fas receptor (CD95) in the three species (). Therefore, baboon CD4+ T lymphocytes express CD28 in their naïve as well as in their memory subsets, similarly to humans.
Figure 1.CD28 expression by human, baboon and cynomolgus macaque memory T cells. One representative immunophenotyping analysis of CD28 in CD4+ (upper panel)and CD8+ (lower panel) memory (CD95+) and naïve (CD95-) T lymphocytes of human, baboon and cynomolgus macaque PBMC. Data are representative of at least three independent analyses.
In vitro comparison of human vs baboon T cells responses to anti-CD28 mAbs
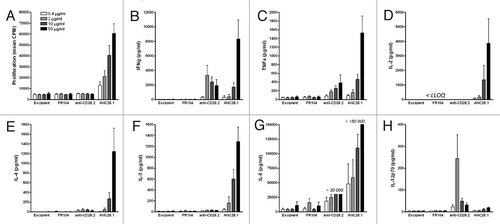
The TGN1412 accident prompted the development, evaluation and comparison of novel in vitro assays for mimicking in vivo cytokine release and lymphocytes proliferation.Citation25,Citation26 The development of co-culture systems, such as human endothelial cell monolayers with human PBMC, although not fully mimicking the cytokine profile observed in vivo, has been considered as a reasonable surrogate assay to obtain cytokine responses predictive of in vivo conditions.Citation27,Citation28 We therefore used this assay to evaluate capacities of baboon PBMC to respond to agonistic or superagonist anti-CD28 stimulation and perform comparison with humans. First, we confirmed that human PBMC cultured over human umbilical vein endothelial cell (HUVEC) monolayers vigorously proliferated and secreted diverse pro-inflammatory cytokines, including interferon (IFN)γ, tumor necrosis factor (TNF), interleukin (IL)-2, IL-4, IL-5,and IL-6, in a dose-response manner in response to superagonist (clone ANC28.1) anti-CD28 mAbs (). In contrast, conventional agonist (clone CD28.2) anti-CD28 mAbs induced moderate cytokine secretion in this assay and did not induce lymphocytes proliferation. Surprisingly, anti-CD28.2 mAbs induced IL-12p70 (p35 + p40 subunits) secretion mainly at lower concentration, while superagonist anti-CD28 mAbs did not. This induction was not the result of possible endotoxin contamination in the antibody preparation, which was similar to the background of the culture medium. As expected, antagonist Fab’ anti-CD28 fragments (FR104) did not elicit any cytokines release or T lymphocytes proliferation ().
Figure 2.Proliferation and cytokine release of human PBMC co-cultured with human endothelial cells in response to anti-CD28 mAbs. (A) Proliferation at 72 h, (B) IFNγ, (C) TNF,(D) IL-2, (E) IL-4, (F) IL-5, (G) IL-6 and (H) IL-12p70 release at 48 h after addition of 0.4, 2, 10 or 50 µg/ml of humanized pegylated Fab’ fragment FR104, divalent agonist anti-CD28.2 mAb and superagonist anti-CD28 ANC28.1 mAb or of an equivalent volume of excipient. Histogram bars are means ± SEM from data obtained with four different blood donors. LLOQ: Lower limit of quantification.
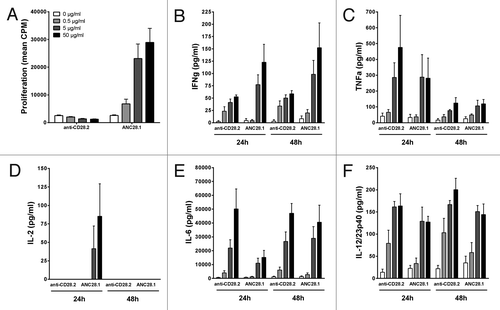
We then evaluated the response of baboon PBMC in the same HUVEC monolayers co-culture assay. Baboon PBMC responded very similarly to human cells in regard to lymphoproliferation, since superagonist anti-CD28 mAbs induced important proliferation in a dose-response manner, while agonistic anti-CD28 mAbs did not (). However, although both agonistic and superagonist anti-CD28 mAbs induced measurable cytokine (IFNγ, TNF, IL-6 and IL-12 or IL-12/IL-23p40) secretion at 24 or 48 h of co-culture in a dose-response manner, the magnitude of the release with baboon PBMC was overall weaker than with human cells. As observed with human PBMC, agonistic anti-CD28 antibody did not elicited measurable IL-2 release with baboon PBMC.
Figure 3.Proliferation and cytokine release of baboon PBMC co-cultured with human endothelial cells in response to anti-CD28 mAbs. (A) Proliferation at 72 h, (B) IFNγ, (C) TNF, (D) IL-2, (E) IL-6 and (F) IL-12/23p40 release at either 24 and 48 h after addition of excipient (0),0.5, 5 or 50 µg/ml of divalent agonist anti-CD28.2 and superagonist anti-CD28 ANC28.1 mAbs. Data are mean ± SEM of 4 different blood donors.
Trans vivo comparison of human vs baboon T cells responses to anti-CD28 mAbs
We recently described that humanized mice (immunodeficient mice reconstituted by human PBMC injection: HuPBL mice) models enable evaluation and detection of superagonist anti-CD28 mAbs activity by measuring T lymphocytes activation markers and pro-inflammatory cytokines release in vivo.Citation7 To confirm in vivo that baboon PBMC still respond to superagonist anti-CD28 mAbs, we conducted similar experiments in immunodeficient non-obese diabetic/severe combined immunodeficiency (NOD/SCID) IL-2rγ knockout (NSG) mice reconstituted with freshly isolated baboon PBMC. We observed that baboon PBMC engrafted with a lower efficiency than human PBMC in NSG mice. Indeed, two weeks after infusion of 50 x 106 human PBMC, human T cell engraftment reached 75.2 ± 7.7% in the blood (). In contrast, although some mice reconstituted with baboon PBMC showed significant (> 10%) T lymphocytes engraftment one week after infusion, engraftment at two weeks stayed moderate (17.7 ± 11.1%) (). Considering the low number of blood leukocytes in naïve NSG mice (120 ± 20 x 103 cells/ml, n = 57, data not shown), we calculated that baboon blood T lymphocytes count was 14 times lower (26 ± 15 x 103 cells/ml) compared with human T cell count (364 ± 10 x 103 cells/ml) two weeks after reconstitution.
Figure 4.In vivo stimulation of human or baboon PBMC with anti-CD28 mAbs in NSG mice. One representative flow cytometry analysis (upper panel) and quantification of engraftment (low panel) of (A) human (n = 20) and (B) baboon (n = 18) cells in NSG mice two weeks after PBMC transfer. Each mark represents an individual mouse. (C, D) Percentage of activated human and baboon T lymphocytes expressing CD25 and CD69 among CD3+ cells in recipient blood and spleen 48 h after drug injection. Two weeks after PBMC transfer, mice received either 50 µg i.p. of superagonist anti-CD28 ANC28.1 mAb (red square symbols; n = 5 for human and n = 7 for baboon), 150 µg of humanized pegylated Fab’ fragment FR104 (blue dots; n = 6 for human and n = 5 for baboon) or an equivalent volume of excipient (open diamond; n = 5 for human and baboon). Each symbol represents an individual mouse. Horizontal bars indicate the mean. (E) Human and (F) baboon cytokine release after treatment in same experiments as in C and D; data are means ± SEM.*P < 0.05 and **P < 0.01 compared with control conditions. Cumulative data were obtained from three independent series of experiments using three different human and three baboon blood donors.
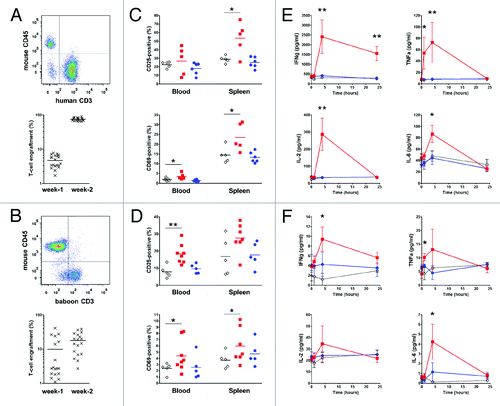
Two weeks after PBMC injection, these mice were randomly assigned to a treatment group consisting of a single injection of either superagonist anti-CD28 mAbs at 1 mg/kg or FR104 at 5 mg/kg or an equivalent volume of excipient. Injection of a superagonist anti-CD28 mAbs, but not of FR104, significantly increased the frequency of CD69+ and CD25+ activated T lymphocytes in the spleen or blood of NSG mice reconstituted by human or baboon PBMC (). Similarly, whereas no difference could be observed in term of cytokines secretion between mice treated with FR104 or excipient, administration of a superagonist anti-CD28 mAbs resulted in a rapid (one to four hours after injection) and significant release of pro-inflammatory cytokines measured in the plasma of NSG mice reconstituted by human or baboon PBMC (). However, the level of baboon cytokines was significantly lower than human cytokines (even at T0 before administration of treatment), in accordance with the lower engraftment of baboon T cells in these mice.
In vivo immunotoxicity evaluation of FR104 in baboon
Three baboons were injected once intravenously with FR104 at 20 mg/kg and immunotoxicity parameters were compared with two baboons that received an equal volume of excipient. The pharmacokinetics and pharmacodynamics analysis showed that C-max concentration in sera reached between 160 to 542 µg/ml within one hour. The Tβ1/2 elimination half-life ranged between 8.6 and 9.9 d (mean: 9.3 d) and receptor occupancy in periphery was maintained at 100% over two months, as long as through levels were above 1 μg/ml ().
Figure 5.Immunotoxicity evaluation of FR104 after intravenous injection in baboon. (A) Serum concentration (black square; left y-axis) of FR104 and receptor occupancy (open square; right y-axis) on blood T lymphocytes measured by flow cytometry after a single intravenous administration at 20 mg/kg in three different baboons. Data are mean ± SEM (B) Serum concentration of indicated cytokines measured after FR104 injection in the three animals described in (A). Data are mean ± SEM. Dotted line represents the highest lower limit of quantification (LLOQ). (C) leukocytes count, (D) lymphocytes count, (E) platelets count, (F) hemoglobin count, (G) body temperature, (H) cardiac frequency, (I) mean arterial pressure and(J) oxygen saturation recorded after FR104 injection in the three animals described in (A; black symbols and solid lines) as well as in two other excipient-treated baboons (open symbols and dotted lines). Each animal is represented by a different symbol. The horizontal lines indicate the range of normal values in baboons.Citation39
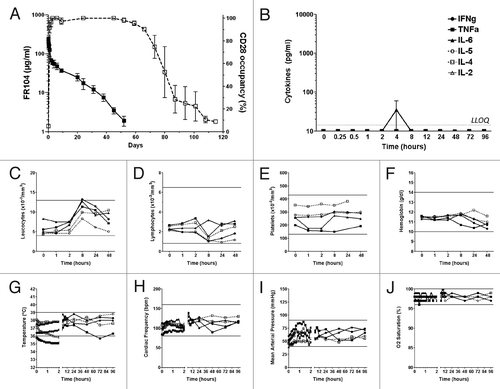
Proinflammatory cytokines (IFN-γ, TNF, IL-2, IL-4, IL-5 and IL-6) remained undetectable (under the limit of detection: 13.2 pg/ml for IFN-γ, 1.6 pg/ml for TNF, 14.4 pg/ml for IL-2, 3.6 pg/ml for IL-4, 1.2 pg/ml for IL-5) in all recipient baboons that received FR104 or excipient (), with the exception of IL-6, which was detected at low level in the sera of all animals four hours after intravenous (IV) injections (levels ranging from 1.1 to 84.7 pg/ml), and therefore probably related to the procedure, including anesthesia.
Finally, we did not observe clinical symptoms of cytokine release syndrome based on hematology (), body temperature, blood pressure, cardiac frequency and oxygen saturation () parameters, which all stayed within physiological ranges for both FR104 and excipient treated baboons. It should be noted that for two baboons (one treated with FR104, one with excipient), we experienced a fault in a warming electric blanket (used for the first two hours of general anesthesia to avoid hypothermia), explaining a significant drop in body temperature during this period.
In vitro immunotoxicity assessment in the presence of anti-drug antibodies
Due to overall concerns related to mAbs immunogenicity,Citation29 we investigated if creating immune complexes and cross-linking monovalent anti-CD28 mAbs with anti-drug antibodies (ADA) would modify monovalent-related antagonist properties and induce proliferation or cytokines release of human PBMC. We purified IgG from several ADA-positive sera from two baboons (titers determined by ELISA were 1/800 for ADA#1+ and 1/50 for ADA#2+ sera; data not shown) previously exposed to FR104. In parallel, we purified IgG from a pool of sera derived from up to 10 naive baboons as negative control (ADA-). Importantly, we verified that ADA IgG had conserved their anti-FR104 activity after purification (data not shown).
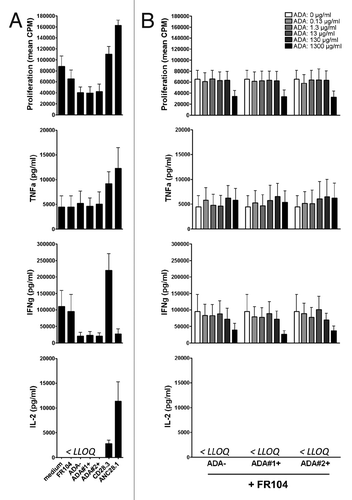
To assess potential agonist properties monovalent anti-CD28 mAbs could acquire in the presence of ADA, we added antibodies to anti-CD3 stimulated human PBMC cultured in polypropylene plate that we previously described as the most stringent assay to detect agonist properties.Citation7 As expected, agonist as well as superagonist anti-CD28 mAbs increased lymphoproliferation and cytokines release, more particularly IFNγ for agonist mAbs (). In contrast, addition of FR104 had no effect even in the presence of purified IgG ADA+ at a concentration up to 1.3 mg/ml (). Notably, at very high concentration (1.3 mg/ml) polyclonal immunoglobulins exercised immunoregulatory function, observed also in the absence of FR104 (data not shown).
Figure 6.In vitro response of human PBMC to antagonist anti-CD28 mAbs and ADA. Human PBMC were stimulated with anti-CD3 antibodies and different types of anti-CD28 mAbs plus indicated concentrations of IgG containing ADA. (A) Proliferation at 72 h and cytokine release at 48 h in the presence of excipient, FR104 (10µg/ml), divalent agonist anti-CD28.3 mAbs (10 µg/ml), superagonist anti-CD28 ANC28.1 mAbs (10µg/ml), ADA-negative purified IgG (1.3 mg/ml), or ADA-positive purified IgG from two different baboons (1.3 mg/ml).(B) Same experiments as in (A) where human PBMC were cultured with 10 µg/ml of FR104 and increasing concentrations of ADA-negative or ADA-positive purified IgG (ranging from 0 to 1300 µg/ml as indicated in the upper panel).Data are means ± SEM of 3 different human blood donors. LLOQ: Lower limit of quantification.
Discussion
Selective CD28 antagonists have demonstrated superior efficacy over CD80/86 antagonists in experimental preclinical models of transplantation and autoimmune diseases.Citation3,Citation8 However, such selective antagonists have not yet been formerly evaluated in the clinic due to the difficulty of assessing the innocuity of antagonist anti-CD28 mAbs, in relation to the deleterious effects of superagonists anti-CD28 mAbs in humans.
A series of sensitive immunotoxicity assays, including mAb immobilization, culture of T cells at high density, evaluation in whole blood or co-culture assays with endothelial cells, have been developed and characterized to avoid a repeat of the TGN1412 incident with new therapeutics.Citation25,Citation26 Following these recommendations, evaluation of a humanized monovalent pegylated anti-CD28 Fab’ fragment (FR104) did not show induction of T lymphocytes proliferation or cytokines release, even in the presence of polyclonal activation (ex. anti-CD3).Citation7,Citation19 Using the co-culture assay with endothelial cell, Findlay et al.Citation28 described that CD28.2 (an agonist anti-CD28 mAb that has no superagonist activity) did not induce T-cell proliferation or cytokine release. In our study, we confirmed the absence of induced proliferation with CD28.2, but recorded significant levels of IFNγ, TNF, IL-6 and IL-12 release. Otherwise, whereas the severity of the adverse response of TGN1412 was correlated with the level of IL-2 release,Citation30 we observed that level of IFNγ release could be more predictive of agonist properties of divalent anti-CD28 mAbs.
In spite of these sensitive assays, current non-human primate models used for preclinical toxicological evaluation are not predictive of clinical outcome in man, in part because they use monkeys, which do not express CD28 on a significant part of their TEM cells, which are cells that rapidly secrete cytokines after stimulation. For that purpose, we developed a trans vivo model where severe immunodeficient mice (NSG) were infused with human PBMC.Citation7 NSG mice reconstitution by human PBMC, although incomplete, allowed pertinent mAbs immunotoxicity evaluation in vivo on human cells, since early engraftment was predominantly composed of human CD4+ and CD8+ T lymphocytes as well as natural killer (NK) cells. Furthermore, in contrast to conventional in vivo models in naïve animals, in this humanized mice model, human T lymphocytes undergo proliferation and are activated due to homeostasis and xenoreactivity leading to a florid graft-vs.-host-disease.Citation7 Indeed, besides superagonist anti-CD28 mAbs, other therapeutics known to induce CRS in some patients, such as alemtuzumab (anti-CD52), rituximab (anti-CD20) and muromonab-CD3 (OKT3), were described to stimulate cytokine release from NK cells and T lymphocytes.Citation20,Citation25 In this trans vivo model, the humanized monovalent pegylated anti-CD28 Fab’ fragment (FR104) remained antagonist, while superagonist or agonist divalent anti-CD28 mAbs were able to induce both T lymphocytes activation and cytokine release.
Even though in vitro assays and humanized rodents are helpful, in vivo immunotoxicity evaluation in a relevant species remains most certainly the best predictive assessment.Citation31 Concerning TGN1412, whereas binding affinity for CD28 was similar between cynomolgus macaques and humans,Citation21,Citation32,Citation33 PBMC from macaques were not stimulated in vitro by immobilized TGN1412, contrary to results with human cells.Citation24,Citation34 It was first suggested that the differences could be due to the loss of Siglecs inhibitory molecules during human evolution,Citation35 but Richard Stebbings group later reported that CD4+ effector memory T cells were the predominant source of cytokine release after TGN1412 infusion, and that macaque CD4+ T cells, but not human counterparts, lose CD28 expression during their differentiation into memory cells.Citation20 Whereas it is now obvious that cynomolgus or rhesus macaques cannot constitute relevant species for preclinical immunotoxicity evaluation of emerging drugs targeting CD28, these animals still continue to be used for that purpose.Citation36
In this study, we found that the majority of baboon CD4+ memory T cells expressed CD28, similar to humans. Furthermore, we observed in vitro and trans vivo that baboon PBMC were activated, proliferated and secreted pro-inflammatory cytokines in response to agonist or superagonist anti-CD28 mAb stimulation. Baboon T lymphocytes, however, quantitatively released less cytokines compared with human cells in the same co-culture assay. This could first be explained by altered molecular interactions between baboons PBMC and xenogeneic human endothelial cells, important for anti-CD28-mediated T cells activation, such as ICOS/ICOSL.Citation37 Second, an inhibitory Siglecs molecule lost during human evolutionCitation35 could probably explain why human PBMC seem more prone to produce cytokines in response to anti-CD28 mAb stimulation. In spite of a lower response than in the human system, our study illustrated that assays with baboon cells can be predictive of potential immunological toxicity of anti-CD28 mAbs, and increases the relevance of data showing the presence or absence of cytokines released in the serum of baboons after infusion of an anti-CD28 mAb, since it is difficult due to ethical considerations to consider ANC28.1 or TGN1412 injection to baboon as positive controls. Of note, administration of FR104, a drug candidate antagonist of CD28, to baboons did not induce detectable cytokine release and no clinical signs of CRS.
Immunogenicity and development of ADA is another safety concern when developing monovalent antagonists. To circumvent the lack of representativity of anti-PEG or anti-Vκ antibodies that have been recently used to address that problem,Citation36 we purified IgG from baboons immunized with FR104 and containing ADA and added these antibodies to human cells activated with anti-CD3 and FR104. In these experiments, adding ADA-positive or ADA-negative IgG at either low (0.13 µg/ml), moderate or very high concentration (1.3 mg/ml) did not modify antagonist properties of FR104. This suggested that creating immune complexes of monovalent Fab’ fragments with ADA does not reproduce the agonist effect of dimeric IgG anti-CD28 antibodies on T cells, an effect that might be of conformational origin.Citation19
In conclusion, we reported that Papio anubisis can be considered an elective species for the preclinical assessment of immunotoxicity of therapeutic anti-CD28 mAbs. Moreover, immunodeficient mice reconstituted by human PBMC provide a suitable complementary model by allowing evaluation in vivo, directly on human target-expressing cells, of agonist or superagonist properties of anti-CD28 mAbs. Any translation of a novel CD28 antagonist into clinical application should first take these models into consideration.
Materials and Methods
Flow cytometry
Fluorescent mAbs against mouse CD45 (30-F11) and human CD3 (SP34–2), CD4 (L200), CD8 (RPA-T8), CD25 (M-A251), CD28 (28.6), CD69 (FN50) and CD95 (DX2) were from BD Biosciences. FR104 staining was performed with a polyethylene glycol (PEG) rabbit mAb (Epitomics) followed by a fluorescent goat anti-rabbit IgG (Invitrogen). CD28 receptor occupancy by FR104 on T lymphocytes was determined by performing the ratio of median fluorescent intensity (MFI) of FR104 staining between an unmodified blood sample and a blood sample incubated for 30 min at room temperature with a saturating concentration of FR104 (5 µg/ml). Samples were acquired on a BD FACS CANTOTM flow cytometer (BD Bioscience) and analyzed with FlowJo software.
Endothelial cell co-culture assay
Human umbilical vein endothelial cell (HUVEC), used between the second and fifth passage, were originally obtained from Lonza and cultured in Endothelial Cell Basal Medium (Promocell) supplemented with 10% heat-inactivated human AB sera, 0.4% endothelial cell growth supplement/heparin (Promocell), hydrocortisone (1 μg/ml, Promocell), human basic fibroblast growth factor (1 ng/ml, Promocell), human epidermal growth factor (0.1 ng/ml, Promocell), 100 U/ml penicillin (Life Technologies) and 0.1 mg/ml streptomycin (Life Technologies) at 37 °C in a 5% CO2 humidified air incubator, as previously described.Citation38 HUVEC were seeded at 20 x 103 cells/well in 96-well flat-bottomed microtiter plates (Nunc) precoated with 1% gelatin (Sigma-Aldrich). After 24 h of HUVEC culture, supernatants were aspirated and 125 x 103 PBMC (from healthy human or baboon donors) isolated by density gradient centrifugation, were added to the HUVEC monolayers and cultured in complete medium (RPMI-1640 medium supplemented with L-glutamine, sodium pyruvate, Hepes, antibiotics, all from Gibco) supplemented with either 2% heat-inactivated human AB sera or 2% heat-inactivated baboon sera. Superagonist anti-CD28 mAb (ANC28.1, Calbiochem), divalent IgG anti-CD28 mAb (CD28.2, BD Biosciences), monovalent anti-CD28 Fab’ fragment (FR104, from Effimune as previously describedCitation7) or its excipient were added from day 0 of the co-culture. Fifty µl supernatant were collected from each triplicate at 24 and 48 h and then analyzed for cytokines concentration using ProcartaPlexTM multiplex immunoassays (Affymetrix) specific for human or non-human primate cytokines. At day 3 of co-culture, cells were pulsed with 1μCi of 3H-thymidine during the final eight hours of culture and proliferation was evaluated in a scintillation counter.
Anti-CD3 biological assay
105 human PBMC from healthy volunteers were cultured in 96-well U-bottomed microtiter plates (Nunc) precoated with 1 µg/ml of anti-CD3 antibody (OKT3) in complete medium (described above) supplemented with 2% heat-inactivated human AB sera, in the presence of 10 µg/ml of superagonist anti-CD28 mAb (ANC28.1, Calbiochem), divalent IgG anti-CD28 mAb (CD28.2, BD Biosciences), monovalent anti-CD28 Fab’ fragment (FR104, Effimune) or 1300 µg/ml of Protein G purified IgG from control baboons or from baboon sera previously immunized against FR104. To study the possibility of Fab’ fragment crosslinking by ADA, the same purified baboon IgG preparations were added at either 1300, 130, 13, 1.3 or 0.13 µg/ml in wells already containing FR104 at 10 µg/ml. 25μl supernatant was collected at 48 h of culture to measure IFNγ, TNF and IL-2 concentration with human BD OptEIA Elisa Kits (BD Biosciences). At day 3 of co-culture, cells were pulsed with 1μCi of 3H-thymidine during the final 8 h of culture and proliferation was evaluated in a scintillation counter.
Animals and treatments
Seven to ten week old IL-2rγ knockout mice (Charles River) were irradiated (2 Gy) and infused intraperitoneally (i.p.) with 50x106 freshly isolated human or baboon PBMC from healthy donors, as previously described.Citation7 Mice were then maintained in aseptic conditions and were monitored every week for T-lymphocytes engraftment in the blood. Two weeks after PBMC injection, mice were treated once i.p. with 50 µg of superagonist anti-CD28 (ANC28.1), 150 µg of anti-CD28 Fab’ fragment (FR104), or equivalent volume of excipient. Retro-orbital blood samples were performed before, 1, 4 and 24 h after drug injection and human or baboon cytokines concentration in mouse sera was determined by CBA Flex (BD Bioscience). T-lymphocytes activation was analyzed by flow cytometry on blood and spleen cells harvested 48 h after antibody injection. Baboons (Papio anubis, from the CNRS Primatology Center, Rousset, France), were housed at the large animal facility of our laboratory. All experiments were performed under general anesthesia with Zoletil (Virbac, Carros, France) followed when necessary by ventilation with a mixture of nitrous oxide, oxygen and isoflurane (Forène, Abbot). Three animals received a single IV injection of FR104 at 20 mg/kg and two others animals received an equivalent volume of excipient. Blood samples were drawn at different time point for receptor occupancy, FR104 concentration, hematological and biochemical analyses, as well as cytokines concentration measured by BD CBA Non-human Primate Th1/Th2 Cytokine Kit (BD Biosciences). All experiments in mice and non-human primates were performed in accordance with the recommendations of the Institutional Ethical Guidelines of the “Institut National de la Santé Et de la Recherche Médicale” (France).
Statistical analyses
All variables were expressed as mean ± SEM and were compared when appropriated using the Mann-Whitney non parametric test. P values less than 0.05 were considered statistically significant. All statistical analyses were performed with GraphPad Prism (GraphPad Software).
Disclosure of Potential Conflicts of Interest
NP, CM and BV are shareholders and employees of Effimune, a company developing CD28 antagonists.
Acknowledgments
We thank Dr. B. Charreau, INSERM, Nantes, France, for providing HUVEC cells and E. Merieau for animal cares.
The authors received funding from the Fondation de la Recherche Médicale (FRM grant #DIM20081013832; Paris, France), the Labec IGO (ANR grant ANR-11-LABX-0016–01; Paris, France) and the European project TRIAD (EU-FP7-Health program EC-GA N°281493; www.TRIAD-CD28.eu). This work was also supported by the Progreffe and Centaure foundations (France).
Author contributions
NP designed, organized and performed in vitro and in vivo experiments, analyzed and interpreted the data and prepared the manuscript. CM and SLB performed in vitro experiments on human cells. VD and LB performed in vivo experiments on mice. MC performed flow cytometry experiments. JH and DM assisted with baboon experiments. SV and VC performed in vivo experiments on baboons. GB organized and supervised baboon experiments. BV funded the research, designed experiments, analyzed and interpreted the data and edited the manuscript.
References
- Reichert JM. Marketed therapeutic antibodies compendium. MAbs 2012; 4:413 - 5; http://dx.doi.org/10.4161/mabs.19931; PMID: 22531442
- Nelson AL, Dhimolea E, Reichert JM. Development trends for human monoclonal antibody therapeutics. Nat Rev Drug Discov 2010; 9:767 - 74; http://dx.doi.org/10.1038/nrd3229; PMID: 20811384
- Poirier N, Blancho G, Vanhove B. A more selective costimulatory blockade of the CD28-B7 pathway. Transpl Int 2011; 24:2 - 11; http://dx.doi.org/10.1111/j.1432-2277.2010.01176.x; PMID: 20964725
- Kremer JM, Westhovens R, Leon M, Di Giorgio E, Alten R, Steinfeld S, Russell A, Dougados M, Emery P, Nuamah IF, et al. Treatment of rheumatoid arthritis by selective inhibition of T-cell activation with fusion protein CTLA4Ig. N Engl J Med 2003; 349:1907 - 15; http://dx.doi.org/10.1056/NEJMoa035075; PMID: 14614165
- Vincenti F, Larsen C, Durrbach A, Wekerle T, Nashan B, Blancho G, Lang P, Grinyo J, Halloran PF, Solez K, et al, Belatacept Study Group. Costimulation blockade with belatacept in renal transplantation. N Engl J Med 2005; 353:770 - 81; http://dx.doi.org/10.1056/NEJMoa050085; PMID: 16120857
- Poirier N, Azimzadeh AM, Zhang T, Dilek N, Mary C, Nguyen B, Tillou X, Wu G, Reneaudin K, Hervouet J, et al. Inducing CTLA-4-dependent immune regulation by selective CD28 blockade promotes regulatory T cells in organ transplantation. Sci Transl Med 2010; 2:17ra10; http://dx.doi.org/10.1126/scitranslmed.3000116; PMID: 20371478
- Poirier N, Mary C, Dilek N, Hervouet J, Minault D, Blancho G, Vanhove B. Preclinical efficacy and immunological safety of FR104, an antagonist anti-CD28 monovalent Fab’ antibody. Am J Transplant 2012; 12:2630 - 40; http://dx.doi.org/10.1111/j.1600-6143.2012.04164.x; PMID: 22759318
- Poirier N, Blancho G, Vanhove B. CD28-specific immunomodulating antibodies: what can be learned from experimental models?. Am J Transplant 2012; 12:1682 - 90; http://dx.doi.org/10.1111/j.1600-6143.2012.04032.x; PMID: 22471377
- Zhang T, Fresnay S, Welty E, Sangrampurkar N, Rybak E, Zhou H, Cheng X-F, Feng Q, Avon C, Laaris A, et al. Selective CD28 blockade attenuates acute and chronic rejection of murine cardiac allografts in a CTLA-4-dependent manner. Am J Transplant 2011; 11:1599 - 609; http://dx.doi.org/10.1111/j.1600-6143.2011.03624.x; PMID: 21749640
- Vanhove B, Laflamme G, Coulon F, Mougin M, Vusio P, Haspot F, Tiollier J, Soulillou J-P. Selective blockade of CD28 and not CTLA-4 with a single-chain Fv-alpha1-antitrypsin fusion antibody. Blood 2003; 102:564 - 70; http://dx.doi.org/10.1182/blood-2002-08-2480; PMID: 12649149
- Haspot F, Villemain F, Laflamme G, Coulon F, Olive D, Tiollier J, Soulillou J-P, Vanhove B. Differential effect of CD28 versus B7 blockade on direct pathway of allorecognition and self-restricted responses. Blood 2002; 99:2228 - 34; http://dx.doi.org/10.1182/blood.V99.6.2228; PMID: 11877302
- Li J, Semple K, Suh W-K, Liu C, Chen F, Blazar BR, Yu X-Z. Roles of CD28, CTLA4, and inducible costimulator in acute graft-versus-host disease in mice. Biol Blood Marrow Transplant 2011; 17:962 - 9; http://dx.doi.org/10.1016/j.bbmt.2011.01.018; PMID: 21447398
- Butte MJ, Keir ME, Phamduy TB, Sharpe AH, Freeman GJ. Programmed death-1 ligand 1 interacts specifically with the B7-1 costimulatory molecule to inhibit T cell responses. Immunity 2007; 27:111 - 22; http://dx.doi.org/10.1016/j.immuni.2007.05.016; PMID: 17629517
- Butte MJ, Peña-Cruz V, Kim M-J, Freeman GJ, Sharpe AH. Interaction of human PD-L1 and B7-1. Mol Immunol 2008; 45:3567 - 72; http://dx.doi.org/10.1016/j.molimm.2008.05.014; PMID: 18585785
- Yao S, Zhu Y, Zhu G, Augustine M, Zheng L, Goode DJ, Broadwater M, Ruff W, Flies S, Xu H, et al. B7-h2 is a costimulatory ligand for CD28 in human. Immunity 2011; 34:729 - 40; http://dx.doi.org/10.1016/j.immuni.2011.03.014; PMID: 21530327
- Suntharalingam G, Perry MR, Ward S, Brett SJ, Castello-Cortes A, Brunner MD, Panoskaltsis N. Cytokine storm in a phase 1 trial of the anti-CD28 monoclonal antibody TGN1412. N Engl J Med 2006; 355:1018 - 28; http://dx.doi.org/10.1056/NEJMoa063842; PMID: 16908486
- Lühder F, Huang Y, Dennehy KM, Guntermann C, Müller I, Winkler E, Kerkau T, Ikemizu S, Davis SJ, Hanke T, et al. Topological requirements and signaling properties of T cell-activating, anti-CD28 antibody superagonists. J Exp Med 2003; 197:955 - 66; http://dx.doi.org/10.1084/jem.20021024; PMID: 12707299
- Shiao SL, McNiff JM, Masunaga T, Tamura K, Kubo K, Pober JS. Immunomodulatory properties of FK734, a humanized anti-CD28 monoclonal antibody with agonistic and antagonistic activities. Transplantation 2007; 83:304 - 13; http://dx.doi.org/10.1097/01.tp.0000251426.46312.d5; PMID: 17297405
- Mary C, Coulon F, Poirier N, Dilek N, Martinet B, Blancho G, Vanhove B. Antagonist properties of monoclonal antibodies targeting human CD28: role of valency and the heavy-chain constant domain. MAbs 2013; 5:47 - 55; http://dx.doi.org/10.4161/mabs.22697; PMID: 23221503
- Eastwood D, Findlay L, Poole S, Bird C, Wadhwa M, Moore M, Burns C, Thorpe R, Stebbings R. Monoclonal antibody TGN1412 trial failure explained by species differences in CD28 expression on CD4+ effector memory T-cells. Br J Pharmacol 2010; 161:512 - 26; http://dx.doi.org/10.1111/j.1476-5381.2010.00922.x; PMID: 20880392
- Pallardy M, Hünig T. Primate testing of TGN1412: right target, wrong cell. Br J Pharmacol 2010; 161:509 - 11; http://dx.doi.org/10.1111/j.1476-5381.2010.00925.x; PMID: 20880391
- Pitcher CJ, Hagen SI, Walker JM, Lum R, Mitchell BL, Maino VC, Axthelm MK, Picker LJ. Development and homeostasis of T cell memory in rhesus macaque. J Immunol 2002; 168:29 - 43; PMID: 11751943
- Findlay L, Eastwood D, Stebbings R, Sharp G, Mistry Y, Ball C, Hood J, Thorpe R, Poole S. Improved in vitro methods to predict the in vivo toxicity in man of therapeutic monoclonal antibodies including TGN1412. J Immunol Methods 2010; 352:1 - 12; http://dx.doi.org/10.1016/j.jim.2009.10.013; PMID: 19895813
- Stebbings R, Findlay L, Edwards C, Eastwood D, Bird C, North D, Mistry Y, Dilger P, Liefooghe E, Cludts I, et al. “Cytokine storm” in the phase I trial of monoclonal antibody TGN1412: better understanding the causes to improve preclinical testing of immunotherapeutics. J Immunol 2007; 179:3325 - 31; PMID: 17709549
- Stebbings R, Eastwood D, Poole S, Thorpe R. After TGN1412: recent developments in cytokine release assays. J Immunotoxicol 2013; 10:75 - 82; http://dx.doi.org/10.3109/1547691X.2012.711783; PMID: 22967038
- Vidal J-M, Kawabata TT, Thorpe R, Silva-Lima B, Cederbrant K, Poole S, Mueller-Berghaus J, Pallardy M, Van der Laan J-W. In vitro cytokine release assays for predicting cytokine release syndrome: the current state-of-the-science. Report of a European Medicines Agency Workshop. Cytokine 2010; 51:213 - 5; http://dx.doi.org/10.1016/j.cyto.2010.04.008; PMID: 20471854
- Findlay L, Eastwood D, Ball C, Robinson CJ, Bird C, Wadhwa M, Thorpe SJ, Thorpe R, Stebbings R, Poole S. Comparison of novel methods for predicting the risk of pro-inflammatory clinical infusion reactions during monoclonal antibody therapy. J Immunol Methods 2011; 371:134 - 42; http://dx.doi.org/10.1016/j.jim.2011.06.022; PMID: 21741383
- Findlay L, Sharp G, Fox B, Ball C, Robinson CJ, Bird C, Stebbings R, Eastwood D, Wadhwa M, Poole S, et al. Endothelial cells co-stimulate peripheral blood mononuclear cell responses to monoclonal antibody TGN1412 in culture. Cytokine 2011; 55:141 - 51; http://dx.doi.org/10.1016/j.cyto.2011.03.019; PMID: 21493088
- Brennan FR, Morton LD, Spindeldreher S, Kiessling A, Allenspach R, Hey A, Muller PY, Frings W, Sims J. Safety and immunotoxicity assessment of immunomodulatory monoclonal antibodies. MAbs 2010; 2:233 - 55; http://dx.doi.org/10.4161/mabs.2.3.11782; PMID: 20421713
- Eastwood D, Bird C, Dilger P, Hockley J, Findlay L, Poole S, Thorpe SJ, Wadhwa M, Thorpe R, Stebbings R. Severity of the TGN1412 trial disaster cytokine storm correlated with IL-2 release. Br J Clin Pharmacol 2013; 76:299 - 315; http://dx.doi.org/10.1111/bcp.12165; PMID: 23701319
- Chapman K, Pullen N, Graham M, Ragan I. Preclinical safety testing of monoclonal antibodies: the significance of species relevance. Nat Rev Drug Discov 2007; 6:120 - 6; http://dx.doi.org/10.1038/nrd2242; PMID: 17268483
- Ohresser M, Olive D, Vanhove B, Watier H. Risk in drug trials. Lancet 2006; 368:2205 - 6; http://dx.doi.org/10.1016/S0140-6736(06)69883-8; PMID: 17189022
- Hanke T. Lessons from TGN1412. Lancet 2006; 368:1569 - 70, author reply 1570; http://dx.doi.org/10.1016/S0140-6736(06)69651-7; PMID: 17084746
- Waibler Z, Sender LY, Merten C, Hartig R, Kliche S, Gunzer M, Reichardt P, Kalinke U, Schraven B. Signaling signatures and functional properties of anti-human CD28 superagonistic antibodies. PLoS ONE 2008; 3:e1708.
- Nguyen DH, Hurtado-Ziola N, Gagneux P, Varki A. Loss of Siglec expression on T lymphocytes during human evolution. Proc Natl Acad Sci U S A 2006; 103:7765 - 70; http://dx.doi.org/10.1073/pnas.0510484103; PMID: 16682635
- Suchard SJ, Davis PM, Kansal S, Stetsko DK, Brosius R, Tamura J, Schneeweis L, Bryson J, Salcedo T, Wang H, et al. A monovalent anti-human CD28 domain antibody antagonist: preclinical efficacy and safety. J Immunol 2013; 191:4599 - 610; http://dx.doi.org/10.4049/jimmunol.1300470; PMID: 24081989
- Weissmüller S, Semmler LY, Kalinke U, Christians S, Müller-Berghaus J, Waibler Z. ICOS-LICOS interaction is critically involved in TGN1412-mediated T-cell activation. Blood 2012; 119:6268 - 77; http://dx.doi.org/10.1182/blood-2011-12-401083; PMID: 22577174
- Devallière J, Chatelais M, Fitau J, Gérard N, Hulin P, Velazquez L, Turner CE, Charreau B. LNK (SH2B3) is a key regulator of integrin signaling in endothelial cells and targets α-parvin to control cell adhesion and migration. FASEB J 2012; 26:2592 - 606; http://dx.doi.org/10.1096/fj.11-193383; PMID: 22441983
- www.primateportal.org/normative-values.
