Abstract
To improve recruitment and activation of natural killer (NK) cells to lyse tumor cells, we isolated a human anti-CD16A antibody with similar affinity for the CD16A 158F/V allotypes, but no binding to the CD16B isoform. Using CD16A-targeting Fv domains, we constructed a tetravalent bispecific CD30/CD16A tandem diabody (TandAb®) consisting solely of Fv domains. This TandAb has two binding sites for CD16A and two for CD30, the antigen identifying Hodgkin lymphoma cells. The binding and cytotoxicity of the TandAb were compared with antibodies with identical anti-CD30 domains: (1) a native IgG, (2) an IgG optimized for binding to Fc receptors, and (3) a bivalent bispecific CD30/CD16A diabody. Due to its CD16A-bivalency and reduced koff, the TandAb was retained longer on the surface of NK cells than the IgGs or the diabody. This contributed to the higher potency and efficacy of the TandAb relative to those of the other anti-CD30 antibodies. TandAb cytotoxicity was independent of the CD16A allotype, whereas the anti-CD30 IgGs were substantially less cytotoxic when NK cells with low affinity CD16A allotype were employed. TandAb activation of NK cells was strictly dependent on the presence of CD30+ target cells. Therefore, the CD30/CD16A TandAb may represent a promising therapeutic for the treatment of Hodgkin’s lymphoma; further, anti-CD16A TandAbs may function as potent immunotherapeutics that specifically recruit NK cells to destroy cancer cells.
Introduction
CD16 (FcγRIII), a low-affinity receptor for the IgG Fc domain, facilitates antibody-dependent cell-mediated cytotoxicity (ADCC) and is responsible for triggering the lysis of target cells by natural killer (NK) cells.Citation1 Human NK cells comprise ~10% of all lymphocytes and are defined phenotypically by expression of CD56, absence of CD3,Citation2 and high density of CD16.Citation3 Human CD16 exists in two isoforms, CD16A and CD16B, that share 96% sequence identity in their immunoglobulin-binding regions.Citation4 CD16A is expressed on NK cells, macrophages, and mast cells and is an activating receptor. CD16B is expressed on granulocytes as a GPI-anchored receptor and does not trigger tumor cell killing.Citation4
ADCC is one of the major cytotoxic mechanisms employed by FcγR-expressing effector cells to eliminate tumor cells. Several immunotherapeutic approaches for oncology have explored the mobilization of ADCC-mediating effector cells, expressing FcγRs, by tumor antigen-specific IgGs. Thus, bispecific molecules binding to both tumor cells and either FcγRI (CD64) or CD16 have been developed to improve effector cell recruitment.Citation5 Because NK cells mediate innate immunity and are constitutively activated, they are attractive candidates for cancer immunotherapy, and bispecific antibodies recruiting these cytotoxic cells (CD16/CD30 and CD16/HER-2) have been developed.Citation6,Citation7
Previously, there have been no reports of CD16A-specific antibodies lacking CD16B binding, and the majority of anti-CD16 antibodies bind both CD16 isoforms, CD16A and CD16B, except for two antibodies described as recognizing only CD16B.Citation8-Citation10CD16A-specific antibodies would engage NK cells without being removed from the circulation by CD16B+ granulocytes, which represent up to 70% of leukocytes. Thus, an anti-CD16A antibody should allow the engineering of bi- or multi-specific molecules that target disease-associated cells via specific NK cell recruitment.
CD16A exhibits allelic polymorphisms at positions 48 and 158 of the Fc-binding domain. A previous report has demonstrated that IgG bind with different affinities to CD16A allotypes, due to polymorphism at position 158.Citation11 Since affinity to CD16A is implicated as important for the clinical efficacy of therapeutic antibodies,Citation12-Citation14 and since the frequencies of these low/high-affinity allotypes, 158F/V, are 0.6 and 0.4,Citation11 respectively, an anti-CD16A therapeutic antibody with similar affinity to both allelic forms, and hence potently activating NK cells in all patients, would be advantageous. Here, we report the incorporation of an anti-CD16A single chain antibody (scFv), which recognizes both CD16A allotypes with similar affinity and does not bind to CD16B,Citation15 into a tetravalent bispecific anti-CD30/anti-CD16A (CD30/CD16A) antibody format (TandAb®) comprising solely Fv domainsCitation16 for the recruitment of NK cells to lyse CD30+ Hodgkin Reed-Sternberg (HRS) lymphoma cells. We describe the in vitro properties of this TandAb and compare its efficacy and mode of action to an anti-CD30 full-length IgG, with either native or enhanced Fc receptor binding properties, and a CD30/CD16A bispecific diabody.
Results
Selection of a specific anti-CD16A
To isolate a human antibody specific for CD16A, we screened a phage display library of scFv derived from human PBMCs and identified a CD16A-specific antibody that did not bind CD16B; after affinity maturation, we identified several mutants that bound CD16A with higher affinity than the original scFv with no loss of specificity (Table 1S). Consistent with this specificity profile, affinity-matured scFvs demonstrated strong binding to CD16A+ NK cells and no binding to CD16B+ granulocytes.Citation15
The scFv most potently recruiting NK cells was selected using a cell cytotoxicity assay. We employed an IgG1 antibody that binds the C-terminal scFv His-tags to promote pairwise binding to FcγRII+ P-815 cells;Citation17 this emulates the bivalent binding of the bispecific TandAb, with anti-CD16A moieties, to effector NK cells. The relative amounts of cell lysis, induced by incubation of enriched human NK cells and calcein-labeled P-815 target cells in the presence of the scFvs, are shown in . With no crosslinking by anti-His IgG, lysis was similar to that of the negative controls (anti-CD3 IgG, anti-CD56 IgG, anti-CD3 scFv in the presence or absence of anti-His IgG). However dimerization of anti-CD16A scFvs, promoted by anti-His IgG, enhanced cytotoxicity toward P-815 cells. Thus, simultaneous crosslinking of FcγRII+ target and NK effector cells, induced by the anti-His IgG-dimerized scFv, can trigger activating signaling and subsequent target cell lysis. The most efficacious anti-CD16A scFvs triggered cytotoxicity similar to that of control anti-CD16 and anti-NKp46 IgG. We thus selected the variable domains of LSIV21 (Fig. 1S) for TandAb incorporation.
Figure 1. Selection of anti-CD16A-specific scFv for activation of NK cells and construction of bispecific CD30/CD16A TandAb and diabody. (A) Induction of redirected cell lysis with affinity matured anti-CD16A scFvs. 1 × 104 calcein-labeled FcγRII+ murine mastocytoma P-815 target cells were incubated in triplicate with freshly isolated and enriched human NK cells at an effector-to-target ratio of 10:1 in the presence of 1 µg/mL of the indicated scFv or IgGs with or without the addition of 1 µg/mL anti-His IgG1, clone 13/45/31–2, for 3 h. Percentage of specific target cell lysis was calculated from the measured fluorescence counts in the culture supernatant. Mean values and standard deviations from triplicates are plotted. (B and C) Schematic representation of the gene organization and domain order of the bispecific CD30/CD16A TandAb (B) and diabody (C). VHA and VLA represent the variable anti-CD16A domains that are derived from the human scFv clone LSIV21 isolated from Affimed's phage display library. VHB and VLB stand for the murine anti-CD30 Fv domains that are derived from the HRS-3 IgG. The HRS-3 hybridoma-derived light chain contains two mutations in framework 1: tyrosine at L23, instead of the conserved cysteine that forms a disulfide bond with CysL88, and asparagine at L20, which is a potential glycosylation site. The residues at L20 and L23 were restored to the original amino acids in the germline sequence. The linker peptides (L) are of 9 amino acids length and consist of three Gly-Gly-Ser repeats.
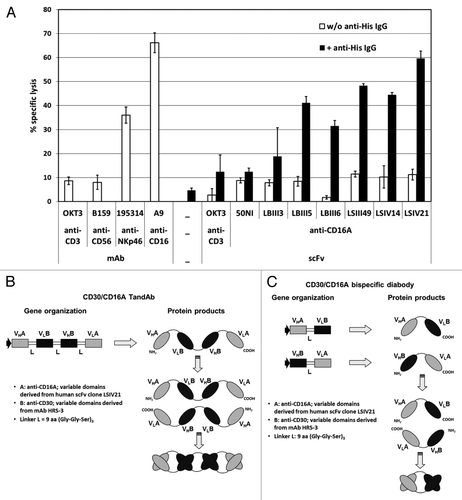
Construction of a CD30/CD16A TandAb and diabody, and anti-CD30 IgGs
To compare the binding and cellular cytotoxicity of: (1) a tetravalent TandAb containing the anti-CD16A Fv described above, (2) a bivalent diabody, also containing this anti-CD16A Fv, and (3) an IgG, we utilized the Fv domains of the anti-CD30 antibody HRS-3.6, Citation6,Citation18The gene organization and gene products for the TandAb and diabody are shown in . The characterization of the purified TandAb employing reducing and non-reducing SDS-PAGE and SE-HPLC revealed a purity of 96.2% homodimers (Fig. 2S). The anti-CD30 IgG with a human IgG1 is referred to as “the native IgG.” The anti-CD30 IgG containing S239D/I332E Fc-mutations, which increase FcγR binding,Citation19 is referred to as “the Fc-enhanced IgG.”
Due to avidity, binding of a CD30/CD16A TandAb is enhanced relative to diabody and IgGs
We compared the affinities of the four constructs using a competitive binding assay employing CD30+ KARPAS-299 target cells. A PE-labeled mAb (HRS-4), binding to the same epitope as HRS-3, from which the anti-CD30 Fv domains were derived,Citation20 served as the reference (). The IC50 values, which were determined by nonlinear regression of the competition experiment between the PE-labeled anti-CD30 mAb, at constant concentration, and the four antibody constructs, added in increasing concentrations, were then used to calculate the KD values of the TandAb, diabody, and IgGs (). The affinities of all three antibodies that exhibited bivalent CD30-binding, the TandAb, the native and Fc-enhanced IgGs, were similar to each other, and more than an order of magnitude higher than that of the monovalent diabody (). Due to bivalent binding, 80% of the TandAb and IgGs are retained after 1 h at 37 °C compared with 10% of the monovalent diabody (). This suggests that the bivalent binding of the antibodies increases apparent affinity due to avidity.
Figure 2. Binding of the TandAb, the diabody and IgGs to CD30 and CD16A. (A) Competition assay. 1x106 KARPAS-299 cells were incubated with 3 µg/mL (~20 nM) PE-conjugated anti-CD30 HRS-4 IgG together with the indicated concentrations of recombinant bispecific antibodies or anti-CD30 IgGs for 45 min at 37 °C. After washing, the fluorescence of 1 × 104 cells was measured with a flow cytometer, corrected for background staining with the corresponding isotype control and analyzed by nonlinear regression for calculation of IC50 and KD values. One out of two experiments is depicted. (B) Cell surface retention at 37 °C. Aliquots of KARPAS-299 cells were incubated with biotinylated CD30/CD16A TandAb and diabody or anti-CD30 IgG for 45 min on ice. After removing excess antibodies by washing, cells were incubated at 37 °C for the indicated periods of time. After repeated washing on ice, remaining antibodies were detected using Dylight488-conjugated streptavidin, and the fluorescence of 1 × 104 cells was analyzed by flow cytometry. The mean fluorescence values from time-point 0 were set to 100% and the percentage of remaining antibodies was determined using nonlinear regression analysis. Data shown are representative of two independent experiments. (C) The CD16A 48R158V Fc-fusion protein was covalently immobilized on a CM5 chip. The different antibodies (CD30/CD16A TandAb and diabody, and anti-CD30 IgGs,) were injected with a flow rate of 10 µL/min for 360 s and the dissociation time was set to 600 s. Background signals in the control flow cell (without immobilized antigen) were subtracted from signals in the test flow cell. Chips were regenerated with 10 mM Glycine-HCL, pH 2.0. (D) The CD16A allotypes, 48R158V and 48R158F, and the CD16B allotypes, SH, NA1 and NA2, were expressed in HEK-293 cells as Fc-fusion proteins and directly immobilized on chips. TandAb was injected with a flow rate of 10 µL/min for 360 s. Background signals in the control flow cell (without immobilized antigen) were subtracted from signals in the test flow cell. Dissociation time was set to 600 s. Chips were regenerated with 10 mM Glycine-HCL, pH 2.0. (E) Cell surface retention assay. 1 × 106 enriched human NK cells were stained with 1 µM of CD30/CD16A TandAb or diabody on ice, washed and incubated for indicated periods of time at 37 °C to allow dissociation. After washing, remaining antibodies were detected by anti-His IgG followed by FITC-conjugated goat anti-mouse IgG. The mean fluorescence values from time-point 0 were set to 100% and the percentage of remaining antibodies was plotted using nonlinear regression.
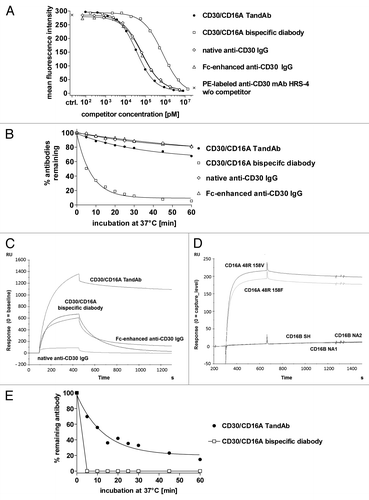
Table 1. Affinities of TandAb, diabody and IgG to CD30 determined in a competition assay on KARPAS-299 cells
To compare the CD16A and CD16B binding kinetics of the antibodies, we performed surface plasmon resonance (SPR) measurements with various allotypes of both CD16 isoforms fused to an Fc domain (; ). The apparent affinity of the TandAb to both allotypes of CD16A was 30-fold higher than that of the Fc-enhanced IgG, and more so compared with the diabody and the native IgG. While the CD16A kon rates of the antibodies were similar, the bivalent-binding TandAb exhibited koff that was substantially less than those of the monovalent-binding diabody and both IgGs (; ). The bivalent TandAb exhibited no measurable binding to any CD16B allotype (), consistent with the original observations of the monovalent anti-CD16A scFv.Citation15 Furthermore, the TandAb displayed a sub-nanomolar apparent affinity to both CD16A allotypes, which was the highest among the assayed antibodies. The Fc-enhanced IgG and the diabody exhibited similar affinities for CD16A, albeit their binding was 30–80-fold lower relative to that of the TandAb; in contrast, the CD16A binding of the native IgG was almost 1000-fold less than that of the TandAb. Both IgGs bound to CD16B, unlike the TandAb and the diabody. These data demonstrate that the TandAb possesses high apparent affinity to both CD16A allotypes and exquisite binding specificity manifested by exclusive binding to CD16A.
Table 2. Affinities of TandAb, diabody and IgGs to different CD16 variants measured by SPR
Cell surface retention assays on primary NK cells confirm the substantially slower dissociation of the bivalent TandAb relative to that of the monovalent diabody (). The fast dissociation of the diabody at 37 °C results in non-measureable FACS signals after a 5 min incubation of the cells in a large volume of medium, which prevents re-binding of dissociated antibodies. Therefore, the TandAb binding characteristics to both CD16A+ NK cells and to CD16A in a cell-free SPR assay are consistent with its bivalent binding.
A CD30/CD16A TandAb exhibits superior cytotoxicity relative to diabody and IgGs
We established an in vitro cytotoxicity assay, employing calcein-labeled targets and enriched human NK effectors, to assess the biological activity of the TandAb, diabody and IgGs. The kinetics of a specific tumor cell line, KARPAS-299, lysis was monitored for 3 h at increasing antibody concentrations. For each antibody, sigmoidal dose-response curves were determined after 5, 15, 30, 60, 120, and 180 min incubation (Fig. 3S), and used for the calculations of EC50 and maximal cell lysis (). The TandAb and the diabody induced significant cytotoxicity of KARPAS-299 cells rapidly, in contrast to the native and Fc-enhanced IgGs. The maximal cell lysis induced by the TandAb and the diabody were similar, and always higher than that of IgG. However, the diabody EC50 was 10–15 times higher than that of the TandAb at every time point, reflecting lower potency. The maximal cell lysis induced by the Fc-enhanced IgG peaked at less than half that of either the TandAb or the diabody.
Figure 3. Comparison of CD30/CD16A TandAb and diabody, anti-CD30 IgG and Fc-enhanced anti-CD30 IgG in cytotoxicity assays. (A) Kinetics of antibody-mediated target cell lysis. 1 × 104 calcein-labeled KARPAS-299 target cells were incubated for the indicated time periods with increasing concentrations of CD30/CD16A TandAb, CD30/CD16A diabody, anti-CD30 IgG and Fc-enhanced anti-CD30 IgG together with freshly isolated human NK cells at an E:T ratio of 5:1. Percent specific target cell lysis was calculated from the fluorescent calcein released into the cell culture supernatant from apoptotic target cells. EC50 values (potency) and maximal target cell lysis (efficacy) were determined by nonlinear regression for all antibodies and plotted. (B) Residual cytotoxicity of pre-opsonized effector cells to measure antibodies’ retentions. Freshly isolated human NK cells were incubated with increasing concentrations of CD30/CD16A TandAb, diabody and Fc-enhanced anti-CD30 IgG for 15 min at 37 °C, washed twice and then incubated for 1 h at 37 °C to allow dissociation of the bound antibodies. After an additional washing step, NK cells were used as effector cells in a 3 h cytotoxicity assay with calcein-labeled KARPAS-299 target cells at an E:T ratio of 4.4:1 (pre-loaded – 1 h dissociation). As a control, NK cells were pre-incubated in the absence of antibodies before they were used as effector cells together with added antibodies in the same cytotoxicity assay (directly added - control). Mean values and SD from duplicates of one out of two independent experiments are shown. (C) Potency of TandAb, diabody and IgG in relation to the CD16A 158 polymorphism. The EC50 values for TandAb, diabody and anti-CD30 IgG were determined in several independent 3 h cytotoxicity assays on KARPAS-299 target cells with NK cells as effector cells isolated from unrelated donors as described and plotted in the diagram together with the mean values shown as bars. The CD16A 158 phenotype of the NK cells was assessed by flow cytometry after staining of the NK cells with the CD16 158V-specific mAb MEM-154. NK cells were rated as CD16A 158F/F when the fluorescence signal was at background level. The asterisk (*) indicates statistical significance (P < 0.05). (D) Cytotoxic potency of the TandAb against a panel of five CD30+ cell lines. The EC50 values of the TandAb were determined in independent 3 h cytotoxicity assays on target CD30+ cells, with NK cells as effectors, isolated from independent donors, at a 1:5 ratio. Mean values for each cell line are shown as horizontal bars.
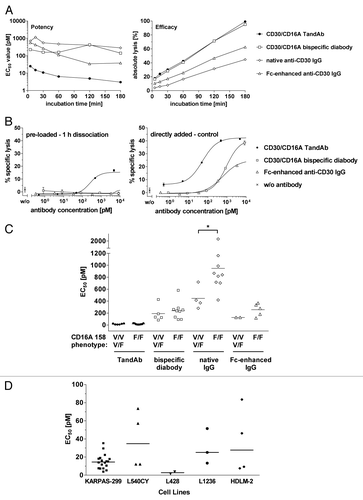
To demonstrate that high affinity CD16A binding correlates with enhanced lytic potency and efficacy, we compared the residual cytotoxic activity of NK cells that were opsonized with three constructs and then permitted to dissociate (). Only NK cells incubated with the TandAb exhibited cytotoxic activity against KARPAS-299 tumor cells. This is in contrast to the observation where the antibodies were directly assayed with no subsequent dissociation step: each antibody exhibited the expected cytotoxic response. These assays demonstrate that the increased CD16A binding is critical to superior tumor cell cytotoxicity; such increased binding is due to higher avidity that reduces koff of the anti-CD16A domains, relative to the Fc domain of the IgGs. Moreover, cytotoxicity assays with KARPAS-299 tumor cells and phenotyped NK cells, presented in , demonstrated similar TandAb potency independent of CD16A NK cell allotype, which is consistent with possessing similar apparent TandAb affinity (158F homozygous: EC50 17.0 pM, mean of n = 9; 158V homo- or heterozygous: EC50 15.7 pM, mean of n = 6). The bispecific diabody exhibited cytotoxic potency, mediated by NK cells, that was independent of their CD16A allotype, as in the case of the TandAb. However, the diabody potency was reduced by an order of magnitude relative to that of the TandAb (158F homozygous: EC50 240 pM, mean of n = 9; 158V homo- or heterozygous: EC50 191 pM, mean of n = 5). In contrast, the native and the Fc-enhanced IgG displayed a 2-fold lower potency when CD16A 158F homozygous NK cells were used (native IgG: 158F homozygous – EC50 948 pM, mean of n = 9; 158V homo- or heterozygous – EC50 446 pM, mean of n = 4, and Fc-enhanced IgG: 158F homozygous: EC50 256 pM, mean of n = 5; 158V homo- or heterozygous: EC50 127 pM, mean of n = 2); a statistically significant difference (P = 0.017) was observed only for the native anti-CD30 IgG.
Finally, we evaluated the cytotoxic activity of the TandAb against a panel of CD30+ cell lines derived from HL or anaplastic large-cell lymphoma tumors (). In all cases the TandAb elicited potent cytotoxicity, in the range of 3–40 pM, confirming its activity across a broad panel of cell lines independent of their origin (KARPAS-299: EC50 = 15 pM [n = 18]; L540CY: EC50 = 39 pM [n = 4]; L428: EC50 = 3 pM [n = 2]; L1236: EC50 = 30 pM [n = 3]; HDLM-2: EC50 = 37 pM [n = 4]).
In the absence of CD30+ targets, CD30/CD16A TandAb elicits neither cytotoxicity nor NK cell activation
To determine whether bivalent CD16A-binding of the TandAb could result in systemic activation of NK cells and non-specific cell lysis, we first assayed cytokine release from human PBMC in the presence and absence of CD30+ KARPAS-299 cells. As a control, KARPAS-299 cells were cultured without human PBMC. shows tumor necrosis factor (TNF) and interferon (IFN)-γ release after incubation with increasing concentrations of TandAb for 24 h. The positive-control anti-CD3 antibody (OKT3), induced strong release of both cytokines, whereas the TandAb induced no or marginal cytokine production in PBMC cultures in the absence of CD30+ cells. When CD30+ cells were added to the cultures, at a PBMC-to-tumor cell ratio of 10:1, a dose-dependent secretion of TNF and IFN-γ was observed in the presence of the TandAb. The TandAb-induced cytokine release, however, was always less than that of OKT3. These data indicate that activation of NK cells is tightly linked to the presence of CD30+ tumor cells.
Figure 4. Specificity and safety in vitro. (A) Cytokine release in PBMC cultures in the presence of the CD30/CD16A TandAb. 5 × 105 human PBMC with and without 1 × 104 CD30+ KARPAS-299 cells, or 1 × 104 KARPAS-299 cells alone were cultured in the presence of increasing concentrations of the CD30/CD16A TandAb (denoted by TandAb; at 0.001–10 µg/mL), 10 µg/mL OKT3, or without antibody (denoted by 0). The concentrations of secreted TNF and IFN-γ were quantified using multiplexing after 24 h incubation. Results from one representative experiment are shown. (B and C) Assessment of bystander cell killing in a cytotoxicity assay with mixed target cells. 1 × 104 calcein-labeled CD20-/CD30+ KARPAS-299 target cells, combined with 1x104 unlabeled CD20+/CD30- Raji target cells (B) or 1 × 104 unlabeled KARPAS-299 combined with calcein-labeled Raji cells (C), were incubated for 3 h, together with increasing concentrations of the CD30/CD16A TandAb or rituximab (MabThera, Roche, Hertfordshire, UK), in the presence or absence of 5 × 104 enriched human NK cells as effectors for 3 h in a cytotoxicity assay. The percentage of specific target cell lysis was calculated and the mean and SD from duplicates were plotted.
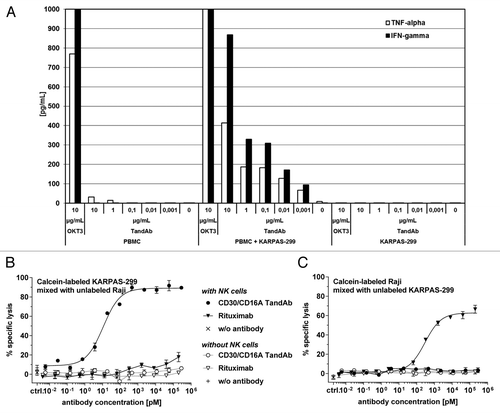
Second, to further assess the specificity of NK cell activation by the TandAb, we performed a bystander cytotoxicity assay by co-culturing CD30+ and CD30- target cells with NK cells at increasing concentrations of the TandAb. When calcein-labeled CD30+/CD20- KARPAS-299 cells were mixed with unlabeled CD30-/CD20+ Raji cells, the TandAb induced dose-dependent lysis, while the rituximab control induced marginal lysis of KARPAS-299 (). In the inverse experiment, with calcein-labeled Raji and unlabeled KARPAS-299 cells, only rituximab mediated lysis of CD20+ Raji cells (). These data demonstrate that: (1) bivalent TandAb binding to NK cells does not trigger non-specific cytolytic activity, and (2) CD30- bystander cells are not affected by TandAb-mediated target cell lysis. The lack of non-specific cytokine release and the absence of bystander cell killing, suggest that the TandAb should not mediate off-target systemic NK cell activation that could lead to severe side effects.
Discussion
Hodgkin lymphoma (HL) is a unique tumor in which the cancerous HRS cells represent a small fraction. The tumor cells appear to regulate their environment by secreting a mixture of inflammatory chemokines and cytokines that attract different leukocytes such as T cells, B cells, plasma cells, eosinophils and mast cells. CD4+ T cells, especially immunosuppressive regulatory T cells, represent the largest population of cells in HL tissue.Citation21-Citation23 CD30, which is highly expressed on HRS and anaplastic large cell lymphoma cells, but otherwise only on activated lymphocytes, has been implicated in the modulation of T cell activation and proliferation.Citation24
Encouraging results for the treatment of HL were obtained in two Phase 1 clinical studies using a bispecific quadroma antibody to recruit immune effector cells via CD16 binding.Citation25,Citation26 The quadroma was made by fusing two hybridomas: the first secreting an anti-CD30 antibody, and the second secreting an anti-CD16 antibody. The program was discontinued due to difficulties with production and purification of this bispecific quadroma antibody, although clinical results showed favorable activity. To overcome the manufacturing challenges, and to avoid potential cytokine release syndrome due to extensive cross-linking of immune effector cells via Fc domains, a bispecific diabody containing the Fv domains of the quadroma was developed.Citation6 It was efficacious at lysing tumor cells, but its low molecular weight (~50 kDa) precluded clinical development due to an unfavorable pharmacokinetic profile. Therefore, we developed an antibody that was twice as large as the diabody, employing a tetravalent bispecific antibody format (TandAb®), also comprised solely of antibody Fv domains.Citation16 TandAbs have two binding domains for each target molecule and a molecular weight of about 105–110 kDa, which is above the threshold for first-pass renal clearance.
To optimize the clinical potential of TandAbs for recruiting NK cells, we screened a human antibody library for a specific anti-CD16A antibody. We isolated an affinity matured antibody with no binding to any CD16B allotype. The CD16A specificity of the TandAb may be advantageous because broad expression of the non-activating CD16B receptor on granulocytes may act as a sink for non-CD16A-specific antibodies, resulting in decreased efficacy. CD16A possesses two clinically-relevant allotypes, 158V and 158F, which bind immunoglobulins with higher and lower affinities, respectively. It was observed that trastuzumab- and rituximab-treated patients homozygous for the high affinity allotype, 158V/V, respond significantly better than patients who were heterozygous or homozygous for the low affinity allotype.Citation12-Citation14 The anti-CD16A antibody described here similarly bound both CD16A allotypes, and it exhibited equivalent cytotoxic potency mediated by NK cells possessing either CD16A allotype. This suggests that the CD30/CD16A TandAb may benefit an expanded patient population.
Bivalent CD16A-binding by the TandAb did not mediate off-target NK cell activation in vitro; the TandAb only lysed cells expressing CD30. In the absence of target cells, there was no substantial release of cytokines, whether measured in the in vitro assay presented here, or during toxicological studies in cynomolgus monkeys, or in a Phase 1 clinical study (data not shown). Due to bivalent CD30 binding, both the TandAb and the corresponding IgGs exhibited similar apparent affinity to CD30+ target cells, which was more than 10-fold higher than that of the CD30-monovalent diabody. Moreover, due to much higher koff, molecules monovalently binding to CD16A (diabody, native, and Fc-enhanced IgGs) displayed lower affinity compared with that of the TandAb.
The higher avidity and the resultant lower koff of the TandAb for NK cells, leading to higher antibody retention, were apparent in the pre-opsonized effector cell cytotoxicity assay. Only NK cells incubated with the TandAb displayed cytotoxic activity. Furthermore, high amounts of the TandAb remained bound to NK cells in the presence of physiological concentrations of serum IgG. In contrast, even Fc-enhanced IgG exhibited substantially lower retention on, and binding at physiological concentrations of serum IgG to, NK cells, and this translated into substantially reduced cytotoxicity relative to the TandAb. This increased avidity of the TandAb may improve clinical efficacy in a broad HL patient population, and it could explain the demonstrated ability of the CD30/CD16A TandAb to overcome inhibition of HL-derived NK cells relative to those from healthy donors.Citation27
In cytotoxicity assays, the TandAb always performed better than the comparator constructs. The cytotoxic potency (EC50) of the diabody and the Fc-enhanced IgG was 10–15-fold lower than that of the TandAb. Meanwhile, the cytotoxic efficacy (maximal cell lysis) of the Fc-enhanced IgG was more than 2-fold less than those of the similarly efficacious TandAb and diabody.
In conclusion, the significantly higher efficacy and potency of the TandAb relative to native and Fc-enhanced antibodies may result in better clinical response in the treatment of HL, and other cancers, through the improved targeted recruitment of immune effector cells.
Methods
Constructs
For constructing a TandAb, a diabody and an anti-CD30 IgG1, the Fv domains of the anti-CD30 IgG HRS-3Citation6,Citation18were modified as follows. Two substitutions, N20T and Y23C (Kabat numbering), were introduced into framework 1 of the Vκ domain to remove a glycosylation site and to introduce a missing Cys residue by splice overlapping PCR, essentially as previously described.Citation28 In the TandAb and the diabody, the anti-CD30 domains were combined with the Fv domains of the affinity-matured anti-CD16A scFv clone LSIV21 that was isolated from a human phage display library as follows.Citation15 Anti-CD16A-specific scFv were isolated by phage display from a naïve IgM library comprising ~2 × 109 independent clones by three rounds of selection on biotinylated CD16A-Fc fusion protein.Citation29 After characterization of several clones for their binding and specificity, a single clone was selected for further affinity maturation by light chain shuffling. Two libraries (2.6 × 106 [Vκ] and 2.2 × 106 [Vλ]) were generated by recombining the VH chain of the selected clone with a repertoire of light chains. The libraries were subjected to four rounds of selection on biotinylated CD16A-Fc fusion protein. Selection pressure favoring high affinity binders was applied by successively limiting the antigen concentration. From six isolated scFv clones showing the best selectivity and affinity for CD16A, clone LSIV21 was selected for construction of a CD30/CD16 diabody and TandAb. The amino acid sequence of the VH and VL from clone LSIV21 is displayed in Figure 1S. In the diabody, the anti-CD16A domain was located as in the anti-CD16 domain of the previously described CD30/CD16 diabody.Citation6 In the TandAb, the anti-CD30 and anti-CD16A domains were located identically as the anti-CD19 and anti-CD3 domains, respectively, of the previously described anti-CD19/anti-CD3 TandAb.Citation16 All three peptide linkers of the CD30/CD16A TandAb comprised nine amino acids, (Gly-Gly-Ser)3. The Fc domain of the anti-CD30 antibody had human IgG1 origin. An anti-CD30 IgG1 with enhanced Fc-binding to CD16 was generated by introducing two Fc-mutations, S239D/I332E.Citation19 The eukaryotic expression plasmid for human CD16A-158V-Fc (pCDM8-CD16A 158V) was provided by Dr. Mandelboim.Citation29 The plasmid encoding the CD16A 158F-Fc fusion protein was obtained by mutating pCDM8-CD16A 158V. The plasmids encoding the ectodomains of the CD16B allotypes (NA1, NA2, and SH) fused to the Fc domain, were constructed from synthetic nucleotide sequences (Geneart), fused with the Fc domain, and cloned into the pSEC2 vector (Invitrogen, catalog number V900–20).
Expression and purification
The expression cassette for the TandAb was cloned into the mammalian expression vector pPBG-GPEX1, and the protein was produced (Rentschler) by perfusion fermentation in CHO cells. The TandAb secreted into the medium was harvested daily, stored at 4 °C, and the fractions collected over three weeks were pooled. After capture by hydrophobic charge induction chromatography, the product was purified by anion exchange and hydroxyapatite chromatography. The diabody was produced as previously described.Citation30 The anti-CD30 IgG expression vectors were generated by subcloning into pEUVH1.2 and pEUVL3.2 (Cambridge Antibody Technology Group Plc). IgG and Fc fusion proteins were secreted by transiently transfected HEK-293 cells and subsequently purified on Protein A.
CD16 affinity determination
SPR analysis was performed (GE Healthcare, BIAcore X100, catalog number BR110073) on CD16-Fc fusion proteins that were either covalently coupled to (GE Healthcare, Amino Coupling Kit, catalog number BR100050) or directly immobilized on a CM5 chip (GE Healthcare, catalog number). The antibodies were diluted in 1x HBS-EP+ buffer with 1 mg/mL NSB reducer (GE Healthcare) to a concentration range of either 0.78–100 nM or 100–1600 nM depending on antibody affinity to CD16.
Biotinylation of antibodies
Biotinylation was performed according to manufacturer’s instructions (GE Healthcare, Biotinylation Module, catalog number RPN2202). Free biotin was removed from the product (GE Healthcare, NAP5 column, catalog number 17–0853–01). The biotinylated product in individual column fractions was detected by dot blot analysis.
Cell culture and transient transfection
HEK-293, Raji, P-815, KARPAS-299, L540CY, L428, L1236, and HDLM-2 cell lines were cultured under standard conditions in DMEM or RPMI 1640 medium supplemented with: 10% heat-inactivated FCS, 100 U/mL penicillin G, 100 µg/mL streptomycin, and 2 mM L-glutamine (all Invitrogen). HEK-293 cells (catalog number ACC 305), KARPAS-299 cells (catalog number ACC 31), L428 cells (catalog number ACC 197), L1236 cells (catalog number ACC 530), HDLM-2 cells (catalog number ACC 17) were purchased (DSMZ). L540CY, Raji, and P-815, were provided by Dr. Moldenhauer (DKFZ Heidelberg).
Isolation of PBMC and enrichment of NK cells
PBMCs were isolated from healthy volunteers’ heparinized peripheral blood or buffy coat (IKTZ Heidelberg) as described.Citation31 NK cells were isolated from PBMC (StemCell Technologies, Inc., EasySEP™ Negative NK Cell Enrichment Kit, catalog number 19055) according to manufacturer’s instructions and phenotyped by flow cytometry using CD16 158V-specific mAb MEM-154 (Fisher Scientific, catalog number MA1–19563).
Cell binding assays and flow cytometric analysis
Affinity to CD30 was determined in a competitive binding assay using phycoerythrin (PE)-labeled IgG HRS-4 (Beckman-Coulter, catalog number IM2033U). 1 × 106 KARPAS-299 cells were incubated with 20 nM PE-labeled HRS-4 in the presence of increasing concentrations of the competitors (CD30/CD16A TandAb, CD30/CD16A diabody, native IgG, or Fc-enhanced IgG) in FACS PBS buffer (Invitrogen, catalog number 14190–094) supplemented with 2% heat-inactivated FCS and 0.1% sodium azide (Roth, catalog number A1430.0100) at 37 °C for 45 min. After repeated washing, the fluorescence intensity of 1 × 104 cells was measured (FC500 MPL flow cytometer with MXP software, Beckman-Coulter, catalog number 774199). The IC50 values were determined by nonlinear regression (GraphPad Prism, GraphPad Software) and Kd were calculated using a formula derived as described:Citation32 Kd(I) = IC50(I)/(1+[{HRS-4}/Kd(HRS-4)]),wherein: I is the unlabeled competitor, [HRS-4] is the concentration of PE-labeled HRS-4 (20 nM), Kd(HRS-4) is 7 nM,Citation33 and IC50(I) is the concentration of the unlabeled competitor causing 50% inhibition of HRS-4 binding.
Cell surface retention assay
To quantify the retention of antibodies on CD30+ cells, aliquots of KARPAS-299 cells were incubated with 70 nM of biotinylated antibodies for 45 min on ice. After repeated washing with ice-cold FACS buffer, aliquots of 1 × 106 cells were suspended in 10 mL FACS buffer supplemented with 10% heat-inactivated FCS at 37 °C for the indicated time periods. After repeated washing, cell-retained antibodies were detected by DyLight™488-conjugated streptavidin (Dianova, catalog number 016–480–084) as described above. For enriched NK cells, the cell surface retention assay was performed as described for KARPAS-299, but the NK cells were stained with 1 µM CD30/CD16A TandAb or diabody for 45 min on ice, and after the 37 °C dissociation phase remaining cell-bound antibodies were labeled with anti-His IgG 13/45/31–2 (Dianova, catalog number Dia910) followed by FITC-conjugated goat anti-mouse IgG (Dianova, catalog number 115–095–062) and detected as described above. The mean fluorescence values at time-point 0 were taken to be 100%, and the remaining antibody was analyzed by nonlinear regression using GraphPad Prism.
Cytotoxicity assays
Target cells were labeled with 10 µM calcein AM (Invitrogen, catalog number C3100MP) for 30 min in RPMI medium at 37 °C, washed, and 1 × 104 cells were seeded together, with NK cells, in 200 µL at the indicated effector-to-target (E:T) ratios in the presence of increasing antibody concentrations. After incubation for the indicated time periods at 37 °C in a humidified 5% CO2 atmosphere, the fluorescence (F) of calcein released into the supernatant was measured by plate reader at 520 nm (Perkin Elmer, Victor 3, catalog number 1420–012). The specific cell lysis was calculated as: (F[sample] – F[spontaneous])/(F[maximum] – F[spontaneous]) × 100%.
F(spontaneous) represents fluorescence released from target cells in the absence of effector cells and antibodies, and F(maximum) represents that released after total cell lysis induced by addition of 1% Triton X 100 (Roth, catalog number 3051.2). Regression curves were fit to calculate EC50 employing GraphPad Prism software. Statistical significance was assessed by a t test using GraphPad Prism software.
The redirected cytotoxicity assay with P-815 cells was performed as described above, employing the anti-CD16A scFv,Citation15 OKT3-derived scFv6,Citation34 anti-His IgG1 13/45/31–2, OKT3 (Bioxcell, catalog number BE 0001–2), A9Citation20 provided by Dr. Moldenhauer (DKFZ Heidelberg), B159 (BD, catalog number 555514), and 195314 (R&D Systems, catalog number MAB1850).
Cytokine release assay
Individual or mixed cultures of PBMC and KARPAS-299 were incubated in a 96-well micro-plate in the presence of the indicated antibodies for 24 h. Cytokines were quantified in the cell culture supernatant employing Procarta Cytokine Assay Kit, human 7-Plex Panomics, catalog number PC1007) and Luminex 100 Analyzer (Luminex, catalog number Lx100).
Additional material
Download Zip (405.8 KB)Disclosure of Potential Conflicts of Interest
All the authors are or were employees of Affimed Therapeutics.
Financial Disclosures
U.R., C.B., I.F., S.H.J.K., M.L., and E.A.Z. are employees of Affimed Therapeutics AG. F.L.G., M.L.G., and K.H. are former employees of Affimed Therapeutics AG. Otherwise there are no potential conflicts of interest.
Acknowledgments
We thank S. Kiprijanov (Affitech Research, Oslo, Norway) for the contributions to the early stages of the project, G. Moldenhauer (Department of Molecular Immunology, German Cancer Research Center (DKFZ) Heidelberg, Germany) for providing cell lines and antibodies, and O. Mandelboim (The Lautenberg Center for General and Tumor Immunology, The Hebrew University Hadassah Medical School, Jerusalem, Israel) for providing plasmids. The technical assistance of Frank Malischewsky, Andreas Müller, Michael Schott, Alexandra Stolarek, Stefanie Wolff, Helena Mennemann, and Tatjana Zingraf (all Affimed Therapeutics AG, Heidelberg, Germany) was gratefully appreciated.
Footnotes
This work was supported by the BioChancePlus grant no. 0313183 of the Federal Ministry of Education and Research (Germany).
Address correspondence and reprint requests to: Affimed Therapeutics AG, Im Neuenheimer Feld 582, Heidelberg D-69120, Germany, Phone: +49–6221–65307–0; Fax: +49–6221–65307–77; Email: [email protected]
References
- Mandelboim O, Malik P, Davis DM, Jo CH, Boyson JE, Strominger JL. Human CD16 as a lysis receptor mediating direct natural killer cell cytotoxicity. Proc Natl Acad Sci U S A 1999; 96:5640 - 4; http://dx.doi.org/10.1073/pnas.96.10.5640; PMID: 10318937
- Robertson MJ, Ritz J. Biology and clinical relevance of human natural killer cells. Blood 1990; 76:2421 - 38; PMID: 2265240
- Cooper MA, Fehniger TA, Caligiuri MA. The biology of human natural killer-cell subsets. Trends Immunol 2001; 22:633 - 40; http://dx.doi.org/10.1016/S1471-4906(01)02060-9; PMID: 11698225
- van de Winkel JG, Capel PJ. Human IgG Fc receptor heterogeneity: molecular aspects and clinical implications. Immunol Today 1993; 14:215 - 21; http://dx.doi.org/10.1016/0167-5699(93)90166-I; PMID: 8517920
- van Spriel AB, van Ojik HH, van De Winkel JG. Immunotherapeutic perspective for bispecific antibodies. Immunol Today 2000; 21:391 - 7; http://dx.doi.org/10.1016/S0167-5699(00)01659-5; PMID: 10916142
- Arndt MA, Krauss J, Kipriyanov SM, Pfreundschuh M, Little M. A bispecific diabody that mediates natural killer cell cytotoxicity against xenotransplantated human Hodgkin’s tumors. Blood 1999; 94:2562 - 8; PMID: 10515858
- McCall AM, Shahied L, Amoroso AR, Horak EM, Simmons HH, Nielson U, Adams GP, Schier R, Marks JD, Weiner LM. Increasing the affinity for tumor antigen enhances bispecific antibody cytotoxicity. J Immunol 2001; 166:6112 - 7; PMID: 11342630
- Ory PA, Goldstein IM, Kwoh EE, Clarkson SB. Characterization of polymorphic forms of Fc receptor III on human neutrophils. J Clin Invest 1989; 83:1676 - 81; http://dx.doi.org/10.1172/JCI114067; PMID: 2523415
- Huizinga TW, Kleijer M, Tetteroo PA, Roos D, von dem Borne AE. Biallelic neutrophil Na-antigen system is associated with a polymorphism on the phospho-inositol-linked Fc gamma receptor III (CD16). Blood 1990; 75:213 - 7; PMID: 2136803
- Tamm A, Schmidt RE. The binding epitopes of human CD16 (Fc gamma RIII) monoclonal antibodies. Implications for ligand binding. J Immunol 1996; 157:1576 - 81; PMID: 8759741
- Koene HR, Kleijer M, Algra J, Roos D, von dem Borne AE, de Haas M. Fc gammaRIIIa-158V/F polymorphism influences the binding of IgG by natural killer cell Fc gammaRIIIa, independently of the Fc gammaRIIIa-48L/R/H phenotype. Blood 1997; 90:1109 - 14; PMID: 9242542
- Musolino A, Naldi N, Bortesi B, Pezzuolo D, Capelletti M, Missale G, Laccabue D, Zerbini A, Camisa R, Bisagni G, et al. Immunoglobulin G fragment C receptor polymorphisms and clinical efficacy of trastuzumab-based therapy in patients with HER-2/neu-positive metastatic breast cancer. J Clin Oncol 2008; 26:1789 - 96; http://dx.doi.org/10.1200/JCO.2007.14.8957; PMID: 18347005
- Cartron G, Dacheux L, Salles G, Solal-Celigny P, Bardos P, Colombat P, Watier H. Therapeutic activity of humanized anti-CD20 monoclonal antibody and polymorphism in IgG Fc receptor FcgammaRIIIa gene. Blood 2002; 99:754 - 8; http://dx.doi.org/10.1182/blood.V99.3.754; PMID: 11806974
- Weng WK, Levy R. Two immunoglobulin G fragment C receptor polymorphisms independently predict response to rituximab in patients with follicular lymphoma. J Clin Oncol 2003; 21:3940 - 7; http://dx.doi.org/10.1200/JCO.2003.05.013; PMID: 12975461
- Knackmuss S, Molkenthin V. Antibodies from IgM libraries. In: Little M, editor. Recombinant antibodies for immunotherapy. Cambridge: Cambridge University Press; 2009. p. 66–74.
- Kipriyanov SM, Moldenhauer G, Schuhmacher J, Cochlovius B, Von der Lieth CW, Matys ER, Little M. Bispecific tandem diabody for tumor therapy with improved antigen binding and pharmacokinetics. J Mol Biol 1999; 293:41 - 56; http://dx.doi.org/10.1006/jmbi.1999.3156; PMID: 10512714
- Benhamou M, Bonnerot C, Fridman WH, Daëron M. Molecular heterogeneity of murine mast cell Fc gamma receptors. J Immunol 1990; 144:3071 - 7; PMID: 2139078
- Schlapschy M, Gruber H, Gresch O, Schäfer C, Renner C, Pfreundschuh M, Skerra A. Gruber H, Gresch O, Schäfer C, Renner C, Pfreundschuh M, Skerra A. Functional humanization of an anti-CD30 Fab fragment for the immunotherapy of Hodgkin's lymphoma using an in vitro evolution approach. Protein Eng Des Sel 2004; 12:847 - 60; PMID: 15708864
- Lazar GA, Dang W, Karki S, Vafa O, Peng JS, Hyun L, Chan C, Chung HS, Eivazi A, Yoder SC, et al. Engineered antibody Fc variants with enhanced effector function. Proc Natl Acad Sci U S A 2006; 103:4005 - 10; http://dx.doi.org/10.1073/pnas.0508123103; PMID: 16537476
- Hombach A, Jung W, Pohl C, Renner C, Sahin U, Schmits R, Wolf J, Kapp U, Diehl V, Pfreundschuh M. A CD16/CD30 bispecific monoclonal antibody induces lysis of Hodgkin’s cells by unstimulated natural killer cells in vitro and in vivo. Int J Cancer 1993; 55:830 - 6; http://dx.doi.org/10.1002/ijc.2910550523; PMID: 8244580
- Marshall NA, Christie LE, Munro LR, Culligan DJ, Johnston PW, Barker RN, Vickers MA. Immunosuppressive regulatory T cells are abundant in the reactive lymphocytes of Hodgkin lymphoma. Blood 2004; 103:1755 - 62; http://dx.doi.org/10.1182/blood-2003-07-2594; PMID: 14604957
- Gandhi MK, Lambley E, Duraiswamy J, Dua U, Smith C, Elliott S, Gill D, Marlton P, Seymour J, Khanna R. Expression of LAG-3 by tumor-infiltrating lymphocytes is coincident with the suppression of latent membrane antigen-specific CD8+ T-cell function in Hodgkin lymphoma patients. Blood 2006; 108:2280 - 9; http://dx.doi.org/10.1182/blood-2006-04-015164; PMID: 16757686
- Küppers R. The biology of Hodgkin’s lymphoma. Nat Rev Cancer 2009; 9:15 - 27; http://dx.doi.org/10.1038/nrc2542; PMID: 19078975
- Su CC, Chiu HH, Chang CC, Chen JC, Hsu SM. CD30 is involved in inhibition of T-cell proliferation by Hodgkin’s Reed-Sternberg cells. Cancer Res 2004; 64:2148 - 52; http://dx.doi.org/10.1158/0008-5472.CAN-03-1337; PMID: 15026356
- Hartmann F, Renner C, Jung W, Deisting C, Juwana M, Eichentopf B, Kloft M, Pfreundschuh M. Treatment of refractory Hodgkin’s disease with an anti-CD16/CD30 bispecific antibody. Blood 1997; 89:2042 - 7; PMID: 9058726
- Hartmann F, Renner C, Jung W, da Costa L, Tembrink S, Held G, Sek A, König J, Bauer S, Kloft M, et al. Anti-CD16/CD30 bispecific antibody treatment for Hodgkin’s disease: role of infusion schedule and costimulation with cytokines. Clin Cancer Res 2001; 7:1873 - 81; PMID: 11448899
- Reiners KS, Kessler J, Sauer M, Rothe A, Hansen HP, Reusch U, Hucke C, Köhl U, Dürkop H, Engert A, et al. Rescue of impaired NK cell activity in hodgkin lymphoma with bispecific antibodies in vitro and in patients. Mol Ther 2013; 21:895 - 903; http://dx.doi.org/10.1038/mt.2013.14; PMID: 23459515
- Le Gall F, Reusch U, Little M, Kipriyanov SM. Effect of linker sequences between the antibody variable domains on the formation, stability and biological activity of a bispecific tandem diabody. Protein Eng Des Sel 2004; 17:357 - 66; http://dx.doi.org/10.1093/protein/gzh039; PMID: 15126676
- Mandelboim O, Lieberman N, Lev M, Paul L, Arnon TI, Bushkin Y, Davis DM, Strominger JL, Yewdell JW, Porgador A. Recognition of haemagglutinins on virus-infected cells by NKp46 activates lysis by human NK cells. Nature 2001; 409:1055 - 60; http://dx.doi.org/10.1038/35059110; PMID: 11234016
- Kipriyanov SM, Cochlovius B, Schäfer HJ, Moldenhauer G, Bähre A, Le Gall F, Knackmuss S, Little M. Synergistic antitumor effect of bispecific CD19 x CD3 and CD19 x CD16 diabodies in a preclinical model of non-Hodgkin’s lymphoma. J Immunol 2002; 169:137 - 44; PMID: 12077238
- Fuss IJ, Kanof ME, Smith PD, Zola H. Isolation of whole mononuclear cells from peripheral blood and cord blood. Curr Protoc Immunol 2009; Chapter 7: Unit7.1.
- Schodin BA, Kranz DM. Binding affinity and inhibitory properties of a single-chain anti-T cell receptor antibody. J Biol Chem 1993; 268:25722 - 7; PMID: 8245009
- Engert A, Burrows F, Jung W, Tazzari PL, Stein H, Pfreundschuh M, Diehl V, Thorpe P. Evaluation of ricin A chain-containing immunotoxins directed against the CD30 antigen as potential reagents for the treatment of Hodgkin’s disease. Cancer Res 1990; 50:84 - 8; PMID: 2152774
- Le Gall F, Reusch U, Moldenhauer G, Little M, Kipriyanov SM. Immunosuppressive properties of anti-CD3 single-chain Fv and diabody. J Immunol Methods 2004; 285:111 - 27; http://dx.doi.org/10.1016/j.jim.2003.11.007; PMID: 14871540
