Abstract
The Fc receptor (FcγRIIb) inhibits B cell responses when coengaged with B cell receptor (BCR), and has become a target for new autoimmune disease therapeutics. For example, BCR and FcγRIIb coengagement via the Fc-engineered anti-CD19 XmAb5871 suppresses humoral immune responses. We now assess effects of XmAb5871 on other activation pathways, including the pathogen-associated molecular pattern receptor, TLR9. Since TLR9 signaling is implicated in autoimmune diseases, we asked if XmAb5871 could inhibit TLR9 costimulation. We show that XmAb5871 decreases ERK and AKT activation, cell proliferation, cytokine, and IgG production induced by BCR and/or TLR9 signals. XmAb5871 also inhibited differentiation of citrullinated peptide-specific plasma cells from rheumatoid arthritis patients. XmAb5871 may therefore have potential to suppress pathogenic B cells in autoimmune diseases.
Introduction
Coengagement of the B cell receptor (BCR) and the Fcγ receptor IIb (FcγRIIb) by immune complexes selectively suppresses activation of B cells recognizing the cognate antigen.Citation1-Citation3 FcγRIIb is a negative coreceptor for BCR, having an immunoreceptor tyrosine-based inhibitory motif (ITIM) on its intracellular tail.Citation4,Citation5 ITIM is phosphorylated after coligation of FcγRIIb and BCR, and recruits SH2 domain-containing inositol 5-phosphatase (SHIP) to inhibit signal transduction.Citation6-Citation9 CD19 is a B cell-specific transmembrane glycoprotein that is expressed from the pre-B cell until the plasma cell stage.Citation10-Citation12 In contrast to FcγRIIb, CD19 functions as a positive coreceptor of BCR, and its intracellular domain contains several immunoreceptor tyrosine-based activation motif (ITAM)-like regions that may serve as targets for tyrosine kinases.Citation13,Citation14 After being phosphorylated, CD19 recruits Lyn tyrosine kinase, phosphatidyl inositol 3 kinase (PI3-K), and the guanine nucleotide exchange factor Vav to the signaling complex, thus lowering the threshold for B cell activation.Citation15 However, crosslinking of CD19 may also inhibit B cells, depending on the context of activation.Citation16
To mimic the inhibitory effect of immune complexes, a series of humanized monoclonal CD19-specific antibodies have previously been generated with modified Fc domains.Citation17 The leading antibody, XmAb5871, binds to human FcγRIIb with >400 higher affinity than native IgG1 antibody, and is now in clinical trials as a treatment for rheumatoid arthritis (RA).Citation18-Citation21 CD19 and CD32b coengagement by XmAb5871 on the B cell surface results in recruitment of SHIP to the phosphorylated ITIM of FcγRIIb.Citation18 SHIP dephosphorylates phosphatidylinositol (3,4,5)-triphosphate (PIP3), consequently suppressing activation of pleckstrin homology (PH) domain-containing molecules such as AKT.Citation22 Phosphorylated SHIP also binds Dok1, another adaptor that inactivates Ras and thus inhibits the Ras-MAPK pathway.Citation23
Dysfunction of FcγRIIb is critical in the development of autoimmune diseases such as RA.Citation3,Citation24,Citation25 B cells play crucial roles in RA, for example, by producing auto-antibodies against citrullinated self proteins. Consequently, the most sensitive and specific marker for RA is the presence of anti-citrullinated protein/peptide antibodies (ACPAs) in sera.Citation26-Citation28
By suppressing CD19+ B cell functions via the physiological FcγRIIb inhibitory pathway, XmAb5871 has the potential to suppress autoimmunity without chronic B cell depletion. Indeed, Horton et al. have shown that XmAb5871 suppressed humoral immunity against tetanus toxoid and reduced serum antibody levels in SCID mice engrafted with human peripheral blood mononuclear cells (PBMC) from patients with systemic lupus erythematosus (SLE).Citation19 More recent studies have demonstrated similar B cell inhibition and suppression of humoral responses in patients with RA.Citation20
We have now expanded these previous studies by investigating if XmAb5871 is able to suppress not only BCR, but also TLR9-mediated activation signals, when these stimuli are acting independently or synergistically. The innate receptor TLR9 is expressed on intracellular endosomal membranes, and recognizes unmethylated DNA from bacteria or released from apoptotic cells.Citation29,Citation30 TLR9 and BCR act synergistically in human B cells, inducing enhanced proliferation, cytokine, and antibody production; thus, TLR9 plays a crucial role in breaking tolerance and developing autoimmunity.Citation31,Citation32 We show here that XmAb5871 inhibits proliferation and cytokine secretion of B cells induced by TLR9 and by synergistic stimulation via both BCR and TLR9. Additionally, XmAb5871 reduced citrullinated peptide-specific IgG production by B cells of RA patients, suggesting that it has potential to suppress autoantibody production in this and other immune-mediated diseases.
Results
XmAb5871 downregulates AKT and ERK phosphorylation in an FcγRIIb-dependent manner
XmAb5871 has been previously shown to mediate phosphorylation of FcγRIIb and SHIP in human B cells.Citation18,Citation19 To investigate the inhibitory potential of XmAb5871 on the AKT and Ras/MAPK pathways activated by anti-Ig and CpG ODN, we first assessed FcγRIIb Y292 phosphorylation, which contributes to the transduction of FcγRIIb-mediated inhibitory signals. Purified tonsillar resting B cells pretreated with XmAb5871 showed a strong FcγRIIb phosphorylation at tyrosine 292 as detected by specific antibody (). Treatment with the CD19-specific control monoclonal antibody (mAb) XENP6187, which has mutations in the Fc domain (G236R and L328R) and thus is unable to bind to Fcγ receptors (anti-CD19-FcKO),Citation18 anti-Ig, and CpG ODN stimulation (alone or in combination), did not induce FcγRIIb phosphorylation, indicating that high-affinity coengagement of FcγRIIb and CD19 by XmAb5871 is necessary and sufficient to stimulate FcγRIIb inhibitory signaling.
Figure 1. XmAb5871 suppresses BCR and TLR9-induced signals through phosphorylation of FcγRIIb. Purified human resting tonsillar B cells were pre-treated with media or 10 μg/ml XENP6187 or XmAb5871, respectively, for 1 h before activation with anti-Ig (2.5 μg/ml) or CpG (1 μg/ml) for 30 min. Cell lysates were western-blotted to assess phosphorylation and activation of FcγRIIb, AKT and ERK. One representative experiment from three independent ones is shown.
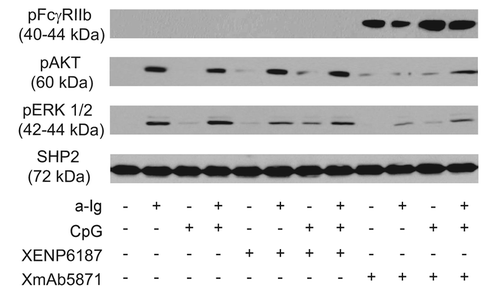
To elucidate the inhibitory potential of phosphorylated FcγRIIb on BCR and TLR9 induced activation of the AKT and Ras/MAPK pathways, parallel samples were tested for phosphorylation of AKT and ERK kinases. Anti-Ig, but not CpG, induced strong AKT phosphorylation, which was approximately similar after dual anti-Ig and CpG stimuli. The Fc-KO antibody XENP6187 slightly enhanced AKT phosphorylation in all samples including the nonstimulated control and the CpG ODN-induced ones, presumably due to CD19 crosslinking,Citation33 while in marked contrast, XmAb5871 blocked both CD19 and BCR-induced AKT phosphorylation. Anti-Ig was more effective than CpG for ERK activation. CpG ODN somewhat enhanced anti-Ig-stimulated ERK phosphorylation, and XENP6187 slightly enhanced phospho-ERK signal in the CpG stimulated sample, while XmAb5871 inhibited ERK phosphorylation in all samples, even dual-stimulated ones, compared with the XENP6187-treated cells (). These data indicate that coligation of FcγRIIb and CD19 upon stimulation via BCR and TLR9 induces phospho-ITIM-dependent downregulation of AKT and ERK activation in B cells.
Intracellular calcium mobilization induced by BCR and CD19 coengagement is inhibited by the enhanced binding of XmAb5871 to FcγRIIb
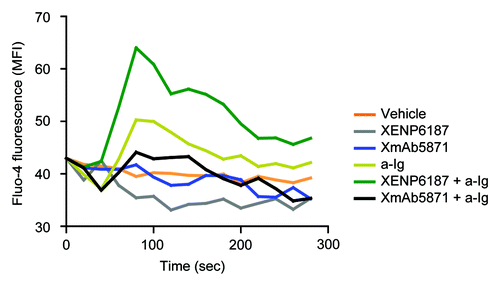
Crosslinking of BCR with CD19 on B cells increases intracellular calcium ion concentration [Ca2+]i.Citation9 We compared the effect of XmAb5871 and the Fc-KO control XENP6187 on the BCR-induced calcium response. CD19 engagement alone in samples treated with XENP6187 or XmAb5871 did not induce Ca2+ mobilization in B cells. Compared with untreated cells, XENP6187 pretreatment enhanced [Ca2+]i in response to anti-Ig stimulation that crosslinks BCR and CD19, while XmAb5871 diminished calcium mobilization, indicating that its high affinity binding to FcγRIIb overcomes the CD19-induced enhancing effect ().
Figure 2. XmAb5871 inhibits while XENP6187 enhances calcium mobilization when CD19 is coligated with BCR. Fluo-4 loaded B cells were treated with either XmAb5871 or the Fc-KO XENP6187 antibodies and then stimulated with 10 μg/ml anti-Ig. Calcium flux kinetics was recorded using FACSCalibur flow cytometer and data were analyzed by FlowJo software. Data represent the average of two independent experiments.
XmAb5871 inhibits B cell proliferation triggered by anti-Ig and CpG ODN
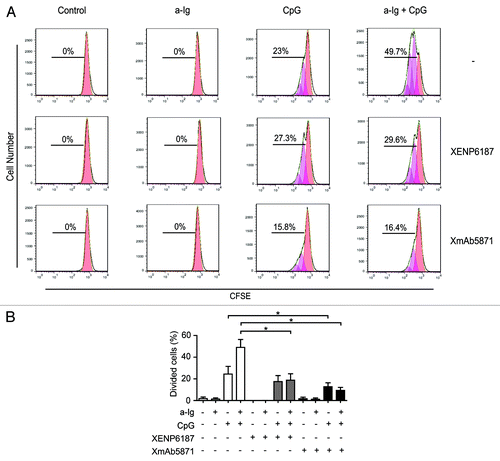
To test if the FcγRIIb-enhanced anti-CD19 antibody can interfere with cell proliferation, purified B cells were treated with XmAb5871 or XENP6187 and stimulated via BCR, TLR9, or both receptors. A CFSE dilution assay was used for assessing cellular proliferation (). In agreement with previous results,Citation32,Citation34 anti-Ig synergistically enhanced CpG-induced cell proliferation, which was significantly inhibited by both the Fc-KO control antibody XENP6187 and by XmAb5871 (). However, the number of dividing cells in XmAb5871-treated and anti-Ig plus CpG-stimulated samples was approximately half that of the XENP6187-treated ones. XmAb5871 treatment significantly (P < 0.05) reduced proliferation of both CpG- and anti-Ig plus CpG-stimulated samples compared with the untreated control cells.
Figure 3. Effect of XmAb5871 on BCR and TLR9-induced proliferation. (A) Representative flow cytometry histograms of CFSE-labeled human blood B cells stimulated with combinations of 2.5 μg/ml anti-Ig and 1 μg/ml CpG in the presence of 10 μg/ml XENP6187 or XmAb5871 for 5 d. Peaks shifted to the left represent cell populations undergoing increasing numbers of cell division. Total percentages of dividing cells are shown. (B) Percentages of dividing cells (mean ± SD) in three independent experiments. B cells were stimulated with anti-Ig, CpG ODN or with the combination of the two in the presence or absence of XENP6187 or XmAb5871. XmAb5871 significantly reduced proliferation of both CpG and anti-Ig plus CpG stimulated cells, *: P < 0.05.
XmAb5871 blocks cytokine secretion induced by synergistic BCR and TLR9-mediated signals
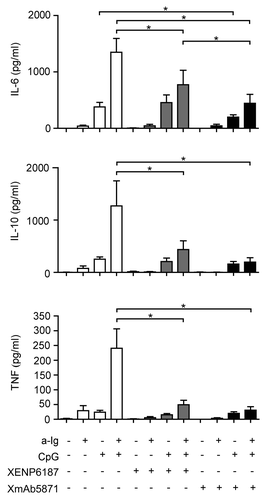
B cells stimulated via BCR and TLR9 can secrete both pro-inflammatory and anti-inflammatory cytokines, and such dual stimulation has a synergistic effect on interleukin (IL)-6, tumor necrosis factor (TNF) and IL-10 secretion. We therefore compared the effect of the control XENP6187 and FcγRIIb-enhanced anti-CD19 antibodies on B cell secretion of IL-6, TNF and IL-10. Both TLR9-stimulated and the synergistic BCR and TLR9-induced IL-6 production were significantly inhibited by XmAb5871 (). The Fc-KO antibody XENP6187 had no effect on TLR9 induced IL-6, but inhibited the dual signal-stimulated IL-6; however, IL-6 production by the XmAb5871-treated dual-stimulated cells was significantly lower. IL-10 and TNF secretion induced by the dual anti-Ig and CpG ODN signals was significantly inhibited by XmAb5871 and XENP6187 as well. However, the effect of XmAb5871 was more pronounced in all cases. These data indicate that efficient FcγRIIb – CD19 coligation significantly diminished TLR9 and BCR-TLR9 dual signal-induced inflammatory cytokine secretion by human B cells; however, BCR and CD19 coengagement in the absence of FcγRIIb binding might also be inhibitory.
Figure 4. XmAb5871 inhibits BCR and TLR9-induced IL-6, IL-10 and TNF production by B cells. B cells cultured in 96-well plates were activated by 2.5 μg/ml anti-Ig or 1 μg/ml CpG ODN in the presence of XmAb5871 or the control antibody XENP6187. IL-6, IL-10 and TNF secreted from the culture supernatants were measured after 48 h using the Flow Cytomix bead array. Data represent the mean ± SD of four independent experiments. XmAb5871 significantly inhibits IL-6 production induced by anti-Ig and CpG, as compared with XENP6187 treated cells. *: P < 0.05.
Plasma cell differentiation is significantly reduced by XmAb5871
We next tested the IgG-producing cells in stimulated B cell cultures by ELISPOT assay. CpG ODN and CpG ODN plus anti-Ig in the presence of cytokines IL-2 and IL-10 both stimulated differentiation of B cells into IgG-producing plasma cells; under the conditions used the dual signals were less efficient. XENP6187 did not significantly influence IgG production, while the XmAb5871-treated, CpG ODN-stimulated samples showed a significantly lower number of IgG-synthesizing cells relative to the untreated ones ().
Figure 5. The effect of XmAb5871 on total and citrullinated peptide-specific IgG production as detected by ELISPOT assay (A) Purified human B cells were cultured with anti-Ig (2.5 μg/ml) or CpG (0.5 μg/ml) for 3 d in the presence of IL-2 (50 ng/ml) and IL-10 (50 ng/ml), and XmAb5871 or XENP6187 (10 μg/ml), respectively. The number of IgG-secreting cells was evaluated by ELISPOT assay on anti-IgG-coated nitrocellulose plates. Data represent the mean ± SD of seven independent experiments. *: P < 0.05. (B) B cells were stimulated by IL-2 (10 ng/ml) and R848 (1 μg/ml) for 3 d in the presence of XENP6187 or XmAb5871 antibodies. The number of IgG-secreting cells was assessed as above. Data represent the mean ± SD of seven independent experiments. *: P < 0.05. (C) Citrullinated filaggrin peptide-specific IgG-producing B cells were tested upon activation with IL-2 (10 ng/ml) and R848 (1 μg/ml) for 3 d with or without XmAb5871. The citrullinated filaggrin peptide-specific IgG secreting cells were detected on peptide-coated nitrocellulose plates. The results for B cells from six different ACPA-positive RA patients are shown. XmAb5871 significantly inhibited the development of citrullin-containing filaggrin peptide-specific antibody-forming cells, *: P < 0.05.
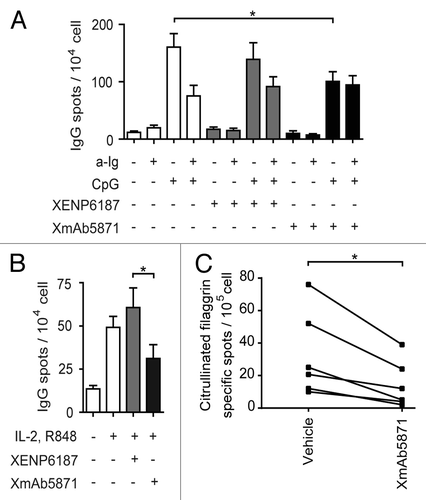
We compared the effect of XmAb5871 and XENP6187 in another B cell activation system. R848 is a synthetic molecule that activates immune cells via the TLR7/TLR8 MyD88-dependent signaling pathway.Citation35 R848 in the presence of IL-2 induces differentiation of IgG antibody-forming cells (AFC). XmAb5871 significantly inhibited (P < 0.05) the differentiation of AFC as compared with XENP6187-treated samples ().
XmAb5871 diminished the frequency of citrulline-containing peptide-specific antibody-forming cells (AFC) triggered by TLR7/TLR8 agonist R848 in in vitro cultures of RA B cells
Approximately 70% of RA patients produce autoantibodies against citrullinated self-proteins, furthermore, anti-citrullinated protein/peptide-specific antibodies (ACPA) play a central role in the pathogenesis of the disease and are presently the most specific and sensitive marker for RA.Citation26-Citation28 We and others have previously shown that in vitro-activated B cells from ACPA-positive RA patients produce citrullinated peptide-specific IgG.Citation36,Citation37 To see if XmAb5871 has an effect on citrulline peptide-specific antibody production, purified B cells from selected RA patients were cultured in the presence of IL-2 and R848 to stimulate memory B cells. The number of antibody-producing cells was estimated on citrulline-containing filaggrin-peptide coated plates. XmAb5871 significantly diminished the number of peptide-specific spots, indicating that FcγRIIb-dependent inhibition of autoreactive B cells may indeed block autoantibody production ().
Discussion
B cells have a crucial role in adaptive immunity and play an important part in the pathogenesis of certain autoimmune diseases such as RA and SLE. Autoreactive B cells produce autoantibodies against self-peptides; furthermore, they have antibody-independent regulatory functions, especially in autoimmune diseases. For example, they play a role in activating pathogenic T cells,Citation38 produce pro-inflammatory cytokines,Citation39 and form ectopic lymphoid organs such as germinal centers in the synovium of RA patients.Citation40 B cells are central players in maintaining autoimmune inflammation; thus, targeting of B cell-specific surface molecules and blocking of B cell activation and survival are important tools to develop new and efficient therapies.Citation41 Rituximab, a chimeric monoclonal antibody specific for CD20, is the first selective B cell therapy for RA.Citation42,Citation43 Rituximab therapy, however, has two drawbacks: (1) it has no effect on antibody-forming plasma cells, because plasma cells are negative for CD20; and (2) this therapy results in the depletion of peripheral B cells, which leads to a temporary immunosuppressed stage of the patients, leaving them vulnerable to infections.Citation44,Citation45 Therefore, therapies based on targeting BCR signaling and leading to specific inhibition of B cell activation, without B cell depletion, may be advantageous.
The Fc engineered anti-CD19 XmAb5871 was developed to bind to FcγRIIb with ~400 higher affinity compared with its native IgG1 counterpart.Citation18-Citation20 XmAb5871 has the capacity to block BCR signaling, to suppress humoral immunity, and to reduce serum IgM, IgG, and IgE levels in SCID mice engrafted with SLE, RA, or healthy human PBMC without depleting B cells.Citation18-Citation20 The mouse surrogate of XmAb5871 reduces BCR-induced calcium mobilization and cell activation in FcγRIIb transgenic mice.Citation19 Furthermore, XmAb5871 stimulates the known FcγRIIb-mediated inhibitory signaling pathway in B cells of SLE patients, suppressing calcium flux and B cell proliferation. However, the effect of XmAb5871 on TLR9 mediated signals, which are suggested to contribute to the development of autoimmunity, was not previously tested.
Receptors of the adaptive and the innate immune system interact in regulating the immune response. Pathogen-associated molecular pattern receptors (PAMP) such as TLR9 may activate B cells independent of antigen; moreover, TLR9 acts synergistically with BCR, enhancing proliferation, cytokine and antibody production, thus promoting uncontrolled B cell activation that ultimately results in the development of autoimmunity. Indeed, signaling through the TLR pathway has been implicated in the pathogenesis of autoimmune diseases including RA and SLE.Citation31 Therefore it is crucial to examine if XmAb5871 influences TLR9-mediated signals in B cells. Our goal here was to study the effect of XmAb5871 on B cell functions mediated by the synergistic action of BCR and TLR9.
We first confirmed that XmAb5871, but not the IgG1 control Fc-KO antibody, XENP6187, induced phosphorylation of Y292 in FcγRIIb, a prerequisite for inhibition. We also detected reduced ERK phosphorylation and a more significant decrease of AKT phosphorylation in all samples treated with XmAb5871. This could be a result of the decreased level of PIP3 in the cell membrane, evoked by FcγRIIb-recruited SHIP1. CD19, when phosphorylated by Lyn after coligation with BCR, recruits PI3K and enhances PI3K activity, elevating PIP3 level in the cell membrane, which can explain the increased AKT phosphorylation in the XENP6187-treated samples. This activatory effect of CD19 crosslinking was more than negated by the amplified FcγRIIb-dependent signal in XmAb5871-treated B cells.
Intracellular calcium mobilization is induced by the crosslinking of BCR or CD19. XENP6187 control antibody, lacking binding to Fcγ receptors, enhanced [Ca2+]i in BCR and CD19 co-crosslinked B cells, while in agreement with previous data, XmAb5871 inhibited the BCR stimulated response, indicating that high-affinity binding of this antibody to FcγRIIb blocks the calcium signal mediated by BCR-CD19 interaction.
To study the functional consequences of XmAb5871-mediated impaired signal transduction by TLR9 and BCR, we assessed this antibody's effects on cell proliferation, cytokine production and plasma-cell differentiation. XmAb5871 inhibited all three functions albeit by different degrees.
Surprisingly, anti-CD19 antibody XENP6187 still inhibited B cell functions, although less efficiently than XmAb5871. These results are in agreement with earlier findings that crosslinking CD19 may have an inhibitory effect on later events of B cell activation, depending on the context of stimulation.Citation16,Citation46-Citation48 It was suggested that anti-CD19 mAb exerts its inhibitory effect on anti-IgM induced B cell proliferation in late G0 or G1, after the initial signaling events.Citation48 This might explain the apparent inconsistency between the lack of inhibition of early signaling events and the partial inhibition of late events of B cell activation by XENP6187.
Furthermore, CD19 can physically interact with BTK following BCR ligation, which is important for the prolonged phosphorylation of the kinase.Citation49 A recent paper claims that TLR9 and BCR cosignaling also depend on BTK, which is essential for the colocalization of BCR and TLR9.Citation32 Taking all of these results together, we suggest that in the case of BCR-CD19 crosslinking by anti-IgG and XENP6187, BTK is exhausted by CD19 and is not available for BCR-TLR9-induced synergistic signaling. Alternatively, XENP6187 antibody, by crosslinking CD19, might induce phosphorylation via the Lyn kinase of some other negatively regulating molecule on B cells, such as CD22.Citation50
It is notable that the Fc-enhanced anti-CD19 antibody significantly inhibited the inflammatory cytokine IL-6 and reduced TNF secretion from B cells stimulated via BCR and TLR9. This might have functional significance in RA therapy because RA patients have increased levels of IL-6 and TNF in their circulation that promotes inflammation. XmAb5871 also reduced the number of IgG-producing cells in TLR9-stimulated B cell cultures of healthy individuals. The combined stimuli of IL-2 and R848, a synthetic ligand for TLR7/TLR8, selectively stimulate human memory B cells, leading to differentiation of IgG-secreting AFC. XmAb5871 treatment significantly reduced the number of AFC in R848-stimulated healthy B cell culture as compared with XENP6187-treated cells. Thus, the simultaneous ligation of CD19 and FcγRIIb inhibits TLR9- and TLR7/TLR8-mediated B cell activation and differentiation as well.
Approximately 70% of RA patients produce antibody specific for citrullinated proteins/peptides (ACPA); furthermore, in vitro production of citrulline peptide-specific antibodies by RA B cells was shown previously. When XmAb5871-treated, R848 prestimulated B cells of RA patients were tested, we detected a significant reduction in the number of antibody-forming cells specific for citrullinated filaggrin peptide, a characteristic epitope recognized by sera of RA patients, indicating that XmAb5871 is able to suppress autoantibody production in RA B cells.
Taking together, we show here that XmAb5871 inhibits BCR-, TRL9- and dual signal-induced B cell activation, proliferation and inflammatory cytokine and IgG production. Moreover, XmAb5871 inhibits the differentiation of citrullinated filaggrin peptide-specific antibody-producing cells from RA patients, suggesting that this antibody has potential as an alternative B cell suppressive therapy in RA.
Materials and Methods
Ethics statement and patients
Blood samples were taken from healthy blood donors and from RA patients after written consent with ethical permission of the Scientific Research Ethics Committee of the Medical Scientific Board of the Hungarian Ministry of Human Resources (ETT TUKEB 5257–0/2010–1018EKU 376/PI/010). The diagnosis of the disease was established on the basis of the revised ACR/EULAR classification criteria.Citation51 Purified B cells from six RA patients (male/female: 1/5, age: 38–66 y, ACPA titer determined with anti-CCP2 test: 135–3200) were tested. Additional blood samples were taken from healthy blood donors, and tonsils were obtained from patients undergoing tonsillectomy after written consent with ethical permission as above (ETT TUKEB 84–402/2008–1018EKU 1010/PI2008). The Scientific Research Ethics Committee of the Ministry specifically approved all studies.
Reagents and antibodies
Affinity-purified F(ab')2 fragment of goat anti-human IgG + IgM (H+L) (109–006–1270) was purchased from Jackson ImmunoResearch Laboratories, phosphorothioated unmethylated CpG oligodeoxynucleotide (ODN-2006) (5′-tcgtcgttttgtcgttttgtcgt-3′) was purchased from Sigma-Aldrich, and IL-2 (11340025) and IL-10 (11340105) were obtained from ImmunoTools. Anti-human CD19 with the Fc-KO mutations, XENP6187 and anti-human CD19 with FcγRIIb-enhanced IgG1 Fc (XmAb5871) were produced by Xencor. CD19-APC (21270196) was purchased from BD Biosciences. Phospho-AKT (Ser473, 4060) and phospho-ERK (Thr202/Tyr204, 4370)-specific antibodies were purchased from Cell Signaling Technology, and phospho-FcγRIIb (Tyr292, 2308–1) was from Epitomics. Rabbit anti-SHP2 (sc-30687) was from Santa Cruz Biotechnology, secondary goat anti-rabbit IgG-HRP (7074)-conjugated antibodies were from Cell Signaling Technology, and Luminata Forte HRP substrate (WBLUF0500) was purchased from Millipore. Biotinylated and citrullinated epitope peptide of filaggrin 306SHQESTXGXSXGRSGRSGS326 (X stands for citrulline) was a generous gift from Dr Anna Magyar (Research Group of Peptide Chemistry, Hungarian Academy of Sciences, Eötvös Loránd University).
Isolation and activation of B cells
PBMCs were isolated by density gradient centrifugation on Ficoll-Paque PLUS (GE Healthcare, 17–1440–03) and B lymphocytes were purified by negative selection using Magnetic Bead-Activated Cell Sorting (MACS, 130–091–151) according to the manufacturer's protocol (Miltenyi Biotec). B cell purity was assessed by flow cytometry using anti-human CD19-APC antibody. B cells with purity over 95% were used in most experiments, while B cells with purity over 99% were used in experiments investigating cytokine production.
Tonsils were obtained from patients undergoing tonsillectomy. Mononuclear cells were isolated by Ficoll-Paque PLUS density gradient centrifugation and T cells were depleted after rosette formation with sheep red blood cells followed by a second density gradient centrifugation. For western blot analysis, resting tonsil B cells were isolated by Percoll gradient centrifugation.Citation52 The purity of the resultant B cell suspension was over 95%. Tonsil B cells were further purified by positive magnetic bead separation for the cytokine production experiments.
To analyze synergistic activation, suboptimal doses of stimulants were used. B cells were activated by crosslinking the BCR with 2.5 μg/ml F(ab')2 fragment affinity-purified anti-human IgG + IgM in the presence or absence of 1 μg/ml CpG oligonucleotide (ODN). To test the effect of Fc-engineered antibodies on FcγRIIb, AKT and ERK phosphorylation, cells were pre-treated with XmAb5871 or the Fc-KO XENP6187 for 30 min at 37 °C (10 μg/ml). For functional assays, anti-human IgG + IgM, CpG ODN and respective anti-CD-19 antibodies were mixed before adding to the cells. Cell types, concentrations and times are indicated below the figures.
Western blot analysis
2 × 106 resting tonsillar B cells were activated per sample. After 30 min pretreatment of cells with XmAb5871 or XENP6187, the cells were activated via BCR or TLR9 for 30 min, then pelleted and lysed in 50 μl lysis buffer (20 mM Tris (pH 7.5), 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, and 1% Triton X-100, supplemented with protease and phosphatase inhibitors (2.5 mM Na3VO4, 1 mM PMSF, 1.5 mM aprotinin, 10 mM leupeptin). Cell lysates were mixed at 4:1 with 5 times concentrated reducing sample buffer. Samples were subjected to SDS-PAGE under reducing condition, and proteins were transferred electrophoretically to nitrocellulose membranes (Bio-Rad Laboratories, 162–0115). Membranes were blocked with 5% bovine serum albumin (BSA, Sigma-Aldrich, A7030) in TBS with 0.1% Tween 20 for 1 h, then incubated overnight with primary antibodies specific for the phosphorylated forms of various proteins. After several washings with Tris-washing buffer (TWB-Tween), horseradish peroxidase (HRP)-conjugated species-specific antibodies were added, and reactive protein bands were visualized using Luminata Forte HRP substrate. SHP2 was used as loading control.
Calcium mobilization
Intracellular free calcium concentration ([Ca2+]i) was measured by flow cytometry using Fluo-4 (Invitrogen)-stained purified blood B cells. Cells were resuspended at 5 × 106 cells/ml in calcium assay buffer and loaded with Fluo-4 dye for 30 min at room temperature. After incubation with 10 μg/ml anti-CD19 antibodies, cells were stimulated by addition of 10 μg/ml of anti-Ig. Calcium flux kinetics were recorded using a FACSCalibur flow cytometer (Becton-Dickinson) and data were analyzed using FlowJo software (TreeStar).
Proliferation assay
106 – 107 purified peripheral blood B cells were incubated with 5 μM carboxyfluorescein diacetate succinimidyl ester (CFSE, Biolegend, 422701) in 5% FCS containing PBS for 10 min at 37 °C. After repeated washings with ice-cold RPMI 1640 medium, cells were transferred into 96-well plates (4 × 105 cells/well) and cultured for 5 d at 37 °C in complete medium containing different stimuli with or without the respective anti-CD19 antibody. Cell cultures were harvested and CFSE staining was analyzed by flow cytometry. Dead cells were stained with propidium iodide (PI, Sigma-Aldrich, P-4170) and gated out. The results were evaluated by FlowJo software.
Cytokine production
Secretion of IL-6, IL-10, and TNF cytokines was measured in the supernatants of purified blood B cells stimulated under various conditions. 2 × 105 cells were stimulated per sample. The supernatants were collected after 48 h and stored at –70 °C until assayed by Flow Cytomix bead array (Bender MedSystem, BMS8213FF, BMS8215FF, and BMS8223FF) according to the manufacturer’s instructions.
Total IgG ELISPOT assay
105 purified blood B cells were added per well of a 96-well round-bottom plate in 200 μl culture media containing IL-2, IL-10 (50 ng/ml), and different combinations of activators as indicated in the figure. Alternatively, B cells were stimulated in the presence of 10 ng/ml rhIL-2 and 1 μg/ml R848 (Sigma-Aldrich, SML0196) a polyclonal activator. Cells were cultured for three days at 37 °C and in 5% CO2 containing atmosphere. The frequencies of IgG secreting cells were evaluated by ELISPOT assay.Citation53 Briefly, 96-well MultiScreenHTS-IP filter plates (Millipore, MAHAN4550) were pre-treated with 70% ethanol and washed 3 times in sterile PBS before coating overnight at 4 °C with 5 μg/ml mouse anti-human IgG (BD Biosciences, 555784). Plates were washed in sterile PBS and blocked with PBS containing 3% BSA. A serial dilution of cultured B cells was added and incubated at 37 °C. After 20 h incubation, cells were aspirated and plates were washed with PBS containing 0.1% Tween 20. Horseradish peroxidase (HRP) conjugated mouse anti-human IgG (BD Biosciences, 555788) detection antibody (1:1000) was added, and after two hours of incubation plates were subjected to several washes, then spots were developed with TMB substrate (MabTech, 3651–10), and counted using the CTL Immunospot Reader (Cellular Technologies Ltd.).
Peptide-specific ELISPOT assay
The assay was performed as previously described.Citation37 Briefly, purified B cells from RA patient blood were isolated using MACS. 106 cells/ml were cultured in RPMI 1640 containing 10% FCS in the presence of 10 ng/ml rhIL-2 and 1 μg/ml R848 (Sigma-Aldrich, SML0196) polyclonal activator with or without XmAb5871.Citation54 Cells were harvested on the third day, washed and counted, and then were transferred into the wells of ELISPOT plates precoated with neutravidin and the biotinylated citrulline-containing 19mer filaggrin peptide (SHQESTXGXSXGRSGRSGS, X stands for citrulline).Citation37 The spots were developed after 20 h.
Statistical analysis
Data are reported as means ± standard deviation and were analyzed using GraphPad Prism 4. Paired Student’s t test was used to compare groups. P values of <0.05 were considered statistically significant.
| Abbreviations: | ||
| ACPA | = | anti-citrullinated protein/peptide antibody |
| BCR | = | B cell receptor |
| FcγRIIb | = | Fcγ receptor IIb |
| ITAM | = | immunoreceptor tyrosine-based activation motif |
| ITIM | = | immunoreceptor tyrosine-based inhibitory motif |
| MAPK | = | mitogen-activated protein kinase |
| PH | = | pleckstrin homology |
| PI3-K | = | phosphatidylinositol 3-kinase |
| PIP3 | = | phosphatidylinositol (3,4,5)-triphosphate |
| RA | = | rheumatoid arthritis |
| SHIP | = | SH2 domain-containing inositol 5-phosphatase |
| SHP2 | = | SH2 domain containing protein tyrosine phosphatase |
| SLE | = | Systemic lupus erythematosus |
| TAK1 | = | TGFβ-activated kinase |
| TNF | = | tumor necrosis factor |
Disclosure of Potential Conflicts of Interest
David E. Szymkowski is an employee of and holds stock and stock options in Xencor, Inc.
Acknowledgments
This work was supported by the Hungarian Scientific Research Fund (OTKA 104846 and National Development Agency /www.nfu.hu/ -OTKA CK 80689 to G.S.); and the European Union and the European Social Fund have provided financial support to the project under the grant agreement no. TÁMOP 4.2.1./B-09/1/KMR-2010–0003. /www.nfu.hu/. For the synthesis of the 19mer filaggrin peptides, the authors thank Anna Magyar and Ferenc Hudecz at the Research Group of Peptide Chemistry, Eötvös Loránd University, Hungarian Academy of Science, Budapest, Hungary.
References
- Heyman B. Feedback regulation by IgG antibodies. Immunol Lett 2003; 88:157 - 61; http://dx.doi.org/10.1016/S0165-2478(03)00078-6; PMID: 12880686
- Ravetch JV. Fc receptors. Curr Opin Immunol 1997; 9:121 - 5; http://dx.doi.org/10.1016/S0952-7915(97)80168-9; PMID: 9039776
- Nimmerjahn F, Ravetch JV. Fcgamma receptors as regulators of immune responses. Nat Rev Immunol 2008; 8:34 - 47; http://dx.doi.org/10.1038/nri2206; PMID: 18064051
- Daëron M. Building up the family of ITIM-bearing negative coreceptors. Immunol Lett 1996; 54:73 - 6; http://dx.doi.org/10.1016/S0165-2478(96)02652-1; PMID: 9052857
- Van den Herik-Oudijk IE, Capel PJ, van der Bruggen T, Van de Winkel JG. Identification of signaling motifs within human Fc gamma RIIa and Fc gamma RIIb isoforms. Blood 1995; 85:2202 - 11; PMID: 7718892
- Famiglietti SJ, Nakamura K, Cambier JC. Unique features of SHIP, SHP-1 and SHP-2 binding to FcgammaRIIb revealed by surface plasmon resonance analysis. Immunol Lett 1999; 68:35 - 40; http://dx.doi.org/10.1016/S0165-2478(99)00027-9; PMID: 10397153
- Ono M, Bolland S, Tempst P, Ravetch JV. Role of the inositol phosphatase SHIP in negative regulation of the immune system by the receptor Fc(gamma)RIIB. Nature 1996; 383:263 - 6; http://dx.doi.org/10.1038/383263a0; PMID: 8805703
- Sármay G, Koncz G, Pecht I, Gergely J. Cooperation between SHP-2, phosphatidyl inositol 3-kinase and phosphoinositol 5-phosphatase in the Fc gamma RIIb mediated B cell regulation. Immunol Lett 1999; 68:25 - 34; http://dx.doi.org/10.1016/S0165-2478(99)00026-7; PMID: 10397152
- Koncz G, Gergely J, Sármay G. Fc gammaRIIb inhibits both B cell receptor- and CD19-induced Ca2+ mobilization in Fc gammaR-transfected human B cells. Int Immunol 1998; 10:141 - 6; http://dx.doi.org/10.1093/intimm/10.2.141; PMID: 9533441
- Tedder TF, Isaacs CM. Isolation of cDNAs encoding the CD19 antigen of human and mouse B lymphocytes. A new member of the immunoglobulin superfamily. J Immunol 1989; 143:712 - 7; PMID: 2472450
- Otero DC, Anzelon AN, Rickert RC. CD19 function in early and late B cell development: I. Maintenance of follicular and marginal zone B cells requires CD19-dependent survival signals. J Immunol 2003; 170:73 - 83; http://dx.doi.org/10.4049/jimmunol.170.1.73; PMID: 12496385
- Otero DC, Rickert RC. CD19 function in early and late B cell development. II. CD19 facilitates the pro-B/pre-B transition. J Immunol 2003; 171:5921 - 30; http://dx.doi.org/10.4049/jimmunol.171.11.5921; PMID: 14634103
- Matsumoto AK, Martin DR, Carter RH, Klickstein LB, Ahearn JM, Fearon DT. Functional dissection of the CD21/CD19/TAPA-1/Leu-13 complex of B lymphocytes. J Exp Med 1993; 178:1407 - 17; http://dx.doi.org/10.1084/jem.178.4.1407; PMID: 7690834
- Tedder TF, Zhou LJ, Engel P. The CD19/CD21 signal transduction complex of B lymphocytes. Immunol Today 1994; 15:437 - 42; http://dx.doi.org/10.1016/0167-5699(94)90274-7; PMID: 7524521
- Fujimoto M, Poe JC, Jansen PJ, Sato S, Tedder TF. CD19 amplifies B lymphocyte signal transduction by regulating Src-family protein tyrosine kinase activation. J Immunol 1999; 162:7088 - 94; PMID: 10358152
- Karnell JL, Dimasi N, Karnell FG 3rd, Fleming R, Kuta E, Wilson M, Wu H, Gao C, Herbst R, Ettinger R. CD19 and CD32b differentially regulate human B cell responsiveness. J Immunol 2014; 192:1480 - 90; http://dx.doi.org/10.4049/jimmunol.1301361; PMID: 24442430
- Lazar GA, Dang W, Karki S, Vafa O, Peng JS, Hyun L, Chan C, Chung HS, Eivazi A, Yoder SC, et al. Engineered antibody Fc variants with enhanced effector function. Proc Natl Acad Sci U S A 2006; 103:4005 - 10; http://dx.doi.org/10.1073/pnas.0508123103; PMID: 16537476
- Chu SY, Vostiar I, Karki S, Moore GL, Lazar GA, Pong E, Joyce PF, Szymkowski DE, Desjarlais JR. Inhibition of B cell receptor-mediated activation of primary human B cells by coengagement of CD19 and FcgammaRIIb with Fc-engineered antibodies. Mol Immunol 2008; 45:3926 - 33; http://dx.doi.org/10.1016/j.molimm.2008.06.027; PMID: 18691763
- Horton HM, Chu SY, Ortiz EC, Pong E, Cemerski S, Leung IW, Jacob N, Zalevsky J, Desjarlais JR, Stohl W, et al. Antibody-mediated coengagement of FcγRIIb and B cell receptor complex suppresses humoral immunity in systemic lupus erythematosus. J Immunol 2011; 186:4223 - 33; http://dx.doi.org/10.4049/jimmunol.1003412; PMID: 21357255
- Szili D, Bankó Z, Tóth EA, Nagy G, Rojkovich B, Gáti T, Simon M, Hérincs Z, Sármay G. TGFb activated Kinase 1 (TAK1) at the crossroad of B cell receptor and Toll-like receptor 9 signaling pathways in human B Cells. PLoS One 2014; 9:e96381; http://dx.doi.org/10.1371/journal.pone.0096381; PMID: 24801688
- European Medicines Agency. EudraCT: European Clinical Trials Database; 2013.
- Aman MJ, Lamkin TD, Okada H, Kurosaki T, Ravichandran KS. The inositol phosphatase SHIP inhibits Akt/PKB activation in B cells. J Biol Chem 1998; 273:33922 - 8; http://dx.doi.org/10.1074/jbc.273.51.33922; PMID: 9852043
- Tridandapani S, Phee H, Shivakumar L, Kelley TW, Coggeshall KM. Role of SHIP in FcgammaRIIb-mediated inhibition of Ras activation in B cells. Mol Immunol 1998; 35:1135 - 46; http://dx.doi.org/10.1016/S0161-5890(98)00097-2; PMID: 10395202
- Takai T. Roles of Fc receptors in autoimmunity. Nat Rev Immunol 2002; 2:580 - 92; PMID: 12154377
- Tarasenko T, Dean JA, Bolland S. FcgammaRIIB as a modulator of autoimmune disease susceptibility. Autoimmunity 2007; 40:409 - 17; http://dx.doi.org/10.1080/08916930701464665; PMID: 17729034
- Sebbag M, Chapuy-Regaud S, Auger I, Petit-Texeira E, Clavel C, Nogueira L, Vincent C, Cornélis F, Roudier J, Serre G. Clinical and pathophysiological significance of the autoimmune response to citrullinated proteins in rheumatoid arthritis. Joint Bone Spine 2004; 71:493 - 502; http://dx.doi.org/10.1016/j.jbspin.2004.07.004; PMID: 15589429
- Yamada R, Suzuki A, Chang X, Yamamoto K. Citrullinated proteins in rheumatoid arthritis. Front Biosci 2005; 10:54 - 64; http://dx.doi.org/10.2741/1506; PMID: 15574347
- Snir O, Widhe M, von Spee C, Lindberg J, Padyukov L, Lundberg K, Engström A, Venables PJ, Lundeberg J, Holmdahl R, et al. Multiple antibody reactivities to citrullinated antigens in sera from patients with rheumatoid arthritis: association with HLA-DRB1 alleles. Ann Rheum Dis 2009; 68:736 - 43; http://dx.doi.org/10.1136/ard.2008.091355; PMID: 18635594
- Peng SL. Signaling in B cells via Toll-like receptors. Curr Opin Immunol 2005; 17:230 - 6; http://dx.doi.org/10.1016/j.coi.2005.03.003; PMID: 15886111
- Ries M, Schuster P, Thomann S, Donhauser N, Vollmer J, Schmidt B. Identification of novel oligonucleotides from mitochondrial DNA that spontaneously induce plasmacytoid dendritic cell activation. J Leukoc Biol 2013; 94:123 - 35; http://dx.doi.org/10.1189/jlb.0612278; PMID: 23610148
- Meyer-Bahlburg A, Rawlings DJ. B cell autonomous TLR signaling and autoimmunity. Autoimmun Rev 2008; 7:313 - 6; http://dx.doi.org/10.1016/j.autrev.2007.11.027; PMID: 18295736
- Kenny EF, Quinn SR, Doyle SL, Vink PM, van Eenennaam H, O’Neill LA. Bruton’s tyrosine kinase mediates the synergistic signalling between TLR9 and the B cell receptor by regulating calcium and calmodulin. PLoS One 2013; 8:e74103; http://dx.doi.org/10.1371/journal.pone.0074103; PMID: 23967355
- Otero DC, Omori SA, Rickert RC. CD19-dependent activation of Akt kinase in B-lymphocytes. J Biol Chem 2001; 276:1474 - 8; http://dx.doi.org/10.1074/jbc.M003918200; PMID: 11042164
- Ruprecht CR, Lanzavecchia A. Toll-like receptor stimulation as a third signal required for activation of human naive B cells. Eur J Immunol 2006; 36:810 - 6; http://dx.doi.org/10.1002/eji.200535744; PMID: 16541472
- Jurk M, Heil F, Vollmer J, Schetter C, Krieg AM, Wagner H, Lipford G, Bauer S. Human TLR7 or TLR8 independently confer responsiveness to the antiviral compound R-848. Nat Immunol 2002; 3:499; http://dx.doi.org/10.1038/ni0602-499; PMID: 12032557
- Bellatin MF, Han M, Fallena M, Fan L, Xia D, Olsen N, Branch V, Karp D, Stastny P. Production of autoantibodies against citrullinated antigens/peptides by human B cells. J Immunol 2012; 188:3542 - 50; http://dx.doi.org/10.4049/jimmunol.1100577; PMID: 22345652
- Szarka E, Babos F, Magyar A, Huber K, Szittner Z, Papp K, et al. Recognition of new citrulline containing peptide epitopes by autoantibodies produced in vivo and in vitro by B cells of Rheumatoid arthritis patients. Immunology 2013; PMID: 24116744
- Panayi GS. B cells: a fundamental role in the pathogenesis of rheumatoid arthritis?. Rheumatology (Oxford) 2005; 44:Suppl 2 ii3 - 7; http://dx.doi.org/10.1093/rheumatology/keh616; PMID: 15851524
- Bugatti S, Codullo V, Caporali R, Montecucco C. B cells in rheumatoid arthritis. Autoimmun Rev 2007; 7:137 - 42; http://dx.doi.org/10.1016/j.autrev.2007.02.017; PMID: 18035324
- Weyand CM, Goronzy JJ. Ectopic germinal center formation in rheumatoid synovitis. Ann N Y Acad Sci 2003; 987:140 - 9; http://dx.doi.org/10.1111/j.1749-6632.2003.tb06042.x; PMID: 12727633
- Burmester GR, Feist E, Dorner T. Emerging cell and cytokine targets in rheumatoid arthritis. Nat Rev Rheumatol 2013.
- Keystone EC. B cells in rheumatoid arthritis: from hypothesis to the clinic. Rheumatology (Oxford) 2005; 44:Suppl 2 ii8 - 12; http://dx.doi.org/10.1093/rheumatology/keh617; PMID: 15851525
- Looney RJ, Anolik J, Sanz I. B cells as therapeutic targets for rheumatic diseases. Curr Opin Rheumatol 2004; 16:180 - 5; http://dx.doi.org/10.1097/00002281-200405000-00003; PMID: 15103242
- Brinkman IH, van de Laar MA, Jansen TL, van Roon EN. The potential risk of infections during (prolonged) rituximab therapy in rheumatoid arthritis. Expert Opin Drug Saf 2011; 10:715 - 26; http://dx.doi.org/10.1517/14740338.2011.562188; PMID: 21401437
- Mattila PK, Feest C, Depoil D, Treanor B, Montaner B, Otipoby KL, Carter R, Justement LB, Bruckbauer A, Batista FD. The actin and tetraspanin networks organize receptor nanoclusters to regulate B cell receptor-mediated signaling. Immunity 2013; 38:461 - 74; http://dx.doi.org/10.1016/j.immuni.2012.11.019; PMID: 23499492
- Callard RE, Rigley KP, Smith SH, Thurstan S, Shields JG. CD19 regulation of human B cell responses. B cell proliferation and antibody secretion are inhibited or enhanced by ligation of the CD19 surface glycoprotein depending on the stimulating signal used. J Immunol 1992; 148:2983 - 7; PMID: 1374445
- Pezzutto A, Dörken B, Rabinovitch PS, Ledbetter JA, Moldenhauer G, Clark EA. CD19 monoclonal antibody HD37 inhibits anti-immunoglobulin-induced B cell activation and proliferation. J Immunol 1987; 138:2793 - 9; PMID: 2437199
- Rigley KP, Callard RE. Inhibition of B cell proliferation with anti-CD19 monoclonal antibodies: anti-CD19 antibodies do not interfere with early signaling events triggered by anti-IgM or interleukin 4. Eur J Immunol 1991; 21:535 - 40; http://dx.doi.org/10.1002/eji.1830210302; PMID: 1707000
- Fujimoto M, Poe JC, Satterthwaite AB, Wahl MI, Witte ON, Tedder TF. Complementary roles for CD19 and Bruton’s tyrosine kinase in B lymphocyte signal transduction. J Immunol 2002; 168:5465 - 76; http://dx.doi.org/10.4049/jimmunol.168.11.5465; PMID: 12023340
- Fujimoto M, Poe JC, Hasegawa M, Tedder TF. CD19 regulates intrinsic B lymphocyte signal transduction and activation through a novel mechanism of processive amplification. Immunol Res 2000; 22:281 - 98; http://dx.doi.org/10.1385/IR:22:2-3:281; PMID: 11339363
- Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham CO 3rd, Birnbaum NS, Burmester GR, Bykerk VP, Cohen MD, et al. Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum 2010; 62:2569 - 81; http://dx.doi.org/10.1002/art.27584; PMID: 20872595
- Biró A, Sármay G, Rozsnyay Z, Klein E, Gergely J. A trypsin-like serine protease activity on activated human B cells and various B cell lines. Eur J Immunol 1992; 22:2547 - 53; http://dx.doi.org/10.1002/eji.1830221013; PMID: 1396962
- Czerkinsky CC, Nilsson LA, Nygren H, Ouchterlony O, Tarkowski A. A solid-phase enzyme-linked immunospot (ELISPOT) assay for enumeration of specific antibody-secreting cells. J Immunol Methods 1983; 65:109 - 21; http://dx.doi.org/10.1016/0022-1759(83)90308-3; PMID: 6361139
- Jahnmatz M, Kesa G, Netterlid E, Buisman AM, Thorstensson R, Ahlborg N. Optimization of a human IgG B-cell ELISpot assay for the analysis of vaccine-induced B-cell responses. J Immunol Methods 2013; 391:50 - 9; http://dx.doi.org/10.1016/j.jim.2013.02.009; PMID: 23454005
