Abstract
The humanized monoclonal antibody with high affinity for the human epidermal growth factor receptor (HER) 3, RG7116, is a glycoengineered, IgG1 class antibody. By labeling RG7116 with zirconium-89 (89Zr) we aimed to visualize in vivo HER3 expression and study the biodistribution of this antibody in human tumor-bearing mice. Biodistribution of 89Zr-RG7116 was studied in subcutaneously xenografted FaDu tumor cells (HER3-positive). Dose-dependency of 89Zr-RG7116 organ distribution and specific tumor uptake was assessed by administering doses ranging from 0.05 to 10 mg/kg RG7116 to SCID/Beige mice. Biodistribution was analyzed at 24 and 144 h after injection. MicroPET imaging was performed at 1, 3, and 6 days after injection of 1.0 mg/kg 89Zr-RG7116 in the FaDu, H441, QG-56 and Calu-1 xenografts with varying HER3 expression. The excised tumors were analyzed for HER3 expression. Biodistribution analyses showed a dose- and time-dependent 89Zr-RG7116 tumor uptake in FaDu tumors. The highest tumor uptake of 89Zr-RG7116 was observed in the 0.05 mg/kg dose group with 27.5%ID/g at 144 h after tracer injection. MicroPET imaging revealed specific tumor uptake of 89Zr-RG7116 in FaDu and H441 models with an increase in tumor uptake over time. Biodistribution data was consistent with the microPET findings in FaDu, H441, QG56 and Calu-1 xenografts, which correlated with HER3 expression levels. In conclusion, 89Zr-RG7116 specifically accumulates in HER3 expressing tumors. PET imaging with this tracer provides real-time non-invasive information about RG7116 distribution, tumor targeting and tumor HER3 expression levels.
Introduction
The human epidermal growth factor receptor (HER) transmembrane receptor tyrosine kinase family consists of four members: epidermal growth factor receptor (EGFR), HER2, HER3, and HER4. Members of this family play a critical role in tumor cell survival, proliferation, maturation, metastasis and angiogenesis via diverse cellular pathways.Citation1
A number of targeted therapies consisting of monoclonal antibodies (mAbs) and tyrosine kinase inhibitors against the HER family members EGFR and HER2 are currently used in clinical practice. The EGFR-targeting mAbs cetuximab and panitumumab are used in KRAS wild-type metastatic colorectal cancer, and cetuximab is also used to treat patients with squamous cell cancer of the head and neck.Citation2 Trastuzumab, the mAb targeting HER2, is administered to patients with HER2 positive breast cancer in the adjuvant and metastatic setting and for metastatic gastric cancer.Citation3,Citation4 Tyrosine kinase inhibitors (TKI) targeting EGFR and HER2 are used in the clinic in lung and breast cancer, respectively.Citation2,Citation3 Unfortunately many patients do not respond to EGFR and HER2 targeted therapies or they develop resistance during treatment.Citation2,Citation5 Therefore, several pharmaceutical companies are working on the development of new EGFR and HER2 targeting agents.Citation2,Citation3,Citation6 Moreover, combination therapies with other growth factor receptor targeting drugs are being explored. Based on novel insights, an antibody against HER3 is an interesting option.Citation7
HER family members are inactive in monomers, but can be activated by homo- or heterodimerization after ligand binding.Citation3 HER3 is the only member in the HER family lacking intrinsic tyrosine kinase activity, and therefore has been underestimated for its role in cancer until recently.Citation7 It can only be activated after the formation of heterodimers, which are the most active signaling complexes, with HER1/HER3 and HER2/HER3 heterodimers as the most potent dimers in this family.Citation7 Furthermore, HER3 was upregulated after HER2 targeted therapy, indicating the relevance of HER3.Citation8 The prominent role of HER3 in tumor growth and maintenance, and the fact that it is overexpressed in many solid tumors, makes it a potentially promising target to inhibit HER family signaling.Citation9 HER3 is physiologically expressed in a wide variety of normal human tissues, including cells of the gastrointestinal, urinary, respiratory, and reproductive tracts as well as the skin, endocrine and nervous system.Citation10
RG7116, a humanized glycoengineered IgG1 antibody with high affinity for HER3, is an example of such a new drug. RG7116 binds to the extracellular domain of HER3 (domain I) with high affinity. RG7116 is not cross-reactive for mouse HER3. RG7116 inhibits HER3 and AKT phosphorylation and induces HER3 internalization in tumor cells.Citation11 Moreover, glycoengineering translates into enhanced in vitro antibody-dependent cell-mediated cytotoxicity (ADCC) activity of RG7116 via FcγRIIIA binding on human immune effector cells.Citation12 RG7116 (RO5479599) is currently in Phase 1 clinical testing. For mAbs, which show only limited toxicity in cancer patients, it can be difficult to determine the lowest dose for the individual patient needed for antitumor efficacy. Molecular imaging might support decision-making during clinical development and clinical practice. It also has potential as a tool for patient selection for therapy by showing the presence of the target and uptake of the drug before initiating therapy.
Molecular imaging can visualize the biodistribution of monoclonal antibodies by using these drugs as a tracer after labeling it with zirconium-89 (89Zr; t1/2 = 78.4 h).Citation13 For antibody imaging, 89Zr is a suitable isotope that can be used in mouse models as well as in the clinic. We developed the PET radiotracers 89Zr-bevacizumab to image vascular endothelial growth factor (VEGF) and 89Zr-trastuzumab to image HER2 positive tumors and tested them successfully in preclinical and clinical settings.Citation14-Citation17 This experience and technique has now been used to radiolabel RG7116 with 89Zr. To visualize in vivo HER3 expression and study the biodistribution of RG7116, we performed the in vivo evaluation of 89Zr-RG7116 in human tumor-bearing mice.
Results
Dose-escalation biodistribution study
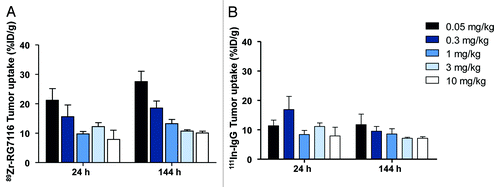
The dose-escalation biodistribution study in sc FaDu tumor-bearing mice showed preferential HER3 driven tumor uptake of 89Zr-RG7116 compared with 111In-IgG. The highest tumor uptake was seen at the 0.05 mg/kg dose group, 144 h after injection (). At 144 h after injection, %ID/g tumor uptake decreased significantly with increasing doses of RG7116, from 27.5 ± 3.5%ID/g at 0.05 mg/kg to 10.1 ± 0.6%ID/g at 10 mg/kg (P < 0.01), indicating that the tumor uptake is saturable. This pattern was also seen in the biodistribution analysis after 24 h, although to a lesser extent. In , non-specific 111In-IgG uptake is shown. When comparing 89Zr-RG7116 uptake vs. 111In-IgG tumor uptake, there was specific uptake of 89Zr-RG7116 in the 0.05 mg/kg dose group already at 24 h (P < 0.01), based on the ratio between 89Zr and 111In tumor values. Between 24 and 144 h after injection, there was a further increase in specific uptake of 89Zr-RG7116 as illustrated by the increasing ratio between 89Zr and 111In tumor uptake. There were significant differences between 89Zr and 111In tumor uptake for all dose groups at 144 h after injection.
Figure 1. Tumor uptake at 24 and 144 h after tracer injection as measured with ex vivo analysis showed dose and time dependent tumor uptake of 89Zr-RG7116 in FaDu tumors (A). Non-specific 111In-IgG uptake was measured in the same tumors (B).
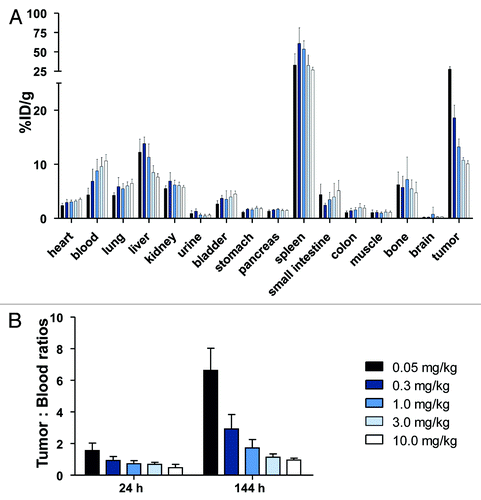
Biodistribution experiments with 89Zr-RG7116 revealed a normal antibody organ distribution except for the exceptionally high spleen uptake found for all doses used (), which occurred already 24 h after injection. 111In-IgG uptake in the spleen was also found to be high, but to a lesser extent than for 89Zr-RG7116 in all dose groups. In the 0.05 mg/kg group, the tumor: blood ratio was 6.6 () and the tumor: muscle ratio was 30.3. With increasing doses, both these ratios decreased at 24 and 144 h after injection, displaying the dose-dependent tracer distribution.
Figure 2. Biodistribution of 89Zr-RG7116 at 144 h as determined by ex vivo analysis (A). Tumor: blood ratios (B) were calculated for the different doses used in the dose-escalation biodistribution study at 24 and 144 h after tracer injection.
Tumor weight measurements at the end of the experiment (144 h after tracer injection) revealed a smaller tumor volume for the highest dose groups compared with the lower dose groups, consistent with dose-dependent tumor inhibitory drug effects.Citation11 Tumor weights were 0.44 ± 0.17 g, 0.54 ± 0.14 g, 0.41 ± 0.18 g, 0.28 ± 0.17 g, and 0.26 ± 0.05 g for doses ranging from 0.05 to 10 mg/kg (not significant). The single antibody administration used in this study already influenced tumor size, and thereby also tumor uptake measured of 89Zr-RG7116.
MicroPET imaging in different tumor models and biodistribution
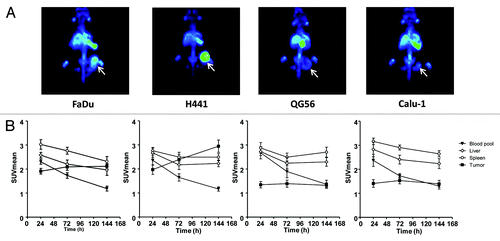
At day 1 after injection of 1.0 mg/kg 89Zr-RG7116, microPET scan analysis revealed high blood pool, spleen and liver activity that could be visualized in all mice bearing xenografted tumors of FaDu, H441, QG-56, and Calu-1. Quantification of PET data showed that the non-specific uptake in these organs decreased over time based on the scans performed at day 3 and 6 after injection of 1.0 mg/kg 89Zr-RG7116 (). Specific HER3-driven uptake was seen in FaDu and H441 tumors, with increasing tumor uptake over time. QG-56 and Calu-1 tumors did show tumor uptake on microPET scans, but minimal differences in tumor uptake were seen over time post-tracer injection. An average tumor SUVmean of 2.1, 2.9, 1.3, and 1.4 was quantified based on microPET scans on day 6 after injection in FaDu, H441, QG-56 and Calu-1, respectively ().
Figure 3.89Zr-RG7116 microPET imaging of FaDu, H441, QG56 and Calu-1 bearing mice. Maximal intensity projection images are shown for all tumor models at 144 h after tracer injection (A). MicroPET data quantification was performed for blood pool, spleen, liver and tumor uptake at 24, 72 and 144 after tracer injection in all mice. (B).
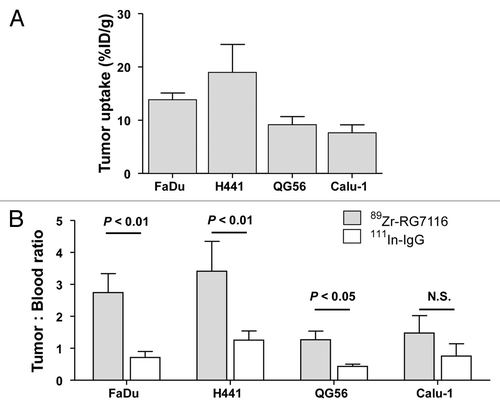
After the last scan, mice were sacrificed for biodistribution analysis. Biodistribution data were fully consistent with the microPET findings, showing ex vivo tumor uptake of 89Zr-RG7116 of 13.9, 19.0, 9.2, and 7.6%ID/g in FaDu, H441, QG56 and Calu-1 xenografts respectively (). 111In-IgG tumor uptake in these models was 9.1 (FaDu), 16.5 (H441), 6.7 (QG56) and 8.3 (Calu-1). Tumor: blood ratios of 89Zr-RG7116 compared with 111In-IgG distribution revealed significant differences in HER3-expressing tumors, indicating specific tumor uptake in these models (). The HER3-negative Calu-1 did not show a significant (P = 0.065) difference between tumor: blood ratios of 89Zr-RG7116 vs. 111In-IgG, indicating non-specific tumor uptake in this model.
Figure 4. Ex vivo tumor uptake of 89Zr-RG7116 in FaDu, H441, QG56 and Calu-1 tumors (A). To compare specific tumor uptake between tumor models, tumor: blood ratios are provided for 89Zr-RG7116 and 111In-IgG (B).
Tumor weight at the end of the experiment were: FaDu tumors, 0.176 ± 0.077 g; H441 tumors, 0.183 ± 0.060 g; QG56 tumors 0.162 ± 0.113 g; and Calu-1 tumors, 0.057 ± 0.043 g. Ex vivo tumor weights showed a correlation of 0.984 (R2) with tumor region of interests (ROIs) volumes used for microPET quantification. Ex vivo 89Zr-RG7116 tumor uptake showed a correlation of 0.812 (R2; P < 0.05) with tumor uptake on microPET quantification.
Ex vivo biomarker analysis
Evaluation of hematoxylin and eosin (H&E) stained slides revealed that fibrosis is similar in FaDu (13% ± 8%), H441 (17% ± 9%), and QG56 (20% ± 12%) tumors, and almost absent in Calu-1 (<3%) tumors. The immunohistochemical (IHC) staining pattern of HER3, which confirmed the HER3 expression levels in the four xenografts, is depicted in .
Figure 5. Ex vivo HER3 IHC staining of FaDu, H441, QG56 and Calu-1 tumors (A). Ex vivo serum levels of RG7116 in the dose-escalation biodistribution study 24 and 144 h after tracer injection (B).
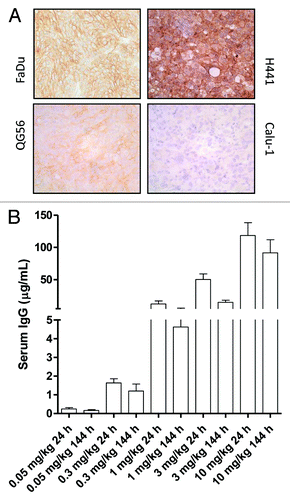
The RG7116 level in mouse serum obtained 24 and 144 h after tracer injection during the dose-escalation biodistribution study is shown in . Serum levels correlate with the different doses used. There was no significant difference in serum RG7116 level 144 h after tracer injection between the different tumor models. This indicates no differences in experimental setup between the tumor models.
Discussion
This in vivo study, using an imaging probe for HER3 expression measurement, shows specific HER3 driven tumor uptake of 89Zr-RG7116. Moreover the tumor uptake of the radiolabeled antibody is related to HER3 expression levels. Our results suggest that 89Zr-RG7116 can be used for non-invasive assessment of the HER3 expression levels in tumor lesions and can provide real-time information about RG7116 distribution and tumor uptake.
AMG-888, another antibody targeting HER3, was labeled with copper-64 (64Cu) for PET imaging. In the human pancreatic cancer xenograft model, microPET imaging showed higher tracer uptake compared with the animals administered a blocking dose. Biodistribution studies in tumor bearing mice show uptake and retention in BxPC3 tumors at 24 and 48 h post injection.Citation18 Furthermore, a cross-reactive 99mTc-labeled Affibody targeting HER3 demonstrated uptake in HER3-expressing organs like the small intestine or the salivary glands (as well as unspecific signals such as in the kidney).Citation19
We used the radionuclide 89Zr for PET imaging of a HER3 targeting antibody, which allowed us to image up to 144 h after tracer injection and to visualize optimal tumor targeting of the antibody. In the absence of other in vivo studies for HER3, it is of interest to compare our findings with results obtained with radiolabeled antibodies against the HER family members HER2 and EGFR. 89Zr labeled trastuzumab and cetuximab both showed specific uptake in a panel xenograft tumor models.Citation15,Citation20 With radiolabeled trastuzumab, a comparative preclinical biodistribution study was performed with 100 µg 89Zr-trastuzumab in SKOV3 (HER2 overexpressing) and GLC4 (HER2 negative); tumor uptake was 33.4 and 7.1%ID/g, respectively.Citation15 This tracer also proved applicable in patients with even higher 89Zr-trastuzumab tumor: background ratios in HER2-positive lesions than in human tumor-bearing animals.Citation15 HER2 is amplified in 15–25% of breast cancer patients. Although no amplification has been shown for HER3 in tumors, we could visualize with 89Zr-RG7116 that tracer tumor uptake is correlated with high HER3 expression levels of the tumors. In FaDu tumors, a 27.5%ID/g at 0.05 mg/kg was obtained 144 h after tracer injection, showing comparable tumor uptake as obtained with trastuzumab in a HER2-positive human ovarian cancer tumor model. 89Zr-labeled cetuximab showed disparity between tumor uptake assessed with PET imaging and in vivo EGFR expression in human epidermoid, breast, glioblastoma and colorectal carcinoma tumors.Citation20 Therefore, the authors concluded that 89Zr-cetuximab represents antibody uptake instead of quantification of EGFR expression levels. Findings were similar for 64Cu labeled cetuximab and panitumumab in HNSCC xenograft models.Citation21-Citation23
Comparison of biodistribution data between 89Zr-RG7116 and other antibodies is hampered given the mouse strain we used. In these Fox Chase SCID Beige mice, there is a disproportional high influence of spleen uptake of 89Zr-RG7116. Ex vivo serum analysis of RG7116 confirmed a dose-dependent presence of RG7116 in the blood pool, indicating that spleen uptake did not affect RG7116 blood pool and tumor uptake. Biodistribution experiments revealed high spleen uptake for RG7116 as well as for non-specific IgG. This may be because the spleen acts as a “sink” for antibodies, for RG7116 even more than for IgG. This is not related to specific HER3-driven uptake in the spleen as the uptake decreased over time on the microPET scans in various different tumor models. High spleen uptake of 82.4 ± 15.3%ID/g 24 h after administration of 111In-labeled mesothelin targeting antibody was observed in C.B-17 SCID mice.Citation24 The mouse strain (SCID mice) used for these experiments, may have affected this relatively high antibody uptake, as far lower IgG uptake in spleen is found in other strains.Citation14,Citation15 The even higher spleen uptake seen with 89Zr-RG7116 compared with 111In-IgG is most likely caused by FcyRIIIA mediated uptake, which occurs as a result of the glycoengineered antibody. SCID/beige mice bear active FcγRIV monocytes and macrophages as effectors (FcγRIV is the murine homolog of human FcγRIIIA).Citation25 This would potentially clarify the difference between IgG non-specific and RG7116 spleen uptake.
Tumor uptake of antibodies is influenced by many factors, such as vascular density and permeability, as well as tumor structure, extracellular matrix and the presence of soluble or shed HER3. We found a correlation between HER3 expression and RG7116 tumor uptake based on tumor: blood ratios and ex vivo tumor analysis of HER3 expression. Tumor uptake, however, is not necessarily correlated with tumor response. This is illustrated in the HER3 overexpressing H441 xenografts, which showed highest 89Zr-RG7116 uptake, but were non-responsive to RG7116. H441 is dependent on other pathways than those involved in HER3 signaling.
Although the presence of a receptor does not preclude resistance in clinical practice, establishing the presence or absence of a growth factor receptor (mostly by means of tumor staining or molecular imaging) is clearly of tremendous importance. In breast cancer, the absence of the estrogen receptor (ER) or HER2 predicts lack of anti-ER or -HER2 treatment effect (far better than the presence of these receptors predicts a good effect of treatment directed at them) and this negative predictive value drives treatment decisions.Citation26,Citation27 Clearly, the negative predictive value is greater than the positive predictive value. Therefore, establishing HER3 presence (for instance by means of molecular imaging) may well have effects on treatment decisions in the future.
In conclusion, our findings show that 89Zr-RG7116 specifically accumulates in HER3 expressing tumors. PET imaging with this tracer provides real-time non-invasive information about RG7116 distribution, tumor targeting and tumor HER3 expression levels. Based on these promising preclinical results, 89Zr-RG7116 PET imaging is being used to provide insight in RG7116 behavior in cancer patients (clinicaltrials.gov Identifier: NCT01482377). In this study, an optimal clinical tracer dose will first be determined. Molecular imaging can likely contribute to dose-finding, and has potential utility in the selection and monitoring of patients for HER3 therapy.
Materials and Methods
Cell lines
Cell lines with various HER3 expression were used. HER3 expression level was defined using western blot and IHC. With IHC the HER3 expression level was analyzed semi-quantitatively and comparatively including the following parameters: estimated percentage of positive tumor cells, staining intensity (1+ weak, 2+ moderate, and 3+ strong), and the subcellular localization (membranous, cytoplasmic, nuclear). The human head and neck squamous cell carcinoma (HNSCC) FaDu cell line and human non-small cell lung cancer (NSCLC) cell line H441 are high HER3-expressing, characterized by membrane-bound (and cytoplasmic) HER3-expression at moderate to strong intensity level of almost all tumor cells. QG-56, a human NSCLC cell line with medium HER3 expression (weak to moderate membranous staining in about 70% of tumor cells) and Calu-1, a human NSCLC cell line without HER3 expression (negative in the IHC staining), were also used. These cell lines were chosen based on HER3 expression and antitumor effect of RG7116 treatment. Responsiveness of the subcutaneous (sc) xenografted tumor cell lines in mice to RG7116 treatment was determined by tumor growth inhibition studies.Citation11 FaDu and QG-56 are both responsive in vivo to treatment with RG7116; H441 and Calu-1 are non-responder cell lines to treatment with RG7116. FaDu, QG-56, and H441 cells were cultured in DMEM (Invitrogen), supplemented with 10% fetal calf serum (FCS) (Bodinco BV) and, 2 mM L-glutamine (Invitrogen), Calu-1 in RPMI 1640 (Invitrogen) supplemented with 10% FCS and 2 mM L-glutamine. All cell lines were grown at 37 °C in a fully humidified atmosphere containing 5% CO2.
Synthesis and quality control of conjugated and radiolabeled 89Zr-RG7116
RG7116 (2.7 mg/mL; Roche) was allowed to react with a 5-fold molar excess of a tetrafluorophenol-N-succinyldesferal-Fe active ester (TFP-N-SucDf-Fe; VU University Medical Center) as described earlier.Citation28 The conjugate was radiolabeled with clinical-grade 89Zr-oxalate (IBA Molecular) to obtain a radiochemical purity > 95%. Quality control was performed as described previously.Citation14,Citation15
The immunoreactive fraction of the radiolabeled compound was determined by a competition assay performed on HER3 extracellular domain (ECD; Roche) coated well-plates. HER3 ECD was diluted in sodium phosphate buffer (PBS; 140 mmol/L NaCl, 9 mmol/L Na2HPO4, 1.3 mmol/L NaH2PO4; UMCG) resulting in a concentration of 5 µg/mL. The solution was adjusted to pH 9.2–9.5 with 50 mmol/L Na2CO3 and 50 µL was added to the wells, incubated overnight at 4 °C, and then blocked with 1% human serum albumin (HSA; Sanquin) in PBS. After blocking, the plates were washed with 0.1% polysorbate 80 (Sigma-Aldrich) in PBS. 89Zr-RG7116 and RG7116 were mixed and diluted in PBS to result in a fixed concentration of 500 ng/mL (3.33 nM) 89Zr-RG7116 and varying concentrations of unlabeled RG7116, ranging from 3.33 pM to 3.33 μM. These samples were added to the wells, and incubated for 2 h. Subsequently, the samples were collected from the wells in 2 wash steps. Both the 89Zr- RG7116 bound to the HER3 ECD-coated wells and the collected samples containing unbound 89Zr-RG7116 were measured by a calibrated well-type γ-counter. The percentage of HER3 binding was calculated as the fraction of radioactivity bound to HER3 ECD-coated wells divided by the total amount of radioactivity added. At equal doses of labeled and unlabeled RG7116, binding of 35.5% of radiolabeled RG7116 was found, which indicates an immunoreactivity of 70.9 ± 5.8%.
IgG conjugation and indium-111 labeling
Human IgG served as an aspecific control in the experiments, which reflects the non-specific uptake of antibodies in the tumor. Human IgG (Sanquin) conjugation with the bifunctional conjugating agent 2-(4-isothiocyanatobenzyl)-diethylenetriaminepentaacetic acid (p-SCN-Bn-DTPA, Macrocyclics) was performed as described by Ruegg et al.Citation29 Conjugated human IgG was radiolabeled with indium-111 (111In) chloride (Covidien).
Animal experiments
For sc tumor inoculation, cells were harvested by trypsinization and suspended in PBS. In vivo imaging and ex vivo experiments were conducted using female Fox Chase SCID Beige Mouse (CB17.Cg-PrkdcscidLyst bg/Crl) obtained from Charles River (Germany). Mice were sc injected with 5 × 106 FaDu, 4 × 106 QG-56, 4 × 106 H441, or 5 × 106 Calu-1 cells in 0.1 mL in PBS. Tumor growth was followed with caliper measurements. The tracer injection was performed 2 wk after tumor cell inoculation for FaDu; for QG-56 and H441, 3 wk after inoculation; and 10 wk after inoculation for Calu-1.
A dose escalation biodistribution study with 89Zr-RG7116 (1 MBq) was performed in sc FaDu tumor-bearing mice to evaluate dose-dependent tumor uptake of the antibody. Five dose groups of 0.05, 0.3, 1.0, 3.0, and 10.0 mg/kg antibody were included. Each dose was administered to 2 groups (n = 4–6) of animals via intravenous (iv) tail vein injection. In case of doses above 0.3 mg/kg, unlabeled RG7116 antibody was added to reach the desired antibody dose. Biodistribution analyses were performed at 24 and 144 h after injection. 144 h was chosen for biodistribution experiments because, during earlier tracer validations for bevacizumab and trastuzumab in the subcutaneous tumor models, it became clear that the optimal tumor uptake for antibody imaging is measured 6–7 d after tracer injection.Citation14,Citation15 For ex vivo biodistribution experiments, organs are collected and counted for radioactivity of 89Zr-RG7116.
To assess HER3 specific uptake in different tumor models with differential HER3 expression and to assess the time dependent organ distribution of 89Zr-RG7116, microPET imaging was performed in sc xenografted mice (n = 4–6). Mice bearing FaDu, H441, QG-56 and Calu-1 tumors, were scanned at 1, 3, and 6 d after injection of 1.0 mg/kg 89Zr-RG7116 (5 MBq). After the last scan, mice were sacrificed for biodistribution analysis.
To discriminate between specific and non-specific tumor uptake and biodistribution, 111In-IgG (1 MBq) was co-injected with all 89Zr-RG7116 administrations. A ratio between 89Zr and 111In tumor values was calculated for all dose groups at 24 and 144 h after injection in the dose-escalation biodistribution study performed using the FaDu tumor model. To compare specific tumor uptake of 89Zr-RG7116 in different tumor models, tumor: blood ratios were calculated. This enabled comparison of tumor uptake with the amount of antibody present in blood that is able to bind specific or non-specifically in tumor tissue.
Animals were imaged using a microPET Focus 220 rodent scanner (CTI Siemens). After image reconstruction, in vivo quantification was performed with AMIDE Medical Image Data Examiner software (version 0.9.1, Stanford University).Citation30 The data are presented as the mean standard uptake value (SUVmean), which is calculated by dividing the mean ROI activity by the injected dose (corrected for decay) per gram body weight. For ROI measurements, tumor volumes were drawn based on tumor weight measured ex vivo assuming a tissue density of 1 g/mL. For organ tracer distribution quantification, a ROI was drawn in the representative area of the measured organs. For blood pool measurements, a representative area in the heart was selected. Animals were sacrificed at the time of biodistribution, organs and tissues were excised and weighed, samples and primed standards were counted for radioactivity in a calibrated well-type LKB-1282-Compu-gamma system (LKB Wallac) and corrected for physical decay. Ex vivo tissue activity is expressed as percentage of the injected dose per gram tissue (%ID/g). Half of each harvested tumor was paraffin embedded, the other half of the tumors and collected serum were stored in the -80 °C for further analysis. The animal experiments were approved by the animal experiments committee of the University of Groningen.
Ex vivo analysis
Tumors were fixed in 3.8% buffered formaldehyde solution, embedded in paraffin and stained with H&E for histopathological examinations. After scanning of the slides, intratumoral necrotic areas were measured using the Aperio ImageScope v10.2.1.2314 software. IHC with the mouse mAb DAK-H3-IC (DAKO, M7297) against the intracellular domain of human HER3 was performed semi-automatically on the DAKO autostainer (isotype control monoclonal mouse IgG2a, R&D Systems MAB0031; secondary antibody-detection system: Ultra Vision LP).
The concentration of RG7116 in mouse serum was measured in the samples obtained during the dose-escalation biodistribution study using a generic human IgG ELISA assay. Murine serum samples and reference standards were preincubated with digoxigenylated anti-human Fcγ monoclonal antibody and added to biotinylated anti-human Fcγ monoclonal antibody immobilized on a streptavidin coated microtiter plate. Signal was detected using anti-digoxigenin-horseradish-peroxidase (HRP) antibody-conjugate with ABTS-solution as the substrate for HRP.
Statistical analysis
Data are presented as mean ± standard deviation (SD). Statistical analyses were performed using Mann-Whitney test (GraphPad 5.00). A P value ≤ 0.05 was considered significant.
Disclosure of Potential Conflicts of Interest
Keelara Abiraj, Birgit Bossenmaier, Marlene Thomas, Gabriele Hölzlwimmer, and Thomas Friess are employees of Roche.
Acknowledgments
This study was supported by grant RUG 2010–4603 of the Dutch Cancer Society and by Roche.
References
- Citri A, Yarden Y. EGF-ERBB signalling: towards the systems level. Nat Rev Mol Cell Biol 2006; 7:505 - 16; http://dx.doi.org/10.1038/nrm1962; PMID: 16829981
- Wheeler DL, Dunn EF, Harari PM. Understanding resistance to EGFR inhibitors-impact on future treatment strategies. Nat Rev Clin Oncol 2010; 7:493 - 507; http://dx.doi.org/10.1038/nrclinonc.2010.97; PMID: 20551942
- Baselga J, Swain SM. Novel anticancer targets: revisiting ERBB2 and discovering ERBB3. Nat Rev Cancer 2009; 9:463 - 75; http://dx.doi.org/10.1038/nrc2656; PMID: 19536107
- Fornaro L, Lucchesi M, Caparello C, Vasile E, Caponi S, Ginocchi L, Masi G, Falcone A. Anti-HER agents in gastric cancer: from bench to bedside. Nat Rev Gastroenterol Hepatol 2011; 8:369 - 83; http://dx.doi.org/10.1038/nrgastro.2011.81; PMID: 21647199
- Garrett JT, Arteaga CL. Resistance to HER2-directed antibodies and tyrosine kinase inhibitors: mechanisms and clinical implications. Cancer Biol Ther 2011; 11:793 - 800; http://dx.doi.org/10.4161/cbt.11.9.15045; PMID: 21307659
- Capelan M, Pugliano L, De Azambuja E, Bozovic I, Saini KS, Sotiriou C, Loi S, Piccart-Gebhart MJ. Pertuzumab: new hope for patients with HER2-positive breast cancer. Ann Oncol 2013; 24:273 - 82; http://dx.doi.org/10.1093/annonc/mds328; PMID: 22910839
- Amin DN, Campbell MR, Moasser MM. The role of HER3, the unpretentious member of the HER family, in cancer biology and cancer therapeutics. Semin Cell Dev Biol 2010; 21:944 - 50; http://dx.doi.org/10.1016/j.semcdb.2010.08.007; PMID: 20816829
- Narayan M, Wilken JA, Harris LN, Baron AT, Kimbler KD, Maihle NJ. Trastuzumab-induced HER reprogramming in “resistant” breast carcinoma cells. Cancer Res 2009; 69:2191 - 4; http://dx.doi.org/10.1158/0008-5472.CAN-08-1056; PMID: 19276389
- Campbell MR, Amin D, Moasser MM. HER3 comes of age: new insights into its functions and role in signaling, tumor biology, and cancer therapy. Clin Cancer Res 2010; 16:1373 - 83; http://dx.doi.org/10.1158/1078-0432.CCR-09-1218; PMID: 20179223
- Prigent SA, Lemoine NR, Hughes CM, Plowman GD, Selden C, Gullick WJ. Expression of the c-erbB-3 protein in normal human adult and fetal tissues. Oncogene 1992; 7:1273 - 8; PMID: 1377811
- Mirschberger C, Schiller CB, Schräml M, Dimoudis N, Friess T, Gerdes CA, Reiff U, Lifke V, Hoelzlwimmer G, Kolm I, et al. RG7116, a therapeutic antibody that binds the inactive HER3 receptor and is optimized for immune effector activation. Cancer Res 2013; 73:5183 - 94; http://dx.doi.org/10.1158/0008-5472.CAN-13-0099; PMID: 23780344
- Paz-Ares LG, Gomez-Roca C, Delord JP, Cervantes A, Markman B, Corral J, Soria JC, Bergé Y, Roda D, Russell-Yarde F, et al. Phase I pharmacokinetic and pharmacodynamic dose-escalation study of RG7160 (GA201), the first glycoengineered monoclonal antibody against the epidermal growth factor receptor, in patients with advanced solid tumors. J Clin Oncol 2011; 29:3783 - 90; http://dx.doi.org/10.1200/JCO.2011.34.8888; PMID: 21900113
- van Dongen GA, Vosjan MJ. Immuno-positron emission tomography: shedding light on clinical antibody therapy. Cancer Biother Radiopharm 2010; 25:375 - 85; http://dx.doi.org/10.1089/cbr.2010.0812; PMID: 20707716
- Nagengast WB, de Vries EG, Hospers GA, Mulder NH, de Jong JR, Hollema H, Brouwers AH, van Dongen GA, Perk LR, Lub-de Hooge MN. In vivo VEGF imaging with radiolabeled bevacizumab in a human ovarian tumor xenograft. J Nucl Med 2007; 48:1313 - 9; http://dx.doi.org/10.2967/jnumed.107.041301; PMID: 17631557
- Dijkers EC, Kosterink JG, Rademaker AP, Perk LR, van Dongen GA, Bart J, de Jong JR, de Vries EG, Lub-de Hooge MN. Development and characterization of clinical-grade 89Zr-trastuzumab for HER2/neu immunoPET imaging. J Nucl Med 2009; 50:974 - 81; http://dx.doi.org/10.2967/jnumed.108.060392; PMID: 19443585
- Oosting SF, Nagengast WB, Oude Munnink TH, Lub-de Hooge MN, Brouwers AH, Glaudemans AW, De Jong IJ, Scherer SJ, Gietema JA, De Vries EG. 89Zr-bevacizumab PET imaging in renal cell carcinoma patients: feasibility of tumor VEGF quantification. EORTC-NCI-AACR meeting abstracts 2010, #221
- Dijkers EC, Oude Munnink TH, Kosterink JG, Brouwers AH, Jager PL, de Jong JR, van Dongen GA, Schröder CP, Lub-de Hooge MN, de Vries EG. Biodistribution of 89Zr-trastuzumab and PET imaging of HER2-positive lesions in patients with metastatic breast cancer. Clin Pharmacol Ther 2010; 87:586 - 92; http://dx.doi.org/10.1038/clpt.2010.12; PMID: 20357763
- Sharp TL, Glaus C, Fettig N, Hewig A, Ogbagabriel S, Freeman D, Beaupre D, Hwang D, Welch MJ. Pharmacological evaluation of 64Cu-DOTA-AMG888 (U3-1287) in control and tumor bearing mice using biodistribution and microPET imaging. Proc World Molecular Imaging Congress 2011; abstract #T206.
- Malm M, Kronqvist N, Lindberg H, Gudmundsdotter L, Bass T, Frejd FY, Höidén-Guthenberg I, Varasteh Z, Orlova A, Tolmachev V, et al. Inhibiting HER3-mediated tumor cell growth with affibody molecules engineered to low picomolar affinity by position-directed error-prone PCR-like diversification. PLoS One 2013; 8:e62791; http://dx.doi.org/10.1371/journal.pone.0062791; PMID: 23675426
- Aerts HJ, Dubois L, Perk L, Vermaelen P, van Dongen GA, Wouters BG, Lambin P. Disparity between in vivo EGFR expression and 89Zr-labeled cetuximab uptake assessed with PET. J Nucl Med 2009; 50:123 - 31; http://dx.doi.org/10.2967/jnumed.108.054312; PMID: 19091906
- Niu G, Li Z, Xie J, Le QT, Chen X. PET of EGFR antibody distribution in head and neck squamous cell carcinoma models. J Nucl Med 2009; 50:1116 - 23; http://dx.doi.org/10.2967/jnumed.109.061820; PMID: 19525473
- Ping Li W, Meyer LA, Capretto DA, Sherman CD, Anderson CJ. Receptor-binding, biodistribution, and metabolism studies of 64Cu-DOTA-cetuximab, a PET-imaging agent for epidermal growth-factor receptor-positive tumors. Cancer Biother Radiopharm 2008; 23:158 - 71; http://dx.doi.org/10.1089/cbr.2007.0444; PMID: 18454685
- Cai W, Chen K, He L, Cao Q, Koong A, Chen X. Quantitative PET of EGFR expression in xenograft-bearing mice using 64Cu-labeled cetuximab, a chimeric anti-EGFR monoclonal antibody. Eur J Nucl Med Mol Imaging 2007; 34:850 - 8; http://dx.doi.org/10.1007/s00259-006-0361-6; PMID: 17262214
- Misri R, Saatchi K, Ng SS, Kumar U, Häfeli UO. Evaluation of (111)In labeled antibodies for SPECT imaging of mesothelin expressing tumors. Nucl Med Biol 2011; 38:885 - 96; http://dx.doi.org/10.1016/j.nucmedbio.2011.02.013; PMID: 21843785
- Gerdes CA, Nicolini VG, Herter S, van Puijenbroek E, Lang S, Roemmele M, Moessner E, Freytag O, Friess T, Ries CH, et al. GA201 (RG7160): a novel, humanized, glycoengineered anti-EGFR antibody with enhanced ADCC and superior in vivo efficacy compared with cetuximab. Clin Cancer Res 2013; 19:1126 - 38; http://dx.doi.org/10.1158/1078-0432.CCR-12-0989; PMID: 23209031
- Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet 2005; 365:1687 - 717; http://dx.doi.org/10.1016/S0140-6736(05)66544-0; PMID: 15894097
- Seidman AD, Berry D, Cirrincione C, Harris L, Muss H, Marcom PK, Gipson G, Burstein H, Lake D, Shapiro CL, et al. Randomized phase III trial of weekly compared with every-3-weeks paclitaxel for metastatic breast cancer, with trastuzumab for all HER-2 overexpressors and random assignment to trastuzumab or not in HER-2 nonoverexpressors: final results of Cancer and Leukemia Group B protocol 9840. J Clin Oncol 2008; 26:1642 - 9; http://dx.doi.org/10.1200/JCO.2007.11.6699; PMID: 18375893
- Verel I, Visser GW, Boellaard R, Stigter-van Walsum M, Snow GB, van Dongen GA. 89Zr immuno-PET: comprehensive procedures for the production of 89Zr-labeled monoclonal antibodies. J Nucl Med 2003; 44:1271 - 81; PMID: 12902418
- Ruegg CL, Anderson-Berg WT, Brechbiel MW, Mirzadeh S, Gansow OA, Strand M. Improved in vivo stability and tumor targeting of bismuth-labeled antibody. Cancer Res 1990; 50:4221 - 6; PMID: 2364380
- Loening AM, Gambhir SS. AMIDE: a free software tool for multimodality medical image analysis. Mol Imaging 2003; 2:131 - 7; http://dx.doi.org/10.1162/153535003322556877; PMID: 14649056
