Abstract
Antibodies highly specific to human immunoglobulin (Ig) E are capable of selectively blocking the IgE interaction or eliminating IgE-producing cells, thus providing valuable agents for diagnostics and treatment of various allergic illness. An example is omalizumab, a humanized monoclonal anti-IgE antibody that is approved for the treatment of patients with moderate-to-severe allergic diseases in the United States, European Union and other countries. Here, we describe the generation and characterization of a novel human anti-IgE as a single-chain antibody fragment (scFv). The bacterially-synthesized scFv showed high affinity (86 nM) and specificity to the Fc region of human IgE. To our knowledge, this is the first report of the production of a human anti-IgE scFv in E. coli. Its further development as a potential candidate for medical applications is discussed.
Introduction
The human immune system is designed to secrete specific antibodies to protect bodies from being attacked by foreign toxic molecules, viruses and microorganisms. However, some of the secreted immunoglobulin may act against the body itself, thus causing a variety of autoimmune disorders, including allergies and asthma. Of the four types of allergy (I–IV), type I affects almost 25% of the population worldwide,Citation1 and is typically mediated by immunoglobulin (Ig) E through the following mechanisms: the IgE Fc region binds to a Fc receptor (FcεRI) on mast cells in tissue or basophils in the blood and stimulates those cells to release various biological active mediators (histamine and leukotrienes), causing allergic reactions such as asthma and edema.
Studies of the allergic response mechanism suggest that it is possible to prevent or treat allergy diseases by blocking the binding of IgE to its Fc receptor on mast cells and basophils.Citation2 In the past decade, considerable efforts have been made to identify competitors to specifically inhibit the interaction between IgE and FcεRI.Citation3 These include comprehensive screening of engineered proteins, peptides and nucleic acids,Citation4–Citation6 creation of autoantibody responses against the IgE receptor binding siteCitation7,Citation8 or generation of anti-IgE antibodies.Citation9 Highly specific anti-IgE antibodies that are capable of selectively blocking the IgE-FcεRI interaction have proven to be effective agents for treating allergic illnesses. The humanized monoclonal anti-IgE omalizumab is approved for the treatment of patients with moderate-to-severe allergic diseases in the US, European Union and other countries.Citation10,Citation11
We generated a human anti-IgE antibody by screening a library constructed from patients.Citation12 Here, we describe functional expression of the antibody as a single-chain variable fragment (scFv) in the periplasm of E. coli and demonstrate its affinity and antigen specificity. To our knowledge, this is the first report of the production of a functional, human anti-IgE scFv in E. coli. Our results provide a potential candidate for further development in medical applications.
Results
The human origin of the anti-IgE scFv was confirmed by DNA sequencing (). It also reveals that VH1 and Vk1 are used to form the antigen binding site. A number of point mutations are identified (), which might be introduced by somatic maturation in vivo or polymerase chain reaction (PCR) cloning in vitro.
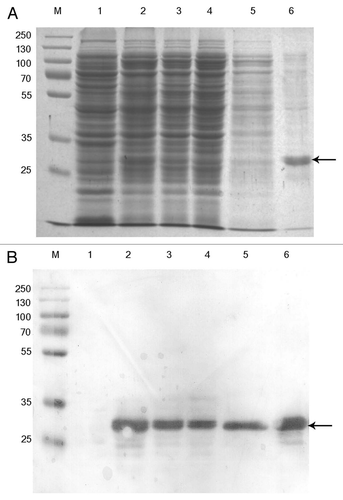
Rosetta™ (DE3) was used to express the scFv fragment in the periplasm and a (His)6 tag was engineered at the c-terminus for detection and purification purposes (). We have shown that, after induction, a protein of the expected size was obtained from the soluble fraction of E. coli by Ni purification under native conditions (), while no corresponding band was detected in the control bacteria (without the induction; , Lane 1). Analysis by western blotting using anti-(His)6 antibody detected the attached c-terminal (His)6-tag (), demonstrating the successful expression of soluble anti-IgE scFv in E. coli.
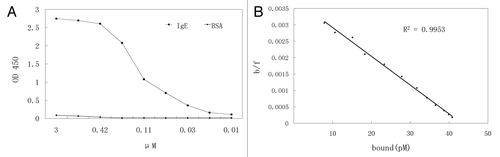
The binding property of the bacterially-synthesised anti-IgE scFv after purification was analysed by ELISA on human IgE-coated wells. This showed that while the binding to IgE coated wells was strongly detected in a series of 2-fold titration, no signal was produced on BSA-coated wells (), which confirmed that the scFv bound specifically to the human IgE molecule.
To examine whether the scFv specifically recognise the Fc region of IgE, a competitive inhibition ELISA was performed in which various amount of free Fc was included to inhibit the interaction between the scFv and IgE-coated wells. The results showed a linear inhibition, i.e., the binding of scFv to IgE decreased in proportion to the increase in the Fc concentration (). Our result demonstrated the specificity of the scFv to the Fc region.
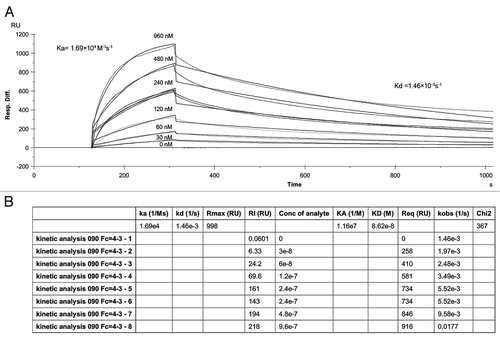
scFv affinity was also determined by using Biacore. The binding kinetics of scFv to the immobilised human IgE molecule are shown in . Based on the Ka of 1.69 × 104 M−1s−1 and Kd of 1.46 × 10−3 s−1 determined by Biacore analysis, the scFv KD (Kd/Ka) was 8.6 × 10−8 M or 86 nM.
Discussion
Interaction between the Fc region of human IgE and its receptor FcεRI on mast cells and basophils plays important roles in initiating allergic responses. A structural study has revealed the IgE-Fc/FcεRI complex at the atomic level.Citation13 Since IgE molecule is well-known as the main mediator responsible for allergic reactions, the identification of inhibitors capable of neutralizing or blocking IgE interactions with the receptor FcεRI could potentially be developed for treatment of the diseases. Although peptides, nucleic acids and small molecules are used as inhibitors, antibodies were proven to be the most effective tools for blocking the specific IgE-Fc/FcεRI interaction.Citation9 Omalizumab (Xolair®), which was directed at the FcεRI-binding site of the human IgE,Citation9,Citation10 was the first humanized monoclonal antibody drug approved for the treatment of patients with allergic asthma. Apart from applications as therapeutics, highly specific anti-IgE antibodies could be valuable reagents for both in vitro and in vivo detection of IgE levels in the blood, e.g., for diagnostics of defined allergen specificities.Citation14–Citation16
As described in this report, we generated a functional human anti-IgE antibody fragment. To our knowledge, this is the first time that a human anti-IgE scFv with high affinity and specificity to the Fc region has been produced. The scFv can be functionally expressed in E. coli (), providing a useful screening system for further improvement of this antibody through molecular evolution. The high affinity (86 nM) of the scFv may also allow for the direct exploitation of its potential for medical applications. For example, it could be used for probing the free IgE molecule level in serum either in vivo or in vitro, or its capacity (either alone or as a fusion partner) for neutralizing/blocking free IgE binding to soluble and membrane FcεRI could be evaluated for therapeutic potential. Alternatively, conjugation of the scFv with a toxin that would lead to elimination of IgE-producing cells in vivo could be examined for possible development.
Materials and Methods
Molecular biology reagents.
Bacterial strain Rosetta™ (DE3) and plasmid PET-22b were from our department's collection. Primers were synthesised from Invitrogen. Restriction enzymes were purchased from TAKARA, China. Taq DNA polymerase was from Qiagen. Human IgE was from CHEMICO, China. Tetramethylbenzidine (TMB) substrate and HRP-linked anti-(His)6 antibody were purchased from Sigma (Cat.# A7058-1). CM5 Chips, N-[3-dimethylaminopropyl]-N9-Ethylcarb-odiimide [EDC], N-hydroxysuccinimide [NHS], 5 M ethanolamine HCl pH 8.5 and Ni sepharose™ 6 Fast Flow were from GE Healthcare. Amicon Ultra-15 (Centrifugal Filter Units-10,000 NMW) was from Millpore.
DNA construction.
Primer HuIgE/B (GCC GGC GAT GGC CAT GGA GGT GCA GCT GGT GCA GTC TGG) and HuAb/F (GCG AAT TCT TAG CAG CCA CCC AGG TGA TGG TGA TGG TGA TGA GAT GGT GCA GCC ACA G) were designed to amplify the single-chain anti-IgE.Citation12 The restriction enzyme sites Msc I and EcoR I are underlined. A hexahistidine (His)6-tag (indicated by italics) was introduced at the c-terminus of scFv for detection and purification purposes. The PCR fragment generated using the two primers was digested by Msc I and EcoR I, followed by ligation into plasmid PET 22b which contains a pelB leader sequence in the front of the scFv for directing the protein into the periplasm of E. coli. The ligation mixture was used to transform E. coli Rosetta™ (DE3) and the resultant clones were screened for the presence of the insert scFv by colony PCR. The correct clone was confirmed by DNA sequencing.
E. coli expression and extraction of the scFv.
Rosetta™ (DE3) carrying the scFv plasmid was grown in 5–10 mL (for a small scale) or 1–2 liter (for a large scale) 2x YT medium containing 50 µg/mL ampicillin, 34 µg/mL chloramphenicol and 0.5% glucose at 37°C overnight. After centrifugation at 1,300x g for 10 min, the bacterial pellet was re-suspended in ½ the original volume of a fresh 2x YT media containing 100 µg/mL ampicillin, 34 µg/mL chloramphenicol and 0.5 mM IPTG. Induction was carried out at 28°C for 4–6 h. After centrifugation at 1,300x g for 10 min, the pellet was collected and used immediately (see below) or stored at −20°C.
To prepare the periplasmic extract, the pellet from 10 mL culture was washed with 1 mL washing buffer containing 20% (w/v) sucrose, 300 mM Tris-HCl (pH 8.0) and 1 mM EDTA. After centrifugation at 1,300x g for 10 min, the pellet was rapidly mixed with 1 ml ice-cold 0.5 mM MgCl2 and incubated on ice for 10 min with a periodic shaking. The supernatant was then recovered as the periplasmic fraction by centrifugation at 16,000x g, 4°C for 10 min. The procedure can be scaled up with a proportional increase of the volumes.
Affinity purification of scFv.
The periplasmic fraction was subjected to Amicon Ultra -15 concentriful filter devices to exchange with a binding buffer (50 mM sodium phosphate buffer containing 500 mM NaCl and 20 mM imidazole, pH 7.4) according to the manufacturer's instruction. Prior to affinity purification, the solution was centrifuged at 16,000x g for 30 min. For the use of Ni column, 20 mL of the solution was added to 1 mL Ni Sepharose 6 Fast Flow which was pre-equilibrated with the binding buffer. After washing with 10 column volumes of the binding buffer supplemented with 50 mM imidazole, the scFv was eluted with 5 column volumes of 50 mM sodium phosphate buffer containing 500 mM NaCl and 500 mM imidazole, pH 7.4. The eluted protein was stored at 4°C or directly used for analysis (see following sections).
ELISA.
96-well polystyrene microtiter plates were coated with 100 µL of human IgE at a concentration of 1 µg/mL in PBS. After overnight at 4°C, the wells were blocked with 100 µL 3% BSA in PBS at 37°C for 1 h. The scFv (either purified or non-purified) was then added to the wells and incubated at 37°C for further 1 h. After three washes with the washing buffer (PBS containing 0.05% Tween 20), a HRP-linked anti-(His)6 antibody in 1:4,000 dilution in PBS, 1% BSA was added and the wells were incubated at 37°C for 1 h. Finally, the wells were washed three times with the washing buffer before the color development, which was performed by detecting the HRP activity using tetramethylbenzidine (TMB) substrate and H2O2 in citrate buffer pH 5.0. The color reaction was stopped with 1 M HCl and optical density read at 450 nm. Competitive ELISA was carried out as described using free Fc as the inhibitor.Citation17 In brief, fixed concentration of scFv (0.16 ug/mL) was mixed with serial dilutions of Fc at concentrations ranging from 1.28–167 nM. After 30 min incubation, the mixtures were transferred to the IgE-coated wells and then follow the procedure described above Bound and free scFv molecules were calculated as the percentage using the reading at OD450.
Biacore analysis.
Human IgE at the concentration of 4 ug/ml was covalently immobilized through free amines to a carboxymethylated sensor chip with a surface density of 4,000–5,000 response units (RU). A Biacore 3000 (GE healthcare) was used to measure the kinetics of scFv over the immobilised human IgE on the chip. Purified scFv were diluted in a series of 2-fold in 10 mM HEPES, 150 mM NaCl, 3 mM EDTA and 0.05% Tween 20. Samples of six different concentrations and assay controls were individually injected sequentially through the IgE coated chip at the rate of 20 µl/min. Biacore data were collected and analyzed by using the BiaEvaluation v. 4.1.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Figures and Tables
Figure 1 (A) DNA and amino acid sequence of the human anti-IgE scFv. IGHV1-2*02 and IGKV1D-12*02 germline sequences are also shown for comparison. Complementarity determining regions (CDRs) are indicated. Residues different to the germline sequences are shown while the identical residues are marked by -. (B) A diagram showing the essential elements of the construct for E. coli expression. T7, T7 promoter; Pel B, the leader sequence; scFv, single-chain antibody fragment. The (His)6-tag and transcription and translation termination region are also indicated.
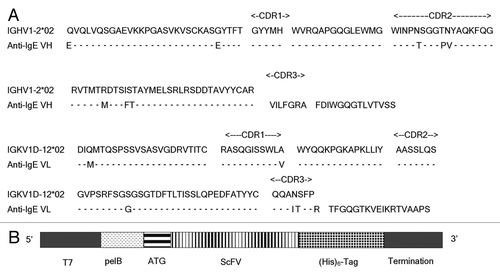
Figure 2 scFv expression in E. coli. (A) Coomassie blue stain. Lane 1, un-induced total cell (prior to IPTG induction); lane 2, induced total cell (at time of the harvest); Lane 3, periplasmic extract; Lane 4, flow through mixture from Ni-sepharose column; Lane 5, washes; Lane 6, elution from Ni-sepharose column. (B) Western blotting of (A) using anti-His antibody as the probe. The samples are in the same order as in (A). The scFv is indicated by arrows. M, the standard markers (KDa).
Acknowledgments
We thank the national “863” scheme, China, for financial support.
References
- Tripathi P, Sigh BP, Arron N. Recombinant allergens-gateway to efficient diagnosis and therapy for atopic asthma and rhinitis. Indian J Allergy Immunol 2006; 20:41 - 51
- Chang TW. The pharmacological basis of anti-IgE therapy. Nat Biotechnol 2000; 18:157 - 162
- Sutton BJ, Beavil RL, Beavil AJ. Inhibition of IgE-receptor interactions. Br Med Bull 2000; 56:1004 - 1018
- Dimov VV, Stokes JR, Casale TB. Immunomodulators in asthma therapy. Curr Allergy Asthma Rep 2009; 9:475 - 483
- Fonseca DE, Kline JN. Use of CpG oligonucleotides in treatment of asthma and allergic disease. Adv Drug Deliv Rev 2009; 61:256 - 262
- Saxon A, Zhu D, Zhang K, Allen LC, Kepley CL. Genetically engineered negative signalling molecules in the immunomodulation of allergic diseases. Curr Opin Allergy Clin Immunol 2004; 4:563 - 568
- Miescher SM, Horn MP, Pachlopnik JM, Baldi L, Vogel M, Stadler BM. Natural anti-FcepsilonRIalpha autoantibodies isolated from healthy donors and chronic idiopathic urticaria patients reveal a restricted repertoire and autoreactivity on human basophils. Hum Antibodies 2001; 10:119 - 126
- Peng P, Liu Q, Wang Q, Rector E, Ma Y, Warrington R. Novel IgE peptide-based vaccine prevents the increase of IgE and downregulates elevated IgE in rodents. Clin Exp Allergy 2007; 37:1040 - 1048
- Hamilton RG Jr, MacGlashan DW, Saini SS. IgE antibody-specific activity in human allergic disease. Immunol Res 2010; 47:273 - 284
- D'Amato G, Salzillo A, Piccolo A, D'Amato M, Liccardi G. A review of anti-IgE monoclonal antibody (omalizumab) as add on therapy for severe allergic (IgEmediated) asthma. Ther Clin Risk Manag 2007; 3:613 - 619
- Nowak D. Management of asthma with anti-immunoglobulin E: a review of clinical trials of omalizumab. Respir Med 2006; 100:1907 - 1917
- Wang MR, Zhang YX, Gao Q, Wu M, He MY. A fully human recombinant anti-IgE antibody 2009; (Patent application no: 200810032639.2, China)
- Garman SC, Wurzburg BA, Tarchevskaya SS, Kinet JP, Jardetzky TS. Structure of the Fc fragment of human IgE bound to its high-affinity receptor FcεRI. Nature 2000; 406:259 - 266
- Qian W, Zhang X, Li B, Zhang D, Tong Q, Chen L, et al. Development and characterization of a novel anti-IgE monoclonal antibody. Biochem Biophys Res Commun 2010; 395:547 - 552
- Kim S, Lee J, Lee SJ, Lee HJ. Ultra-sensitive detection of IgE using biofunctionalized nanoparticle-enhanced SPR. Talanta 2010; 81:1755 - 1759
- Qiao CX, Lv M, Guo LM, Yu M, Li Y, Lin Z, et al. Inhibition of IgE activity to bind its high affinity receptor (FcεRIα) by mouse anti-IgE Cε3∼4 monoclonal antibody (QME5). Int J Biomed Sci 2009; 5:336 - 344
- He MY, Hamon M, Liu H, Corper AL, Taussig MJ. Effects of mutation at the D-JH junction on affinity, specificity and idiotypy of anti-progesterone antibody DB3. Protein Sci 2006; 15:2141 - 2148