Abstract
The DVD-IgTM protein is a dual-specific immunoglobulin. Each of the two arms of the molecule contains two variable domains, an inner variable domain and an outer variable domain linked in tandem, each with binding specificity for different targets or epitopes. One area of on-going research involves determining how the proximity of the outer variable domain affects the binding of ligands to the inner variable domain. To explore this area, we prepared a series of DVD-Ig proteins with binding specificities toward TNFα and an alternate therapeutic target. Kinetic measurements of TNFα binding to this series of DVD-Ig proteins were used to probe the effects of variable domain position and linker design on ligand on- and off-rates. We found that affinities for TNFα are generally lower when binding to the inner domain than to the outer domain and that this loss of affinity is primarily due to reduced association rate. This effect could be mitigated, to some degree, by linker design. We show several linker sequences that mitigate inner domain affinity losses in this series of DVD-Ig proteins. Moreover, we show that single chain proteolytic cleavage between the inner and outer domains, or complete outer domain removal, can largely restore inner domain TNFα affinity to that approaching the reference antibody. Taken together, these results suggest that a loss of affinity for inner variable domains in this set of DVD-Ig proteins may be largely driven by simple steric hindrance effects and can be reduced by careful linker design.
Introduction
Targeting specific disease mediators with monoclonal antibodies (mAbs) is recognized as a very powerful mode of disease modulation. There are currently over 20 US Food and Drug Administration-approved mAb therapeutics on the market for a variety of human diseases.Citation1 As with conventional therapeutics, the increased disease fighting potential of combination therapy with mAbs has also been recognized.Citation2 However, developing two new mAb entities simultaneously could double the cost and effort because it involves preclinical and clinical development, as well as manufacturing, of two independent pharmaceuticals. A potentially more efficient therapeutic modality for targeting two disease mediators simultaneously is the dual specific DVD-Ig protein.
The four chain DVD-Ig protein, first described by Wu et al.Citation3 is a dual-specific, tetravalent immunoglobulin G (IgG)-like molecule that is composed of two heavy chains and two light chains (). Each heavy and light chain has two variable regions connected in tandem by separate linkers; together they form an inner variable domain and an outer variable domain capable of different ligand binding specificities. The inner and outer variable domains of a DVD-Ig protein can be engineered by linking the variable domains from any pair of mAbs while, in many cases, retaining the activities of the parent antibodies. As a therapeutic class, the great potential of the DVD-Ig protein is to simultaneously target two mediators of disease with a single pharmaceutical agent.
Anti-IL12/IL18 and anti-IL1α/IL1β DVD-Ig proteins have been described in references Citation3–Citation5. These reports explain the basic features of DVD-Ig protein design, construction and performance. In each case, the DVD-Ig protein was shown to be capable of simultaneous high affinity binding to both ligands. In addition to being dual-specific, the DVD-Ig protein can be efficiently produced by conventional mammalian expression systems and, as such, can be as easily manufactured as a conventional IgG. Surprisingly, they also exhibit pharmacokinetic and physiochemical properties comparable to conventional IgGs, despite their increased size and complexity.
In 2009 Wu et al.Citation4 described instances where anti-IL1α and anti-IL1β variable domains (VDs) showed reduced affinity when engineered as the inner VD (as compared to either the reference antibody or when engineered as the outer VD). This effect was attributed, at least partially, to steric hindrance due to the proximity of the outer variable domain to the ligand binding site of the inner VD; longer linkers were shown to reduce the affinity loss. Those results pointed to the importance of exploring linker sequence as a key element affecting the function of a DVD-Ig protein. The work presented here was designed to deepen our understanding of this relationship.
Results
DVD-Ig protein production.
A series of DVD-Ig constructs were assembled from the VDs of an anti-TNFα reference mAb and a mAb with specificity toward an alternate therapeutic target. The two VDs were in both possible inner and outer position combinations; DVD-Ig constructs with anti-TNFα as the inner domain were produced with a series of heavy chain (HC) and light chain (LC) linkers as described in . The linkers for DVD-01 and DVD-02 were based on natural linkers found between the constant domain and VD of human antibodies.Citation3 Likewise DVD-03, DVD-04 and DVD-05 contained modified forms of these linkers in which cleavage sites for the endopeptidases thrombin (Thr) or enterokinase (Ek) had been engineered into the light chain linker. In these cases, single-linker cleavage was designed to potentially relieve steric hindrance effects due to the outer domain's proximity to the inner domain without completely removing the outer domain. DVD-06 and DVD-07 were designed with poly-glycine linkers and DVD-08–DVD-12 were designed with linkers based on heavy chain hinge sequences, between CH1 and CH2, from human antibodies.
Proteolytic fragmentation.
The DVD-Ig proteins designed with cleavable light chain linkers were cleaved with either thrombin or enterokinase endopeptidases. Prior to cleavage, reducing SDS-PAGE showed the prominent heavy chain (HC) at ∼68 kDa and a glycosylated form of the light chain (LC) at ∼42 kDa (), as well as a less prominent band at ∼40 kDa that was identified by mass spectrometry as a small population of non-glycosylated light chain (data not shown). Cleavage of the light chain was complete for DVD-05 and nearly complete for DVD-03 and DVD-04 as indicated on reducing SDS-PAGE by the presence of light chain fragments 1 and 2 and the substantial depletion or absence of the original light chain. Fragment 1 is composed of VL2-CL and Fragment 2 is composed of VL1. To evaluate whether the cleaved light chain fragments remained associated, the cleaved proteins were re-purified over protein A. shows that, after washing, the heavy chain and the two light chain fragments of the DVD-Ig protein (including fragment 2) elute together, which suggests that it remains intact after cleavage.
Antigen-binding fragments (Fab) can be routinely generated from mAbs by limited proteolysis with the protease papain.Citation6–Citation8 Papain can cleave antibodies between the CH1 and CH2 domains of the heavy chain generating functional Fab fragments from a given antibody. In a similar way, a Fab of a DVD-Ig protein (DFab) can be generated by papain digestion and purified by protein A flowthrough resulting in a fragment depicted in . A DFab is approximately 75 kDa and retains functional outer and inner VDs. During the process of generating a DFab from DVD-02 via papain digestion a secondary papain cleavage product was also produced, denoted DFab(48k), as depicted in . Both DFab and DFab(48k) fragments from DVD-02 were separately purified by size exclusion chromatography followed by cation exchange. Detailed mass spectrometry analysis (data not shown) revealed the DFab(48k) resulted from proteolysis in both the heavy and light chain linkers (). It is interesting that this secondary cleavage occurs because these linkers were based on natural linkers found in human antibodies and, as such, also occur between the constant domain and the VD in this DVD-Ig protein. However, the linkers between the inner and outer VDs of this DVD-Ig protein are clearly more susceptible to papain cleavage than those between the constant and inner VDs. In this study only DVD-02 was subjected to papain cleavage.
TNFα binding kinetics.
Surface plasmon resonance (SPR) measurements are commonly used to assay the binding kinetics of antibodies. Capture approaches are often used in these assays to achieve oriented surfaces and to avoid avidity effects.Citation9 Here, we used SPR to assay the TNFα binding kinetics for the reference mAb and DVD-Ig proteins using an anti-(Fc) capture approach. For study of fragments that did not contain Fc regions, i.e., DFabs from DVD-02 and the Fab from the reference antibody, an anti-(H+L) capture approach was used. We also assayed intact DVD-02, DVD-01 and reference antibody via anti-(H+L) for direct comparison to the DFab and Fab.
The binding kinetics for the reference mAb and the series of DVD-Ig proteins, partially proteolyzed DVD-Ig proteins, DFab and Fab are listed in and ; the two tables represent two separate studies that employed different assay formats. The binding kinetics reported in were measured from an anti-(Fc) capture format, while those reported in were measured from an anti-(H+L) capture format (as described above and in the materials and methods section). The data revealed interesting relationships for TNFα binding kinetics relative to VD orientation (outer vs. inner), linker sequence, partial structural relaxation (for single linker proteolysis) and complete outer domain removal; for clarity, these relationships were graphed as ka vs. kd plots (–).
The ka vs. kd plot for TNFα binding to the intact reference mAb and DVD-Ig proteins is shown in . The affinities (KDs) of these interactions ranged from 83–13 nM, with the reference mAb at 126 pM. DVD-02 showed the weakest affinity at 13 nM, which was approximately 100-fold weaker than the reference mAb. Off-rates (kd) ranged from 1.47E−04 to 2.39E−04 s−1 (less than a 2-fold range), while the on-rates (ka) ranged from 1.74E+04 to 1.77E+06 M−1s−1 (about a 100-fold range). DVD-01, where the TNFα binding domain is in the outer position, showed very similar binding kinetics for TNFα as the reference mAb. However, the other DVD-Ig proteins, where the TNFα binding domain is in the inner position, showed reduced affinity for TNFα. As shown in , the most striking feature of the affinity differences is that they were largely due to differences in TNFα on-rate (ka). DVD-02 showed the greatest loss of TNFα affinity; this DVD-Ig protein also possessed the shortest pair of linkers between the outer and inner domain. Generally, DVD-Ig proteins with longer linkers (like DVD-05 and DVD-11) show much less loss of TNFα affinity.
Clustering of DVD-Ig proteins can be observed in and correlated to linker sequence and length (); the Table also organizes them according to their affinity, strongest (top) to weakest (bottom). Although there seem to be complex sequence effects on affinity, in general the weaker affinity cluster (Cluster 2) possess shorter linkers than the higher affinity cluster (Cluster 1); also of note, many of the DVD-Ig proteins in cluster 1 contain hinge-based linkers.
Upon light chain cleavage of DVD-03, DVD-04 and DVD-05 with either thrombin or enterokinase the affinity of TNFα for the inner VD is partially restored (). The effect was greatest for DVD-03 and DVD-04, which showed affinity improvements of about 10-fold and 7-fold, respectively, after light chain cleavage. After cleavage, the TNFα affinity of all three cleavable DVD-Ig proteins recovers to a point only about 4-fold lower than the reference mAb, primarily due to on-rate (ka) effects.
DVD-02 was subjected to limited proteolysis by papain to produce a DVD-02-DFab with intact inner and outer VDs and a DVD-02-DFab(48k) in which the outer VD had been removed (). The TNFα binding kinetics for these species, along with their parent molecules and reference mAb and Fab, where measured via the anti-human(H+L) assay format. As shown in , the DVD-02-DFab TNFα affinity is similar to that of the parental intact DVD-02. However, the DVD-02-DFab(48k) shows affinity for TNFα that has recovered to a point that is less than 2-fold weaker compared with the reference mAb in the same assay format.
Discussion
Antibodies are composed of distinct structural domains connected by linkers. The linkers in an antibody molecule are known to impart flexibility between domainsCitation10 and this flexibility is thought to be critical to both the physical properties of the molecule as well as antigen binding. From crystal structures of Fab fragments,Citation11 it is clear that the antigen binding sites of an antibody, are found at the distal position of each arm, facing outward (relative to the constant domains). Likewise, the N-termini of both heavy and light chains are found near the distal portion of each arm. The DVD-Ig protein is constructed with two VDs per arm linked in tandem. The outer VD heavy and light chain C-termini are connected via linkers to the inner VD heavy and light chain N-termini, respectively.Citation3 Positioning and proximity of the outer VD, relative to the inner VD, were found to potentially impact the function of the inner VD.Citation3,Citation4 In this work, we explored the effects of VD orientation and linker sequences further.
The importance of optimizing VD orientation in the DVD-Ig protein, i.e., whether a given VD should be engineered as an inner VD or outer VD, has been previously recognized.Citation3 In some cases, a given VD may retain more activity as an outer VD than as an inner VD. This was clearly the case for the anti-TNFα VD presented in this study, where TNFα binds to DVD-01 (anti-TNFα as outer VD) with kinetics very similar to the reference anti-TNFα mAb; however, it binds to DVD-02 (anti-TNFα as inner VD) with ∼150-fold reduced affinity compared with DVD-01 binding. We also show that the potential negative impact of inner VD orientation may be significantly alleviated by different linker designs. A striking observation about the TNFα affinity difference between DVD-02 and DVD-01 is that the change is almost exclusively due to reduced ka (on-rate); the ka difference between them is ∼100-fold whereas the kd (off-rate) difference is <2-fold. Indeed, the TNFα affinities for the entire set of inner VD anti-TNFα DVD-Ig proteins in this study (DVD-02–DVD-12) were reduced compared with those for DVD-01, primarily due to ka differences. This observation implies that (1) the anti-TNFα binding potential of the inner VD remains intact, (2) the structure of the inner VD is not likely to be significantly perturbed and (3) the reduced affinity may be simply steric in nature due to the proximity of the outer VD.
Linkers between the two VDs of a DVD-Ig protein are critical to both activity and efficient expression.Citation3 Lack of a polypeptide linker between the two VDs may give rise to steric hindrance effects that could cause poor physical properties, aggregation and reduced activity.Citation4 A rational approach to linker design is to use amino acid sequences naturally found in IgGs. Therefore, a linker set engineered into DVD-Ig proteins was based on naturally occurring “elbow” sequences found between the VD and constant domain of human IgG1s. The rationale was that linkers based on these sequences could impart potential structural flexibility between the VDs of a DVD-Ig protein while reducing the likelihood of immunogenicity issues that could arise from unnatural linker sequences. Indeed, it has been demonstrated that DVD-Ig proteins constructed with linkers based on these sequences can show expression yields, physiochemical properties, activity and pharmacokinetics comparable to conventional IgGs.Citation3,Citation4
In this study, DVD-01 and DVD-02 were constructed with linkers of natural origin; however, the considerable reduction in TNFα affinity for DVD-02 led us to develop a series DVD-Ig proteins with linkers designed to potentially restore inner VD TNFα binding activity (). Several approaches were employed in linker design: (1) linkers based on the short and long “elbow” linkers, which also contained protease cleavage sites within the LC linker; (2) longer linkers composed of poly glycine; and (3) longer linkers based on “hinge” sequences naturally found between CH1 and CH2 of human IgGs. The primary hypothesis was that longer linkers would allow the anti-TNFα inner VD to retain a greater fraction of its native affinity. “Hinge” sequences were included as a natural alternative to the “elbow” sequences. Poly glycine linkers were included as a flexible synthetic sequence to probe whether there may be potential sequence-specific effects on activity or if length alone would be a sole driver of affinity restoration. Lastly, single-chain specific proteolytic cleavage sequences were incorporated to allow for targeted proteolysis of the LC only while leaving the HC linkage intact; this was thought to potentially lead to greater steric “relief” resulting in restored inner VD activity.
Longer linkers do generally seem to improve inner VD TNFα affinity relative to shorter linkers for this series of DVD-Ig proteins; however, the relationship also shows a complex dependency on linker sequence. The trend toward higher inner VD TNFα affinity with longer linkers can be appreciated from the loose ka (on-rate) clustering ( and ). On average, DVD-Ig proteins in the higher affinity cluster 1 possess longer linkers than those in the lower affinity cluster 2. The cluster 1 DVD-Ig proteins DVD-05 and DVD-11, which possess the longest two sets of linkers, show the highest inner VD TNFα affinities. Length alone, however, may not be the sole contributor to enhanced inner VD TNFα affinity. For example, DVD-08 was constructed with linkers based on “hinge” sequences while DVD-07's linkers are poly-glycine; these DVD-Ig proteins have nearly identical linker lengths, but show >4-fold difference in TNFα affinity. This was a surprising observation. During the design phase, it was thought that poly-glycine would be the most flexible of linker designs for a given length and provide the greatest degree of freedom to the outer VD, thereby reducing steric effects. Contrary to this hypothesis, it may be that the linker sequences in DVD-08 are semi-ordered and can potentially position the outer VD such that the inner VD is more accessible to the targeted ligand. Future structural studies may shed more light on VD positioning as they relate to linker sequence in the DVD-Ig protein.
Single-chain proteolytic cleavage sites were design features incorporated into the LCs of DVD-03, DVD-04 and DVD-05. This was done to probe potential steric hindrance due to the positioning of the outer VD with respect to the inner VD. As intact DVD-Ig proteins, these showed reduced inner VD anti-TNFα affinity compared with the reference mAb and, again, the loss of affinity seemed to be greatest for those with shorter linkers. After proteolytic cleavage of the LC with the respective endopeptidase, all three of the DVD-Ig proteins showed improved affinity that converged to a point that was only about 4-fold weaker than the reference mAb. The affinity improvement was primarily the result of ka (on-rate) enhancement () and was greatest for DVD-03 and DVD-04, which possess shorter linkers than DVD-05.
The implications of the restored affinity as a result of single-chain proteolytic cleavage are significant. First, it again implies that the inner VD binding potential remains intact and that simple steric hindrance is the root cause for reduced inner VD TNFα affinity. Second, and much more significantly, it was recognized that single chain proteolytic cleavage may provide a means to impart pro-drug like properties to the DVD-Ig protein. For example, there may be instances where reduced inner domain affinity may be desirable during administration of a DVD-Ig protein; however, once at the site of action the DVD-Ig protein could be processed via single-chain cleavage by a specific endogenous protease to “activate” the inner VD affinity. The possible therapeutic applications of engineering specific single-chain proteolytic cleavage sites into the DVD-Ig protein are vast and future studies will likely explore this further.
We also report the generation of a DFab from DVD-02 using papain, which cleaves between CH1 and CH2. A surprising observation during the generation of the DVD-02-DFab was the appearance of a secondary cleavage product, DVD-02-DFab(48k), that we separately isolated and purified. The DVD-02-DFab(48k) arises from papain cleavage of both HC and LC linkers between the inner and outer VDs (). This is a curious observation because the linkers between the inner and outer VDs in DVD-02 are the same sequences as the linkers between the constant domain 1 and the inner VD, yet we observed no indication of cleavage at the later site during DFab generation. The protease sensitivity of this sequence appears to be context dependent. Compared with their counterparts between the constant domain 1 and the inner VD, the VD linkers must be in a different environment or possess different structural features that gives rise to enhanced papain susceptibility. As DVD-02 was the only DVD-Ig protein in this study to be treated with papain, we do not yet know whether the other DVD-Ig proteins are susceptible to this phenomenon. Future structural studies should provide a more detailed assessment of linker structure.
The TNFα binding kinetics of the DVD-02-DFab are very nearly the same as for the intact DVD-02; however, the TNFα affinity for DVD-02-DFab(48k), with the outer domain removed, is almost completely restored to that of the reference mAb (). The rescue of affinity observed for DVD-02-DFab(48k) is startling; the magnitude of the KD change is over 100-fold compared with the intact DVD-02 and over 80-fold compared with the DVD-02-DFab. These observations imply that anti-TNFα as an inner VD is completely competent and linker design is critical to mitigating potential activity losses.
It remains uncertain how generally applicable the linker dependent kinetics results presented here will be to other DVD-Ig proteins and certainly antigen size and cellular location will likely be additional factors to consider. Here, we demonstrated that, for anti-TNFα binding activity, the potential negative impact of inner VD orientation may be significantly mitigated by careful linker design. The variations of inner VD TNFα binding kinetics, as a function of linker sequence and for the different proteolytic products, were almost exclusively due to differences in ka (on-rate). These observations imply that the inner VD remains completely intact and functional and steric hindrance arising from the proximity of the outer VD may be the primary factor for reduced affinity. Indeed, the near complete restoration of affinity observed for DVD-02-DFab(48k) supports this conclusion. The enthalpy and entropy associated with repositioning the outer VD upon ligand binding to the inner VD, as a function of linker sequence, remain to be explored. These are critical components of association that affect the number of productive collisions between the ligand and the inner VD and consequently contribute significantly to the on-rate. Future structural and function studies should shed light on the thermodynamics of inner VD binding as they relate to linker sequence.
Materials and Methods
Cloning.
Amino acid sequences of VDs from an anti-TNFα mAb and a mAb with specificity toward an alternate therapeutic target were codon optimized and synthesized as components for DVD-Ig construct generation. For heavy chain construct design, the two VH sequences were paired, connected with a specific linker sequence and cloned into an Abbott-proprietary pHybE vector with a heavy chain signal peptide and a gamma1 constant region. Similarly, for light chain, the two VL sequences were paired, connected with a specific linker sequence and cloned into a second Abbott-proprietary pHybE vector with a light chain signal peptide and a kappa constant region.
Expression.
DVD-Ig proteins were produced by transient transfection of HEK-293-6E cells. Cells were grown in Freestyle 293 medium (Invitrogen) and transfected with plasmids encoding the light chain and heavy chain of the DVD-Ig construct as per the Invitrogen Freestyle protocol with minor modifications. Media containing the secreted protein was harvested 9–12 days post transfection. The volume of transfection was 0.5 L in a 2 L roller bottle.
Protein purification.
DVD-Ig proteins were purified using MabSelect SuRe columns (GE Healthcare). The proteins were loaded onto MabSelect SuRe, washed with PBS and eluted with 50 mM Citric acid, pH 3.5. The proteins were then dialyzed into PC buffer (10 mM Na2HPO4, 10 mM citric acid, pH 6.0).
Proteolytic fragmentation.
DVD-03 and DVD-05 were cleaved by incubating with 1 U/mg enterokinase for 48 h at room temperature. DVD-04 was cleaved similarly with 5 U/mg thrombin ().
Papain (Worthington Biochemical) was activated by diluting the papain suspension to 1 mg/ml in PBS, pH 7.4, containing 50 mM cysteine and incubating for 30 min at 37°C. The activated papain was added to the DVD-Ig protein at a 1% (w/w) ratio. The DVD-Ig protein concentration was 2 mg/ml in PBS and the final cysteine concentration was 1 mM. The digest was incubated at 37°C and was stopped by adding iodoacetamide to a 5-fold excess over cysteine. Following digestion the material was passed through Protein-A agarose (Sigma) to remove the Fc domain and remaining undigested DVD-Ig protein. The Protein-A flowthough was concentrated and applied to Superdex 200 (GE) to separate the two cleaved products, DFab and DFab(48k), which were then separately purified by SP cation exchange (Tosoh Bioscience).
Binding kinetic measurements.
Biacore 2000 and Biacore T100 instruments were used to measure the kinetics of TNFα binding to the test reagents (anti-TNFα reference mAb, DVD-Ig proteins, partially proteolyzed DVD-Ig proteins, Fabs and DFabs). The assay format was either Fc-based capture via immobilized anti-human(Fc) (Pierce 31125) for intact mAb, DVD-Ig proteins and partially proteolyzed DVD-Ig proteins or (H+L)-based capture via immobilized anti-human(H+L) (Pierce 31119) for Fabs and DFabs. A standard amine coupling protocol was employed to immobilize the capture reagents via primary amines to the carboxy-methyl (CM) dextran surface of CM5 sensorchips (Biacore). The assay buffer was HBS-EP+ (Biacore) [10 mM HEPES, pH 7.4, 150 mM NaCl, 3 mM EDTA, 0.05% P20 surfactant]. During the assay, all measurements were referenced against the capture surface alone. Each assay cycle consisted of the following steps: (1) Capture of test reagent; (2) Analyte injection over both reference and test surface, 240 ul at 80 ul/min, after which the dissociation was monitored for 900 seconds at 80 ul/min; (3) Regeneration of capture surface with low pH glycine. For kinetic determinations, the analyte injections were randomized 3-fold concentrations series of TNFα and buffer only. Data were processed and fit to a 1:1 binding model using Scrubber 2 (BioLogic Software) to determine the binding kinetic rate constants, ka (on-rate) and kd (off-rate) and the affinity, KD. Binding curves with fits to the data can be found in supplemental materials.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Financial Support
This work was funded by Abbott Laboratories.
Abbreviations
| DVD-Ig™ | = | dual variable domain immunoglobulin |
| DVD | = | short notation for a DVD-Ig protein, DVD followed by a number (for example DVD-01) refers to a specific DVD-Ig protein |
| DFab | = | antigen binding fragment of a DVD-Ig protein |
| TNF | = | tumor necrosis factor |
| VD | = | variable domain |
| HC | = | heavy chain |
| LC | = | light chain |
| mAb | = | monoclonal antibody |
Figures and Tables
Figure 1 The schematic depicts the general structure of a DVD-Ig protein where CH and VH refer to constant and variable heavy chain regions, respectively, and CL and VL refer to constant and variable light chain regions, respectively. CD and VD refer to constant domain and variable domain, respectively. The variable regions form tandem inner and outer variable domains, connected by both light and heavy chain linkers, on each arm of the DVD.
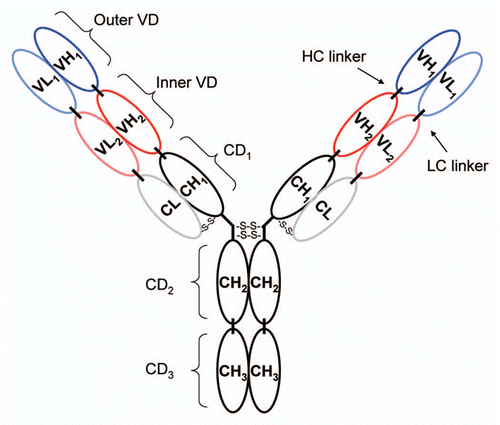
Figure 2 Cleavage of DVD-03, DVD-04 and DVD-05 (referred to simply as 03, 04 and 05) with entorokinase or thrombin visualized by reducing SDS-PAGE. (A) The DVD-Ig proteins before (−) and after (+) cleavage with either enterokinase or thrombin. (B) The cleaved DVD-Ig proteins after purification on Mab Select SuRe columns. HC and LC refer to the heavy and light chains, respectively. Frag 1 refers to the cleavage product of the light chain consisting of the constant region and the inner variable region; Frag 2 refers to the cleavage product of the light chain consisting of the outer variable region.
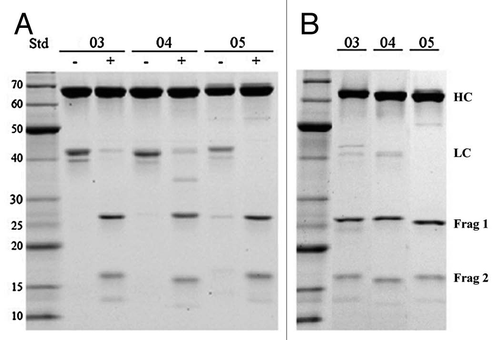
Figure 3 Papain cleavage can be used to generate functional fragments from DVD-Ig proteins. (A) Papain can cleave a DVD-Ig protein between CH1 and CH2 resulting in a product denoted as a DFab that contains intact outer and inner variable domains. (B) Papain cleavage of DVD-02 resulted in an additional secondary product, denoted DFab(48k) where papain cleavage also occurred in both heavy and light chain linkers between the inner and outer variable domains.
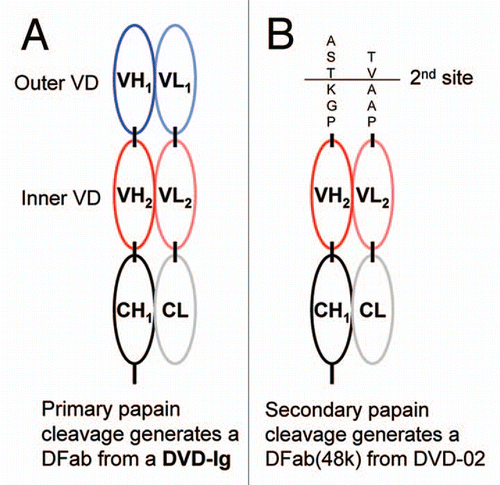
Figure 4 ka vs. kd plot derived from data presented in for TNFα binding kinetics of the intact reference mAb and DVD-Ig proteins. Diagonals show affinities (KD); the vertical and horizontal dashed lines intersect at the reference mAb kinetic constants.
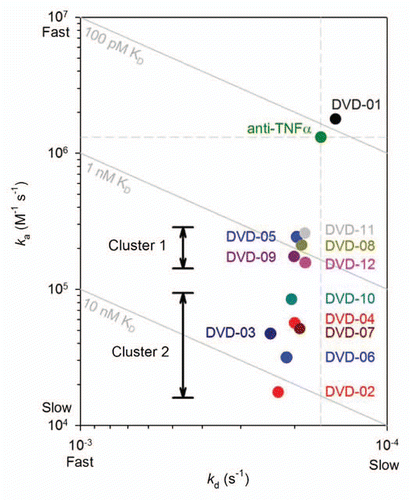
Figure 5 ka vs. kd plot derived from data presented in for TNFα binding kinetics of the intact reference mAb and DVD-03, DVD-04 and DVD-05 before (solid circles) and after (empty circles) cleavage of the light chain linkers with either enterokinase or thrombin proteases. Diagonals show affinities (KD); the vertical and horizontal dashed lines intersect at the reference mAb kinetic constants.
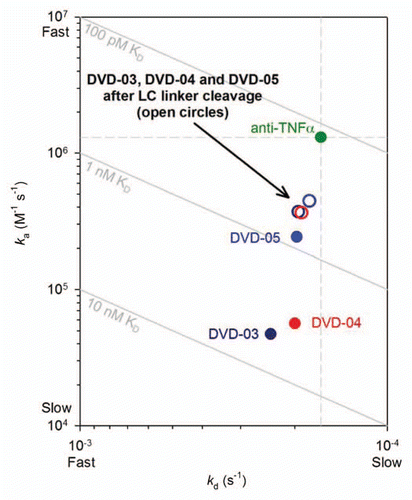
Figure 6 ka vs. kd plot derived from data presented in for TNFα binding kinetics (anti-human(H+L) assay format). Solid circles are the intact reference mAb, DVD-01 and DVD-02; empty circles are the reference Fab, DVD-02-DFab and the DVD-02-DFab(48k). The red arrow highlights the difference in binding kinetics for the DVD-02-DFab and the DVD-02-DFab(48k). Diagonals show affinities (KD); the vertical and horizontal dashed lines intersect at the reference mAb kinetic constants when assayed via anti-human (Fc).
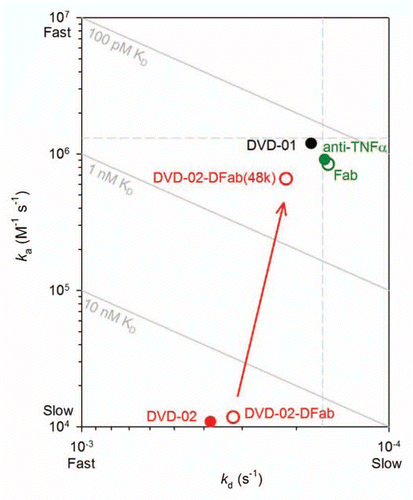
Table 1 DVD-Ig protein identities
Table 2A TNFα binding kinetics (via anti-hu Fc)
Table 2B TNFα binding kinetics (via anti-hu H+L)
Table 3 DVD-Ig protein affinity clusters
Additional material
Download Zip (298.6 KB)Acknowledgments
We would like to thank Dr. Diana Steel (Abbott Bioresearch Center, Worcester, MA) for guidance and assistance during manuscript preparation.
References
- Enever C, Batuwangala T, Plummer C, Sepp A. Next generation immunotherapeutics—honing the magic bullet. Curr Opin Biotechnol 2009; 20:405 - 411
- Uno T, Takeda K, Kojima Y, Yoshizawa H, Akiba H, Mittler RS, et al. Eradication of established tumors in mice by a combination antibody-based therapy. Nat Med 2006; 12:693 - 698
- Wu C, Ying H, Grinnell C, Bryant S, Miller R, Clabbers A, et al. Simultaneous targeting of multiple disease mediators by a dual-variable-domain immunoglobulin. Nat Biotechnol 2007; 25:1290 - 1297
- Wu C, Ying H, Bose S, Miller R, Medina L, Santora L, et al. Molecular construction and optimization of anti-human IL-1alpha/beta dual variable domain immunoglobulin (DVD-Ig) molecules. mAbs 2009; 1:339 - 347
- Wu C, Ghayur T, Salfeld J. Generation and Characterization of a Dual Variable Domain Immunoglobulin (DVD-Ig™) Molecule in Kontermann R, Dübel S, Eds. Antibody Engineering 2010; 2:239 - 250
- Andrew SM, Titus JA. Fragmentation of immunoglobulin G. Curr Protoc Cell Biol 2003; 16:4; PMID: 18228421
- Goding JW. Purification, fragmentation and isotopic labeling of monoclonal antibodies. Monoclonal Antibodies: Principles and Practice 1996; third edition San Diego CA Academic Press 192 - 233
- Parham P. On the fragmentation of monoclonal IgG1, IgG2a and IgG2b from BALB/c mice. J Immunol 1983; 131:2895 - 2902
- Myszka DG. Improving biosensor analysis. J Mol Recognit 1999; 12:279 - 284
- Sandin S, Ofverstedt LG, Wikström AC, Wrange O, Skoglund U. Structure and flexibility of individual immunoglobulin G molecules in solution. Structure 2004; 12:409 - 415
- Evans EJ, Esnouf RM, Manso-Sancho R, Gilbert RJ, James JR, Yu C, et al. Crystal structure of a soluble CD28-Fab complex. Nat Immunol 2005; 6:271 - 279