Abstract
This review illustrates the salutary effects of neonatal Fc receptor (FcRn) overexpression in significantly improving humoral immune responses in the generation of antibodies for immunotherapy and diagnostics. These include: (1) improved IgG protection; (2) augmented antigen-specific humoral immune response with larger numbers of antigen specific B cells, thus offering a wider spectrum of clones; (3) generation of antibodies against weakly immunogenic antigens; (4) significant improvements in the number and substantial developments in the diversity of hybridomas. FcRn transgenesis thus confers a number of practical benefits, including faster antibody production, higher antibody yields and improved generation of hybridomas for monoclonal antibody production. Notably, these efficiencies in polyclonal antibody production were also demonstrated in FcRn transgenic rabbits. Overall, FcRn transgenic animals yield more antibodies and provide a route to the generation of antibodies against antigens of low immunogenicity that are difficult to obtain using currently available methods.
Introduction
Maintenance of antibody (Ab) levels requires continuous secretion of immunoglobulin (Ig) by plasma cells and protection from degradation. IgG is a class of Abs that is unique to mammals. It is the most abundant Ab in serum and is also passively transferred to mammalian offspring. From the standpoint of therapeutic or diagnostic Ab reagent production, it is the most important Ab class when preparing an Ab reagent.
In 1958, Brambell described a saturable receptor that mediates the transport of maternal γ-globulin to the fetus;Citation1 he then inferred the presence of a similar or identical receptor that protected gamma-globulin from catabolism to make it the longest surviving of all plasma proteins.Citation2 At about the same time, it was shown that 7 S γ-globulin (IgG) is the fraction of Ig that was protected by such a mechanism;Citation3 a few years later, it was also shown IgG that mediates maternal immune transport in mammals.Citation4,Citation5
The neonatal Fc receptor (FcRn) was first identified in the 1970s as the protein that mediates transfer of maternal, milk-borne IgGs across the rodent neonatal intestine.Citation6 Subsequently, FcRn was shown to be a heterodimer of two polypeptides that binds IgG in a strictly pH dependent way, with binding occurring at slightly acidic pH and no detectable binding at pH 7.4.Citation7,Citation8 Later it was shown that the functional FcRn is composed of an MHC class-I like α-chain and β2-microglobulin (β2m).Citation9 FcRn orthologs have been isolated from mouse, rat, human, sheep, cow, possum, pig and camel, suggesting that this receptor is present in essentially all mammalian species.Citation10,Citation11 The unique ability of this receptor to modulate the half-life of IgG and albumin has guided engineering of novel therapeutics.Citation12,Citation13 More recently, several publications have shown that FcRn plays major roles in antigen-IgG immune-complex phagocytosis by neutrophils,Citation14 and also in antigen presentation of IgG immune complexes by professional antigen presenting cells.Citation15–Citation18
We and others have shown that higher than normal expression levels of FcRn reduced exogenous IgG catabolism in transgenic animals, resulting in higher circulating levels of IgG.Citation19–Citation21 Our more recent studies have demonstrated that FcRn overexpression in transgenic mice not only augments rescue of antigen-specific IgG, but also enhances the expansion and diversity of antigen-specific B cells and plasma cells in secondary lymphoid organs. Furthermore, we found that these transgenic mice were able to mount substantial humoral responses against weakly immunogenic antigens and to improve hybridoma production efficiency without any sign of autoimmunity.Citation22–Citation24
We summarize here the effects that contribute to the enhancement of IgG formation and protection in transgenic animals that overexpress FcRn. We also discuss the emerging opportunities enabled by these advances for medical and biological applications of monoclonal and polyclonal antibodies.
Ab Reagents are Critical in Immunotherapy and Diagnostics
Abs raised in a number of different species serve as powerful tools in biology and medicine. These molecules were initially produced by immunization of rabbits and guinea pigs with proteins and chemical compounds of interest in the presence of adjuvants. When large volumes were needed, goats and horses were used. In some cases, chicken were used to maximize the phylogenetic distance between the source of the foreign antigen and the host, since closely-related species may be tolerant to shared epitopes. Many schemes have been described for preparing such reagents, each promoted by its inventor as an improvement over previous protocols. These schemes differ in terms of antigen dosage, site of immunization, immunization schedule and choice of adjuvant.
More recently, it has been shown that B cell clones can be directly derived from peripheral blood mononuclear cells (PBMC) and can be recovered from the same species, e.g., human blood.Citation25 However, the ever growing list of targets and the regulatory requirements suggest that scientific and medical research would benefit from a more versatile version of an otherwise well-established monoclonal Ab (mAb) technology. This methodology has the potential advantage of minimizing the regulatory issue since human mAbs are made from human B cells, but it does not provide a means of improving antibody quality since deliberate immunization of humans to improve the quality of the antibodies they make for use by others would evoke ethical concerns. Thus, improved therapeutic antibodies will require improving current mAb technology in mice, some other rodents and rabbits, as well as humanizing them. In an effort to achieve better quality antibodies in such animals, we developed novel FcRn overexpressing transgenic animals that use an FcRn from a species that binds the endogenous IgG of the organism to increase the efficiency of antibody production.
We reported that the transgenic insertion of a construct that contains FcRn in mice is associated with the overexpression of the gene.Citation21 FcRn protein is transcriptionally regulated and multiple copies of the inserted transgenic construct are required to achieve optimal expression levels.
We also showed that the transgenic FcRn overexpression technology can be applied in other species, e.g., rabbit,Citation26 and that animals carrying and expressing multiple copies of FcRn can be safely bred, resulting in offsprings with elevated expression of FcRn and displaying enhanced immune responses, clearly demonstrating that FcRn transgenic lines can be generated for experimental and developmental purposes.
Transgenic Mice that Overexpress FcRn Show Improved IgG Protection
FcRn has been shown to play a central role in regulating the transport of maternal IgG to fetuses or newborns within and across cells of diverse origin, and it also serves to rescue IgG and albumin from degradation, thereby regulating their homeostasis throughout adult life. The multiple functions of FcRn are dependent on its ability to sort monomeric IgG away from lysosomal degradation within cells and release bound cargo during exocytic events at the plasma membrane (). The fact that the FcRn salvage pathway is saturable is a well-known phenomenon referred to as concentration-catabolism effect or fractional catabolic rateCitation27–Citation32 and caused by the fact that the pool of FcRn available in cells to recycle or transport its ligand can be limited. Thus, when FcRn is fully saturated, the unbound ligand is cleared, primarily through lysosomal degradation (). At low to physiological serum IgG concentrations, there is sufficient FcRn to rescue IgG efficiently. However, protocols for the production and subsequent maintenance of high levels of antigen-specific polyclonal antibody require hyperimmunization and even though serum IgG levels may exceed normal levels following immunization, its rate of breakdown is also exponentially increased.Citation2,Citation33 Therefore, frequent immunizations are required to maintain high levels of antigen-specific IgG.
In human therapy, this property can be exploited with benefit in treatment of autoimmune disorders with humoral involvement. High-dose intravenous IgG (IVIg) therapy is thought to act at least partially through FcRn saturation, flushing the body of intact, endogenous IgG, including that which is pathogenic.Citation34 In addition, exploitation of the Fc/FcRn interaction is proving to be a generalized way to extend pharmacokinetics of the therapeutic mAb and Fc-fusion proteins.Citation11,Citation12 Pharmacokinetic studies in mice lacking the endogenous FcRn and transgenic for human FcRn (hFcRn),Citation35 showed that the efficiency of human IgG (hIgG) protection was higher when mice expressed high level of hFcRn. This model also proved useful for studies of the catabolism of different hIgGs.Citation20
In our earlier studies, we cloned the bovine FcRn (bFcRn),Citation36 and confirmed the presence of its transcripts in multiple mucosal epithelial and capillary endothelial cells, which are considered to transport or protect IgG as in other mammalian species.Citation37–Citation39 Furthermore, our in vivo studies in “normal” and transchromosomic cattle expressing hIgGCitation40 indicated that the bFcRn is involved in IgG catabolism in this species, and that the bFcRn binds and efficiently protects both bovine and human IgGs.Citation39 We recently created two types of transgenic mice that overexpressed the bFcRn to analyze its regulation, its role in mammary gland IgG secretion and its function in the humoral immune response.
In our first model, transgenic mice (on Chinese Kunming White genetic background) that carry the endogenous mouse FcRn (mFcRn), and transgenes encoding the bFcRn α-chain and bovine β2m and driven by the mammary gland-specific β-casein promoter, express high levels of the bFcRn in their lactating mammary glands. We used this model to test whether this receptor mediates the transfer of IgG from plasma to milk. Significantly increased IgG levels were observed in the sera and milk from transgenic animals, suggesting that the overexpressed bFcRn could bind and protect endogenous mouse IgG (mIgG) and thus extend its lifespan. We also found that injected hIgG showed a significantly longer half-life (7–8 days) in the transgenic mice than in controls (2.9 days). Altogether, the data suggested that bFcRn could bind both mouse and human IgGs, showing a cross-species FcRn-IgG binding activity. However, we found no selective accumulation of endogenous mIgG or injected bovine IgG (bIgG) in the milk of the transgenic females,Citation19 supporting a previous hypothesis that the role of FcRn in the mammary gland is to recycle IgG from this tissue to the blood instead of secreting it to the milk.Citation41
In our second model, transgenic mice (originally in FVB/N genetic background) carry a 102 kb bovine genomic fragment in a bacterial artificial chromosome (BAC) containing the bFcRn α-chain gene (bFCGRT) with its 44 kb 5′ and 50 kb long 3′ flanking sequences. Mice in one line (#14), carry 2 copies if they are heterozygous (hemizygous) (FVB/N_Tg2) or 4 copies if homozygous (FVB/N_Tg4), while mice in the other line (#19) carry 5 copies if they are hemizygous (FVB/N_Tg5) or 10 copies if homozygous (FVB/N_Tg10) of the bFCGRT, respectively, together with the endogenous mFcRn. bFcRn was detected in multiple tissues that express FcRn endogenously, and its expression was copy-number related (at both the mRNA and protein levels). FVB/N_Tg4 demonstrated significantly extended half-life of mIgG, indicating that bFcRn forms a functional complex with the mouse β2m and thus binds and protects mouse IgG.Citation21 These experiments also suggest that the cytoplasmic domain of the bFcRn is properly involved in signal transduction in mouse cells. Although the cytoplasmic domain of the bFcRn is shorter by 10 amino acid residues than the mFcRn,Citation36 they do not differ in the key cytosolic tail motifs that are important for intracellular trafficking.Citation11 We also found that injected hIgG showed a significantly longer half-life in FVB/N_Tg4 mice (7.6 days) than in controls (4.5 days) (unpublished observation). The IgG clearance results of these transgenic mice that overexpress FcRn clearly suggest a correlation between the levels of expression of FcRn and the protection of IgG ().
Augmented Antigen-Specific Humoral Immune Response and Increased Numbers of Antigen-Specific B Cells in bFcRn Transgenic Mice
It was of greatest interest to know whether better protection of IgG in bFcRn transgenic mice results in increased levels of antigen-specific antibody and B cells following immunization. Using mice carrying extra copies of the bFcRn α-chain,Citation21 we demonstrated that immunization with ovalbumin (OVA), TNP haptenated protein and, interestingly, an influenza vaccine, generated significant increases in the immune response compared with wild-type controls.Citation24 We also created a congenic strain that carries five copies of the bFcRn on BALB/c genetic background (BALB/c_Tg5) that showed similar immune responses, which indicated that these FcRn-mediated effects are not strain dependent. We demonstrated that the transgene (bFCGRT) was integrated as tandem repeats in the two bFcRn transgenic lines (#14 and #19) in two different chromosomes, indicating that the immune phenotype we observed was not due to insertional mutagenesis of unidentified gene(s) at transgene integration sites, but was dependent on bFcRn overexpression. In all our experiments, transgenic mice generated multiple fold higher levels of antigen-specific IgG titers compared with their controls and the average affinity of the antigen-specific Abs generated in transgenic mice were at least as good as in the wild-type controls, implying appropriate affinity maturation in both groups. Importantly, the virus neutralization capability of the influenza specific Abs was doubled in transgenic sera as compared to the wild-type controls. The peak value of the IgG levels in transgenic mice was very high in many cases (around 40 mg/ml in OVA immunization) and persisted relatively long times,Citation24 supporting previous findings that high IgG levels were maintained in transgenic animals overexpressing the bFcRn.Citation19,Citation21
Measurements made during several studies revealed that not only the antigen-specific IgG, but also the IgM titers, were increased during the secondary immune response in transgenic but not in wild-type mice.Citation22–Citation24 Since IgM does not interact with FcRn,Citation10 we concluded that the robust antigen-specific Ab production in these transgenic animals was the result of the additive effect of a better IgG protection and an augmented immune response in lymphoid organs. This assumption was confirmed by the findings that after immunization, the spleens from transgenic mice were significantly larger and contained many more cells compared with the wild-type controls. Moreover, an enhanced expansion of antigen-specific B-cell clones in the spleen of the transgenic mice was observed ().Citation22–Citation24 Pursuit of this finding by analysis of the splenic cell population before and after OVA immunization showed that the spleen of the transgenic mice contained slightly more B and T cells, but twice to three times as many granulocytes, dendritic cells and plasma cells as their wild-type controls.Citation22–Citation24
High Level of Specific Antibodies against Weakly Immunogenic Antigens: Influenza Epitope in bFcRn Transgenic Mice
The higher level of antigen-specific IgG in transgenic animals leads to the formation of more immune complexes (ICs). The ability of these ICs to induce potent humoral immune responses has long been known. Keler et al. have shown that targeting foreign antigen to human FcγRI (CD64) in transgenic mice expressing human CD64 can overcome immunological non-responsiveness to a weak immunogenic antigen.Citation42 In this case the antigen is linked to an IgG/Fc region as a specific targeting molecule and thus, due to feasibility reasons, this approach is intended to facilitate human vaccinationCitation43 instead of routine use in Ab production. Among the possible explanations for the increased B-cell activity in bFcRn transgenic mice is the much increased antigen-specific IgG level that results in more ICs, and thus mimics the natural mechanism to target the antigen to Fc receptors. Furthermore, FcRn overexpression leads to augmented antigen processing in professional antigen presenting cells (unpublished data), which also increases B-cell activation. The elevated antigen-specific IgM level, as well as the many more antigen-specific IgM producer cells (analyzed by ELISPOT assays) observed during the secondary immune response in the transgenic mice, are the result of the more potent activation of naïve B cells in transgenic mice, which suggests increased diversification of the antigen-specific Ab repertoire through the recruitment of novel B-cell clonotypes as was demonstrated in a recent study in reference Citation44.
One of the interesting questions surrounding this augmented immune response is whether these transgenic mice would effectively induce immune responses to weakly immunogenic antigens. To address this question, we immunized the bFcRn transgenic mice with a series of weak antigens from influenza virus and a G protein-coupled receptor.
In one of these experiments, we used a highly conserved hemagglutinin subunit 2 (HA2)-based synthetic peptide that was recently found to be effectively targeted by neutralizing antibodies.Citation45–Citation47 Using an ELISA system, we found that, whereas wild-type mice showed a weak immune response and developed only a de minimis amount of antibody against the epitope, FcRn overexpressing transgenic animals mounted a robust reaction expressed in specific antibody titers on day 28 that continued to rise through day 50. Consistent with our previous data, the enhanced immune response resulting from the FcRn overexpression was also associated with a substantial increase in the number of spleen derived B cells, dendritic cells, granulocytes and plasma cells ().Citation22
Based on this observation, we propose that transgenic mice that overexpress bFcRn offer major advantages in antibody formation by allowing the generation of Abs (and hybridomas) to weakly immunogenic antigens that otherwise would be difficult or even impossible to effectively target. Experiments conducted in collaboration with other teams using a selection of their targeted antigens corroborated these findings.Citation48
Transgenic bFcRn-Mediated Immune Response Augments the Diversity of Antibodies Induced
Encouraged by experimental results that consistently demonstrated a superior immune response capability in FcRn overexpressing animals, we investigated the diversity of the induced antibodies in these animals. We have recently shown by using epitope mapping that the addressed number of epitopes is substantially increased in our transgenic animals (unpublished data).
FcRn Overexpression Improves Hybridoma Production Efficiency
mAbs have become essential tools in scientific, diagnostic and therapeutic applications. Increasing demand for new or more specific mAbs, as well as efforts to reduce the number of laboratory animals, has led to development of genetically modified mouse strains that potentially increase hybridoma production. As antigen-specific B cells in the spleen were multiplied in the bFcRn transgenic mice,Citation22,Citation24 we speculated that this phenomenon might result in higher hybridoma production in these animals. To address this question, we immunized these transgenic mice and their wild-type controls with trinitrophenylated proteins, generated hybridomas and analyzed their numbers and specificities. We observed that transgenic mice generated a 3- to 5-fold increase in antigen-specific IgG titers and had significantly larger spleens containing higher number of antigen-specific B cells and plasma cells, analyzed by ELISA and ELISPOT assays. Fusion of the isolated splenocytes with standard mouse myeloma cells (SP2/0-Ag14) resulted in a 2- to 6-fold increase in number of hapten- or carrier-specific IgM and IgG positive microcultures, indicating that overexpression of the bFcRn does not inhibit the fusion or reduce viability of the hybridomas. We also analyzed the hybridization frequencies (number of hybridoma clones per 108 spleen cells used in the fusion) and found a several-fold increase in antigen-specific microcultures per splenocytes in transgenic mice compared with controls.Citation23 More recent experiments conducted in collaboration with other teams using a selection of their targeted antigens corroborated these findings.Citation48
FcRn Overexpression Does Not Elicit Autoimmunity
Whereas further advances in animal immunization technologies are expected to be slim, transgenic animals have the potential to substantially improve antibody production.Citation49 Previous publications have described the use of genetically altered mouse strains deficient in genes that inhibit B-cell apoptosis or the elimination of the FcγRIIB that inhibits B-cell activation (Fas deficiency, Bcl-2 transgenesis and FcγRIIB deficiency) to improve the efficiency of hybridoma production.Citation50–Citation53 Although, the humoral immune response is augmented in these mice, they generate a large number of autoreactive B cells. In addition, they spontaneously develop immune complex-mediated diseases.Citation54–Citation58 These examples demonstrate that immune hyper-responsiveness can result in vulnerability to autoimmune disease.
To rule out the possibility that enhanced humoral responses in bFcRn transgenic animals are accompanied by dysregulation of B cell selection, we carried out an antibody profiling assay suitable for the monitoring of autoimmune diseases.Citation59 Non-immunized, 7–8 months old bFcRn transgenic animals showed the same general antibody profile as wild type littermates, with no detectable antinuclear antibodies. Thus, enhanced immune responsiveness in these transgenic mice did not result in the development of spontaneous autoimmunity and autoimmune characteristics do not limit the use of these animals in Ab production.Citation23
Transgenic-FcRn Technology also Acts through Immune Complexes Recognized by Neutrophils, Dendritic Cells
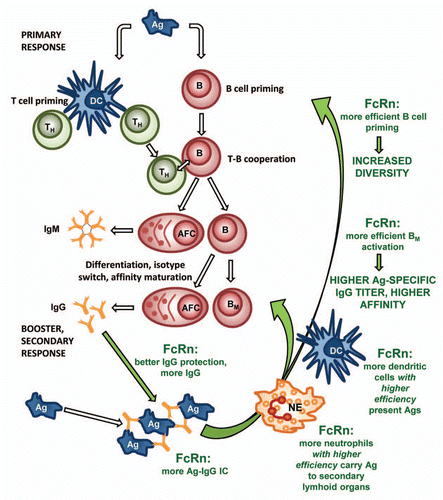
The ability of ICs to induce potent humoral immune responses has long been known. A series of early experimentsCitation60–Citation63 demonstrated the activating capacity of these complexes, finding them able to enhance Ab production. More recently, it was demonstrated that in the presence of ICs formed in vivo between the antigen and pre-existing Abs from the primary response activate naïve B cells, inducing them to respond with accelerated kinetics and increased magnitude during the secondary immune response.Citation44,Citation64 Based on these reports, we propose that the elevated antigen-specific IgM and IgG levels during secondary immune response were the result of the more potent activation of naïve and memory B cells in transgenic mice. An important implication of the augmented naïve B-cell activation during antigen re-exposure in transgenic mice is that it increases diversification of the antigen-specific Ab repertoire.
Our data show a robust neutrophil granulocyte influx in immunized wild-type and transgenic mice. This is consistent with other recent observations, showing that in the presence of ICs the main antigen-specific cells recruited in draining lymph nodes were neutrophils.Citation65–Citation67 Since transgenic mice produced much more antigen-specific IgG than the controls, we concluded that the difference in the number of neutrophils we observed in the transgenic compared with the wild-type mice can be explained at least partly by the greater amount of antigen-IgG ICs formed or transported by neutrophils in transgenic animals. The emerging evidence of the important and multifaceted roles of neutrophil granulocytes in potentiating the adaptive immune response in the secondary lymphoid organs have been recently reviewed in reference Citation68. Furthermore, since FcRn is expressed in neutrophils and plays an active role in phagocytosis,Citation14 we also analyzed this function in transgenic mice. We found that bFcRn is expressed in transgenic neutrophils and they phagocytose IgG immune complexes more efficiently than their wild-type controls.Citation24 We believe that this effect further boosts neutrophil activation, and that their influx into draining secondary lymphoid tissue contributes to the enhanced B-cell activation we observed in transgenic mice.
We also found that bFcRn is expressed in bone marrow derived dendritic cells and they phagocytose IgG immune complexes and activate T cells more efficiently than their wild-type controls (unpublished data). The higher number of dendritic cells in transgenic FcRn animals compared with the wild-type controls after immunization suggests that these cells are more abundant and active in the spleen of transgenic mice and certainly contribute to the augmented immune response we observed.Citation22,Citation24
Transgenic Animals that Overexpress FcRn Act through a Combination of IgG Protection, Expansion of the B-Cell Repertoire and Improved Antigen-Presentation
At the beginning of our research, the role of the FcRn in modulating the immune response via clonal expansion of B cells was a striking and unexpected finding. Studies have variably reported that IgG responses to antigenic stimuli are reducedCitation69–Citation71 or increasedCitation72,Citation73 in β2m-deficient mice that lack functional FcRn. On the other hand, impairment of IgG synthesis was not detected in FcRn α-chain knock-out animals, and the low serum IgG levels were explained by the impaired IgG protection.Citation35 However, FcRn expression and its role in professional APCs, which have essential roles in humoral immune response, have been recently described in references Citation15–Citation18, Citation74 and Citation75. These studies indicate that the FcRn efficiently increases phagocytosis and recycles monomeric IgG out of these cells, and also that it directs IgG-antigen immune complexes into lysosomes.Citation15,Citation16 This latter function is further supported by showing that the MHC class II associated invariant chain, which is generally restricted to APCs, can associate with FcRn and direct it into lysosomes.Citation76 Taken together, the data therefore indicate that FcRn redirects antigens complexed with IgG into degradative compartments that are associated with the loading of antigenic peptides onto MHC class II molecules within cells.Citation11 The higher number of dendritic cells in transgenic FcRn animals compared with the wild-type controls after immunization suggests that these cells are more abundant and active in the spleen of transgenic mice and certainly contribute to the augmented immune response we observed.Citation22,Citation24 This could be due to the higher level of ICs in transgenic miceCitation63 and their increased phagocytosis and antigen presentation (unpublished data).
Based on these results, we suggest that the overexpression of the FcRn not only rescues the antigen-specific IgG at a greater level, but also enhances the priming of naïve B cells, the expansion of antigen-specific memory B cells and plasma cells in the secondary lymphoid organs. A more recent study that demonstrates the FcRn mediated antigen presentation in T-cell proliferation and antigen-specific Ab responsesCitation17 supports our observations ().
Conclusion
For the past 35 years, hybridoma technology has enhanced our capacity for research and development of diagnostics by providing mAb reagents to track, detect and quantify target molecules in cells and serum. Recently, several more advanced methods to harness the immune response have substantially increased the number of antibody-producing cells that can be screened.Citation77–Citation79 Moreover, mAbs isolated from human display libraries have proved extremely useful in the characterization of structural epitopes that mediate neutralization. Caveats to this methodology exist, however, since phage display libraries are generated by random combination of immunoglobulin VH and VL genes and are therefore not restricted, as the in vivo repertoire is, by mechanisms regulating the production of auto-reactive specificities.Citation80
We propose that the effects we observed in transgenic mice that overexpress bFcRn offer major advantages in mAb production, where the goal is to generate a large pool of antigen-specific B cells, especially against weakly immunogenic proteins and peptides.
Our most recent results in transgenic rabbits that overexpress FcRn demonstrate an augmented immune response, similar to that described in this review for mice. This indicates that the adaptation of this technology to larger mammals will bring substantial advantages for the production of polyclonal Ab, as well as for the formation of mAb in species other than mice.
Furthermore, the introduction of overexpressing bFcRn in humanized animal models may endow these animals with an enhanced capacity to mount substantial Ab responses and overcome their intrinsically weakened immune system. It is well known that the immune response in transgenic mice or other transgenic species expressing human IgGs is less robust than in strains that are used to generate homologous mAbs. As a consequence an increased number of immunizations or antibody screens might be requiredCitation81 or there may be a complete failure to generate mAbs in these animals. Based on our current observations and previous findings (e.g., strong binding of the bovine FcRn to human IgGCitation19,Citation39), we believe that there is good reason to expect that the overexpression of the bFcRn has the potential to improve the immune response of the humanized animals.
For the reasons outlined here, bFcRn transgenesis can be expected to enrich the choices for antibodies against many targets that were previously un-addressable. Our studies suggest that the enlarged armamentarium of Abs against critically important antigens and specific epitopes will have a transformational effect in facilitating the derivation and development of antibody-based therapies, diagnostics and other tools. Moreover, the improvements in harvestable quantities of high-quality antibodies will likely afford substantial and enabling efficiencies in making them available as important compounds for medical and biological purposes.
Figures and Tables
Figure 1 (A) The multiple functions of FcRn are dependent on its ability to sort monomeric IgG away from lysosomal degradation within cells and release bound cargo during exocytic events at plasma membrane. (B) The fact that FcRn salvage pathway is saturable is a well-known phenomenon referred to as fractional catabolic rate and caused by the fact that the pool of FcRn available in cells to recycle or transport its ligand can be limited. Thus, when FcRn is fully saturated, the unbound ligand is cleared, primarily through lysosomal degradation. (C) The prolonged IgG half-life results of the transgenic mice that overexpress FcRn clearly suggest a correlation between the levels of expression of FcRn and the protection of IgG.
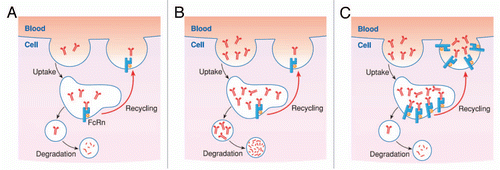
Figure 2 bFcRn overexpression resulted in a robust augmentation of the immune response in tg mice. FVB/N_tg4, FVB/N_tg5 (FVB/N transgenic mice carrying 4 and 5 copies of the bFcRn, respectively) and wild-type mice were immunized i.p. with OVA in CFA and challenged 14 days later with OVA in IFA. (A) OVA-specific IgM and (B) OVA-specific IgG titers were nearly tripled during the secondary immune response in FVB/N_tg4 and FVB/N_tg5 mice compared with the wild-type animals. (C) Transgenic mice produced significantly higher amounts of total IgG compared to the wild-type mice. (D) ELISPOT assays were performed to test for the presence of OVA-specific B cells. The number of OVA-specific cells was calculated taking account of the total spleen cell number. Multiple-fold increase of OVA-specific IgM and IgG producer cells was detected in the spleen of FVB/N_tg4 mice compared with wild-type controls. Significance levels indicate the difference between the tg and wild-type mice. Values shown are the mean ± SEM. (*p < 0.05; **p < 0.01; ***p < 0.001). All the experiments were repeated three times with similar results (Figure is reproduced from reference Citation24 with permission. Copyright 2011. The American Association of Immunologists, Inc.).
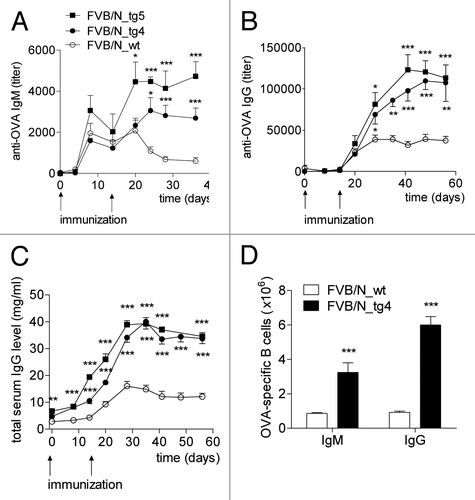
Figure 3 Immunization with HA2-KLH elicits potent anti-peptide immune response in bFcRn tg mice. (A) HA2-specific IgG titers showed a substantial increase in tg mice compared to the negligible IgG titers of wild-type mice. (B) Absolute number of B cells, T cells, neutrophils, dendritic cells and plasma cells were significantly higher in the spleen of transgenic animals as measured by FACS analysis. Values shown are the mean ± SEM. (*p < 0.05; **p < 0.01; ***p < 0.001). All the experiments were repeated twice with similar results (Figure is reproduced from reference Citation22 with permission).
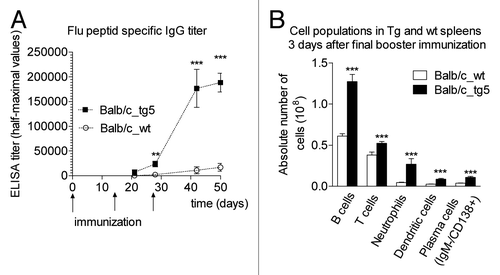
Figure 4 A proposed model for the role of FcRn overexpression in augmented humoral immune response. Better IgG rescue results in higher level of antigen-specific IgG in immunized transgenic animals which leads to the formation of more antigen-IgG ICs. A higher level of ICs and their increased phagocytosis by the transgenic neutrophils (NE) results in a much greater influx of these cells into the regional secondary lymphoid organs, thus potentiating the humoral immune response. Transgenic dendritic cells (DC) that overexpress FcRn phagocytose and present antigens more efficiently to T helper cells (TH) when loaded with antigen-IgG ICs. The higher number of DCs in transgenic FcRn animals compared to the wild-type controls after immunization suggests that these cells are more abundant and active in spleen of transgenic mice and very likely contribute to the augmented immune response observed. Based on these observations, we suggest that the overexpression of the FcRn does more than protect antigen-specific IgG from degradation. It also enhances the priming of naïve B cells, the expansion of antigen-specific memory B cells (BM) and plasma cells (antibody forming cells; AFC) in the secondary lymphoid organs. This results in a more diverse humoral immune response, a higher titer and higher affinity of antigen-specific IgG. (Green texts and arrows indicate cells and effects that contribute in augmenting the humoral immune response by FcRn).
Acknowledgments
Supported by the grant OM-00117-119/2008 from the Hungarian National Office for Research and Technology and ImmunoGenes Ltd., Hungary.
References
- Brambell FWR, Halliday R, Morris IG. Interference by human and bovine serum and serum protein fractions with the absorption of antibodies by suckling rats and mice. Proc R Soc B 1958; 149:1
- Brambell FWR, Hemmings WA, Morris IG. A theoretical model of gammaglobulin catabolism. Nature 1964; 203:1352 - 1355
- Spiegelberg HL, Weigle WO. The catabolism of homologous and heterologous 7s gamma globulin fragments. J Exp Med 1965; 121:323 - 338
- Kraehenbuhl JP, Campiche MA. Early stages of intestinal absorption of specific antibiodies in the newborn. An ultrastructural, cytochemical and immunological study in the pig, rat and rabbit. J Cell Biol 1969; 42:345 - 365
- Butler JE. Hasegawa T, Hayashi M, Ebling FJG, Henderson IW. Transmission of immunity from mother to young. Fertility and Sterility 1971; Amsterdam Excerpta Medica 92 - 98
- Jones EA, Waldmann TA. The mechanism of intestinal uptake and transcellular transport of IgG in the neonatal rat. J Clin Invest 1972; 51:2916 - 2927
- Rodewald R, Kraehenbuhl JP. Receptor-mediated transport of IgG. J Cell Biol 1984; 99:159 - 164
- Simister NE, Rees AR. Isolation and characterization of an Fc receptor from neonatal rat small intestine. Eur J Immunol 1985; 15:733 - 738
- Simister NE, Mostov KE. An Fc receptor structurally related to MHC class I antigens. Nature 1989; 337:184 - 187
- Roopenian DC, Akilesh S. FcRn: the neonatal Fc receptor comes of age. Nat Rev Immunol 2007; 7:715 - 725
- Ward ES, Ober RJ. Chapter 4: Multitasking by exploitation of intracellular transport functions the many faces of FcRn. Adv Immunol 2009; 103:77 - 115
- Roopenian DC, Sun VZ. Clinical ramifications of the MHC family Fc receptor FcRn. J Clin Immunol 2010; 30:790 - 797
- Kuo TT, Baker K, Yoshida M, Qiao SW, Aveson VG, Lencer WI, et al. Neonatal Fc receptor: from immunity to therapeutics. J Clin Immunol 2010; 30:777 - 789
- Vidarsson G, Stemerding AM, Stapleton NM, Spliethoff SE, Janssen H, Rebers FE, et al. FcRn: an IgG receptor on phagocytes with a novel role in phagocytosis. Blood 2006; 108:3573 - 3579
- Qiao SW, Kobayashi K, Johansen FE, Sollid LM, Andersen JT, Milford E, et al. Dependence of antibody-mediated presentation of antigen on FcRn. Proc Natl Acad Sci USA 2008; 105:9337 - 9342
- Mi W, Wanjie S, Lo ST, Gan Z, Pickl-Herk B, Ober RJ, et al. Targeting the neonatal fc receptor for antigen delivery using engineered fc fragments. J Immunol 2008; 181:7550 - 7561
- Liu X, Lu L, Yang Z, Palaniyandi S, Zeng R, Gao LY, et al. The neonatal FcR-mediated presentation of immune-complexed antigen is associated with endosomal and phagosomal pH and antigen stability in macrophages and dendritic cells. J Immunol 2011; 186:4674 - 4686
- Baker K, Qiao SW, Kuo TT, Aveson VG, Platzer B, Andersen JT, et al. Neonatal Fc receptor for IgG (FcRn) regulates cross-presentation of IgG immune complexes by CD8-CD11b+ dendritic cells. Proc Natl Acad Sci USA 2011; 108:9927 - 9932
- Lu W, Zhao Z, Zhao Y, Yu S, Zhao Y, Fan B, et al. Overexpression of the bovine FcRn in the mammary gland results in increased IgG levels in both milk and serum of transgenic mice. Immunology 2007; 122:401 - 408
- Petkova SB, Akilesh S, Sproule TJ, Christianson GJ, Al Khabbaz H, Brown AC, et al. Enhanced half-life of genetically engineered human IgG1 antibodies in a humanized FcRn mouse model: potential application in humorally mediated autoimmune disease. Int Immunol 2006; 18:1759 - 1769
- Bender B, Bodrogi L, Mayer B, Schneider Z, Zhao Y, Hammarstrom L, et al. Position independent and copy-number-related expression of the bovine neonatal Fc receptor alpha-chain in transgenic mice carrying a 102 kb BAC genomic fragment. Transgenic Res 2007; 16:613 - 627
- Vegh A, Cervenak J, Jankovics I, Kacskovics I. FcRn overexpression in mice results in potent humoral response against weakly immunogenic antigen. mAbs 2011; 3:173 - 180
- Schneider Z, Cervenak J, Baranyi M, Papp K, Prechl J, Laszlo G, et al. Transgenic expression of bovine neonatal Fc receptor in mice boosts immune response and improves hybridoma production efficiency without any sign of autoimmunity. Immunol Lett 2011; 137:62 - 69
- Cervenak J, Bender B, Schneider Z, Magna M, Carstea BV, Liliom K, et al. Neonatal FcR overexpression boosts humoral immune response in transgenic mice. J Immunol 2011; 186:959 - 968
- Collarini EJ, Lee FE, Foord O, Park M, Sperinde G, Wu H, et al. Potent high-affinity antibodies for treatment and prophylaxis of respiratory syncytial virus derived from B cells of infected patients. J Immunol 2009; 183:6338 - 6345
- Bosze Z, Hiripi L, Hoffmann O, Kerekes A, Bender B, Kacskovics I. Bosze Z, Fan J, Duranthon V. IgG binding FcRn transgenic rabbits created through BAC transgensis. 4th International Rabbit Biotechnology Meeting 2011; Budapest, Hungary
- Humphrey JH, Fahey JL. The metabolism of normal plasma proteins and gamma-myeloma protein in mice bearing plasma-cell tumors. J Clin Invest 1961; 40:1696 - 1705
- Fahey JL, Robinson AG. Factors controlling serum gamma-globulin concentration. J Exp Med 1963; 118:845 - 868
- Sell S, Fahey JL. Relationship between gamma-globulin metabolism and low serum gamma-globulin in germfree mice. J Imunol 1964; 93:81 - 87
- Junghans RP, Anderson CL. The protection receptor for IgG catabolism is the beta2-microglobulin-containing neonatal intestinal transport receptor. Proc Natl Acad Sci USA 1996; 93:5512 - 5516
- Waldmann TA, Strober W. Metabolism of immunoglobulins. Prog Allergy 1969; 13:1 - 110
- Bleeker WK, Teeling JL, Hack CE. Accelerated autoantibody clearance by intravenous immunoglobulin therapy: studies in experimental models to determine the magnitude and time course of the effect. Blood 2001; 98:3136 - 3142
- Andersen SB, Bjorneboe M. Gamma globulin turnover in rabbits before and during hyperimmunization. J Exp Med 1964; 119:537 - 546
- Jin F, Tayab ZR, Balthasar JP. Pharmacokinetic and pharmacodynamic effects of high-dose monoclonal antibody therapy in a rat model of immune thrombocytopenia. Aaps J 2005; 7:895 - 902
- Roopenian DC, Christianson GJ, Sproule TJ, Brown AC, Akilesh S, Jung N, et al. The MHC class I-like IgG receptor controls perinatal IgG transport, IgG homeostasis and fate of IgG-Fc-coupled drugs. J Immunol 2003; 170:3528 - 3533
- Kacskovics I, Wu Z, Simister NE, Frenyo LV, Hammarstrom L. Cloning and characterization of the bovine MHC class I-like Fc receptor. J Immunol 2000; 164:1889 - 1897
- Mayer B, Zolnai A, Frenyo LV, Jancsik V, Szentirmay Z, Hammarstrom L, et al. Redistribution of the sheep neonatal Fc receptor in the mammary gland around the time of parturition in ewes and its localization in the small intestine of neonatal lambs. Immunology 2002; 107:288 - 296
- Mayer B, Kis Z, Kajan G, Frenyo LV, Hammarstrom L, Kacskovics I. The neonatal Fc receptor (FcRn) is expressed in the bovine lung. Vet Immunol Immunopathol 2004; 98:85 - 89
- Kacskovics I, Kis Z, Mayer B, West AP Jr, Tiangco NE, Tilahun M, et al. FcRn mediates elongated serum half-life of human IgG in cattle. Int Immunol 2006; 18:525 - 536
- Kuroiwa Y, Kasinathan P, Choi YJ, Naeem R, Tomizuka K, Sullivan EJ, et al. Cloned transchromosomic calves producing human immunoglobulin. Nat Biotechnol 2002; 20:889 - 894
- Cianga P, Medesan C, Richardson JA, Ghetie V, Ward ES. Identification and function of neonatal Fc receptor in mammary gland of lactating mice. Eur J Immunol 1999; 29:2515 - 2523
- Keler T, Guyre PM, Vitale LA, Sundarapandiyan K, van De Winkel JG, Deo YM, et al. Targeting weak antigens to CD64 elicits potent humoral responses in human CD64 transgenic mice. J Immunol 2000; 165:6738 - 6742
- Keler T, He L, Graziano RF. Development of antibody-targeted vaccines. Curr Opin Mol Ther 2005; 7:157 - 163
- Goins CL, Chappell CP, Shashidharamurthy R, Selvaraj P, Jacob J. Immune complex-mediated enhancement of secondary antibody responses. J Immunol 2010; 184:6293 - 6298
- Ekiert DC, Bhabha G, Elsliger MA, Friesen RH, Jongeneelen M, Throsby M, et al. Antibody recognition of a highly conserved influenza virus epitope. Science 2009; 324:246 - 251
- Sui J, Hwang WC, Perez S, Wei G, Aird D, Chen LM, et al. Structural and functional bases for broad-spectrum neutralization of avian and human influenza A viruses. Nat Struct Mol Biol 2009; 16:265 - 273
- Throsby M, van den Brink E, Jongeneelen M, Poon LL, Alard P, Cornelissen L, et al. Heterosubtypic neutralizing monoclonal antibodies cross-protective against H5N1 and H1N1 recovered from human IgM+ memory B cells. PLoS One 2008; 3:3942
- Onisk D, Brown M, Keter D, Chambers R, Fancy D, Kacskovics I, et al. DNA immunization in bFcRn transgenic mice results in enhanced immunogenic response. IMPULSE: IMmune-related Pathologies: Understanding Leukocyte Signaling and Emerging therapies 2011; Visegrad, Hungary
- Bradbury AR, Sidhu S, Dubel S, McCafferty J. Beyond natural antibodies: the power of in vitro display technologies. Nat Biotechnol 2011; 29:245 - 254
- Watanabe-Fukunaga R, Brannan CI, Copeland NG, Jenkins NA, Nagata S. Lymphoproliferation disorder in mice explained by defects in Fas antigen that mediates apoptosis. Nature 1992; 356:314 - 317
- Knott CL, Reed JC, Bodrug S, Saedi MS, Kumar A, Kuus-Reichel K. Evaluation of Bcl-2/B cell transgenic mice (B6) for hybridoma production. Hybridoma 1996; 15:365 - 371
- Takai T, Ono M, Hikida M, Ohmori H, Ravetch JV. Augmented humoral and anaphylactic responses in Fc gamma RII-deficient mice. Nature 1996; 379:346 - 349
- Takahashi N, Kakinuma H, Hamada K, Shimazaki K, Yamasaki Y, Matsushita H, et al. Improved generation of catalytic antibodies by MRL/MPJ-lpr/lpr autoimmune mice. J Immunol Methods 2000; 235:113 - 120
- Andrews BS, Eisenberg RA, Theofilopoulos AN, Izui S, Wilson CB, McConahey PJ, et al. Spontaneous murine lupus-like syndromes. Clinical and immunopathological manifestations in several strains. J Exp Med 1978; 148:1198 - 1215
- Vyse TJ, Kotzin BL. Genetic susceptibility to systemic lupus erythematosus. Annu Rev Immunol 1998; 16:261 - 292
- Strasser A, Whittingham S, Vaux DL, Bath ML, Adams JM, Cory S, et al. Enforced BCL2 expression in B-lymphoid cells prolongs antibody responses and elicits autoimmune disease. Proc Natl Acad Sci USA 1991; 88:8661 - 8665
- Bolland S, Yim YS, Tus K, Wakeland EK, Ravetch JV. Genetic modifiers of systemic lupus erythematosus in FcgammaRIIB(-/-) mice. J Exp Med 2002; 195:1167 - 1174
- Tiller T, Kofer J, Kreschel C, Busse CE, Riebel S, Wickert S, et al. Development of self-reactive germinal center B cells and plasma cells in autoimmune Fc{gamma}RIIB-deficient mice. J Exp Med 2010; 207:2767 - 2778
- Papp K, Vegh P, Tchorbanov A, Vassilev T, Erdei A, Prechl J. Progression of lupus-like disease drives the appearance of complement-activating IgG antibodies in MRL/lpr mice. Rheumatology (Oxford) 2010; 49:2273 - 2280
- Kunkl A, Klaus GG. The generation of memory cells. IV. Immunization with antigen-antibody complexes accelerates the development of B-memory cells, the formation of germinal centres and the maturation of antibody affinity in the secondary response. Immunology 1981; 43:371 - 378
- Laissue J, Cottier H, Hess MW, Stoner RD. Early and enhanced germinal center formation and antibody responses in mice after primary stimulation with antigen-isologous antibody complexes as compared with antigen alone. J Immunol 1971; 107:822 - 831
- Coulie PG, Van Snick J. Enhancement of IgG anti-carrier responses by IgG2 anti-hapten antibodies in mice. Eur J Immunol 1985; 15:793 - 798
- Getahun A, Heyman B. How antibodies act as natural adjuvants. Immunol Lett 2006; 104:38 - 45
- Chappell CP, Jacob J. Identification of memory B cells using a novel transgenic mouse model. J Immunol 2006; 176:4706 - 4715
- Maletto BA, Ropolo AS, Alignani DO, Liscovsky MV, Ranocchia RP, Moron VG, et al. Presence of neutrophil-bearing antigen in lymphoid organs of immune mice. Blood 2006; 108:3094 - 3102
- Calabro S, Tortoli M, Baudner BC, Pacitto A, Cortese M, O'Hagan DT, et al. Vaccine adjuvants alum and MF59 induce rapid recruitment of neutrophils and monocytes that participate in antigen transport to draining lymph nodes. Vaccine 2011; 29:1812 - 1823
- Beauvillain C, Cunin P, Doni A, Scotet M, Jaillon S, Loiry ML, et al. CCR7 is involved in the migration of neutrophils to lymph nodes. Blood 2011; 117:1196 - 1204
- Soehnlein O. An elegant defense: how neutrophils shape the immune response. Trends Immunol 2009; 30:511 - 512
- Israel EJ, Patel VK, Taylor SF, Marshak-Rothstein A, Simister NE. Requirement for a beta 2-microglobulin-associated Fc receptor for acquisition of maternal IgG by fetal and neonatal mice. J Immunol 1995; 154:6246 - 6251
- Ghetie V, Hubbard JG, Kim JK, Tsen MF, Lee Y, Ward ES. Abnormally short serum half-lives of IgG in beta 2-microglobulin-deficient mice. Eur J Immunol 1996; 26:690 - 696
- Christianson GJ, Brooks W, Vekasi S, Manolfi EA, Niles J, Roopenian SL, et al. Beta2-microglobulin-deficient mice are protected from hypergamma-globulinemia and have defective antibody responses because of increased IgG catabolism. J Immunol 1997; 159:4781 - 4792
- Lehmann-Grube F, Lohler J, Utermohlen O, Gegin C. Antiviral immune responses of lymphocytic choriomeningitis virus-infected mice lacking CD8+ T lymphocytes because of disruption of the beta2-microglobulin gene. J Virol 1993; 67:332 - 339
- Noble A, Zhao ZS, Cantor H. Suppression of immune responses by CD8 cells. II. Qa-1 on activated B cells stimulates CD8 cell suppression of T helper 2 responses. J Immunol 1998; 160:566 - 571
- Zhu X, Meng G, Dickinson BL, Li X, Mizoguchi E, Miao L, et al. MHC class I-related neonatal Fc receptor for IgG is functionally expressed in monocytes, intestinal macrophages and dendritic cells. J Immunol 2001; 166:3266 - 3276
- Liu X, Ye L, Christianson GJ, Yang JQ, Roopenian DC, Zhu X. NF-{kappa}B Signaling Regulates Functional Expression of the MHC Class I-Related Neonatal Fc Receptor for IgG via Intronic Binding Sequences. J Immunol 2007; 179:2999 - 3011
- Ye L, Liu X, Rout SN, Li Z, Yan Y, Lu L, et al. The MHC class II-associated invariant chain interacts with the neonatal Fc gamma receptor and modulates its trafficking to endosomal/lysosomal compartments. J Immunol 2008; 181:2572 - 2585
- Love JC, Ronan JL, Grotenbreg GM, van der Veen AG, Ploegh HL. A microengraving method for rapid selection of single cells producing antigen-specific antibodies. Nat Biotechnol 2006; 24:703 - 707
- Jin A, Ozawa T, Tajiri K, Obata T, Kondo S, Kinoshita K, et al. A rapid and efficient single-cell manipulation method for screening antigen-specific antibody-secreting cells from human peripheral blood. Nat Med 2009; 15:1088 - 1092
- Reddy ST, Ge X, Miklos AE, Hughes RA, Kang SH, Hoi KH, et al. Monoclonal antibodies isolated without screening by analyzing the variable-gene repertoire of plasma cells. Nat Biotechnol 2010; 28:965 - 969
- Wang TT, Tan GS, Hai R, Pica N, Petersen E, Moran TM, et al. Broadly protective monoclonal antibodies against H3 influenza viruses following sequential immunization with different hemagglutinins. PLoS Pathog 2010; 6:1000796
- Carter PJ. Potent antibody therapeutics by design. Nat Rev Immunol 2006; 6:343 - 357