Abstract
An immunotoxin (IT) constructed with RFB4, a murine anti-CD22 monoclonal antibody, and the “deglycosylated” A chain of ricin has shown activity at safe doses in patients with non-Hodgkin lymphoma and in children with acute lymphoblastic leukemia. The dose limiting toxicity is vascular leak syndrome (VLS), which appears to be due to a unique amino acid motif in the ricin toxin A (RTA) chain that damages vascular endothelial cells. We mutated recombinant (r) RTA to disable this site, but await testing of the IT prepared with this mutant RTA in humans. Another possible approach to reducing IT-induced VLS is to shorten the half-life of the IT in vivo. We previously constructed a mouse-human chimeric RFB4 by grafting the variable genes of RFB4 onto the human IgG1k constant regions. Here, we report the expansion of our panel of mutant chimeric RFB4s (mcRFB4s) that lack the ability to bind to the neonatal Fc receptor (FcRn). In comparison with cRFB4, which had a T1/2 of 263 h, the mcRFB4s had T1/2s ranging from 39 to 106 h. ITs were constructed with these mcRFB4s and rRTA. The mcRFB4-RTA ITs retained their cytotoxicity in vitro and had shorter half lives than the parental cRFB4-RTA IT. In addition, the mcRFB4 IT with the shortest T1/2 induced less pulmonary vascular leak in mice, which we have postulated is a surrogate marker for VLS in humans.
Introduction
Immunotoxins (ITs) are hybrid molecules that consist of monoclonal antibodies (mAbs) linked to toxins or their subunits.Citation1 ITs can bind to and kill target cells without killing the surrounding normal cells. Because of the liver's role in clearing toxins, the maximum tolerated dose (MTD) of ricin and some first generation ITs was defined by hepatotoxicity.Citation2,Citation3 Second generation ITs constructed with the deglycosylated A chain of ricin (dgRTA) did not cause hepatotoxicity because the liver-binding sugars in the toxin were chemically altered.Citation4 ITs prepared with dgRTA and several murine mAbs are extremely potent. They have been evaluated in patients with refractory relapsed lymphoma or leukemia and have shown anti-tumor activity, including partial and complete responses.Citation5–Citation9 The safety and efficacy of these ITs is limited by vascular leak syndrome (VLS), which is the most common dose-limiting toxicity (DLT) of ITs and is characterized by hypoalbuminemia, weight gain and, in the most severe cases, pulmonary edema and hypotension.Citation10–Citation14
Three approaches have been tested to prevent VLS. The first approach was to use prophylactic corticosteroids. Their use did not prevent the occurrence of severe VLS and did not lead to an increase in the MTD.Citation15
The second approach was based on observations that VLS was the result of RTA-mediated damage to vascular endothelial cells. This activity was associated with a unique amino acid sequence in RTA: Leu74-Asp75-Val76 (LDV). Altering this sequence led to reduction of pulmonary vascular leak (PVL), a surrogate marker for VLS in mice.Citation13,Citation16 However, mutations to this LDV sequence also caused a significant decrease in the specific cytotoxicity of the IT against tumor cells.Citation17 Therefore, mutations were made to amino acids that were spatially near the “VLS” site. One recombinant (r) RTA mutant, rRTA-N97A reduced PVL induced by RFB4-rRTA but did not reduce the cytotoxic activity of this IT either in vitro or in SCID mice xenografted with a human lymphoma cell line.Citation17 This IT must now be tested in humans.
The third potential approach to decreasing VLS is to reduce the in vivo half-life of ITs in order to prevent their prolonged contact with vasculature. We previously reported that an IT constructed with the Fab' fragment of the RFB4 mAb had a shorter T1/2 and a higher MTD in humans than an IT prepared with the IgG of RFB4. The former was cleared very rapidly and gave even better clinical responses,Citation9,Citation19 but it was expensive to prepare. Therefore, another approach was used to reduce the T1/2. This approach involved chimerization and modification of the Fc region to shorten the T1/2.
The catabolism of IgG is controlled by the interaction of IgG with the neonatal Fc receptor (FcRn) that is expressed predominantly in vascular endothelial cells.Citation20 Once internalized by the cells, IgG molecules bind to FcRn at the slightly acidic pH of the endosomes and they are then returned to the cell membrane where they are released into the circulation. If IgG molecules do not bind to FcRn, they are destroyed in the lysosomes.Citation21 When a single mutation (H435A) was introduced into the FcRn binding site of chimeric RFB4 (cRFB4), the affinity of the mutant cRFB4 (mcRFB4) for FcRn was not detectable and the T1/2 was decreased by approximately 2.6-fold.Citation22 Furthermore, the T1/2 of a rRTA-containing IT prepared with mcRFB4 was approximately 2.3-fold shorter than the T1/2 of an IT prepared with cRFB4. The cytotoxicities of the two ITs were equivalent. These results demonstrated the feasibility of producing mAbs with mutations in and around the FcRn binding site that do not bind to FcRn and, when used to construct ITs, have reduced T1/2s without loss of cytotoxicity.
In this study, we generated a panel of mcRFB4s that contained mutations in or around the FcRn binding site in a search for mcRFB4s that had shorter T1/2s than our previous mutants. These new mcRFB4 had decreased T1/2s in mice. Their stability in vitro and in vivo was not affected. Therefore, ITs were prepared with two mutants, mcRFB4-P247W and mcRFB4-H310A, and the wild-type cRFB4 by conjugating them to rRTA. Compared with cRFB4-rRTA, the mutant ITs had reduced T1/2s in mice, but equivalent cytotoxicity in vitro. The mcRFB4-H310A-rRTA, which had the shortest T1/2, induced less PVL in mice compared with both the cRFB4-rRTA and the murine RFB4-rRTA.
Results
Design, construction and expression of recombinant RFB4 mutants.
The major aim of this study was to construct an anti-CD22 IT that induced less PVL while retaining full cytotoxicity. Our approach was to eliminate the binding of the anti-CD22 mAb to the FcRn receptor. We generated mcRFB4 constructs by introducing mutations into the Fc region involved in the IgG-FcRn interaction.Citation23–Citation25 Five mutant constructs were derived from cRFB4: (1) mcRFB4-P247W with a single mutation in residue 247 (Pro247Trp), (2) mcRFB4-I253A with a single mutation in residue 253 (Ile253Ala), (3) mcRFB4-H310A with a single mutation in residue 310 (His310Ala), (4) mcRFB4-H435A with a single mutation in residue 435 (His435Ala) and (5) mcRFB4-AAA with mutations in residues 253 (Ile253Ala), 310 (His310Ala) and 435 (His435Ala).
All RFB4 constructs were expressed in CHO/DHFR− cells and purified to homogeneity by affinity chromatography on Protein G-Sepharose. There were no differences in the purity or the molecular weights between the mAbs as determined by sodium dodecyl sulfate PAGE (SDS-PAGE) (). All mAbs had a molecular weight of 150 kDa under non-reducing conditions, while under reducing conditions all samples contained two bands of 50 and 25 kDa corresponding to IgG heavy and light chains, respectively. In addition, there were no aggregates or degradation as determined by size-exclusion chromatography (, S1 and S2).
Binding activity of mcRFB4 constructs for FcRns and CD22+ human B-cell lymphoma Daudi cells.
The mcRFB4 constructs were designed to have a reduced affinity for FcRns. The binding of all constructs to human and rat FcRns at pH 6.0 was below the limit of detection of the assay. cRFB4 mAb and human IgG controls exhibited comparable affinity for these FcRns (data not shown).
Amino acids of IgG that are in the vicinity of the FcRn binding site are located on or around the CH2-CH3 interface.Citation23–Citation25 Therefore, mutation of these residues should not affect binding to CD22. The binding of mcRFB4 constructs to CD22 was evaluated using the CD22+ human B-cell lymphoma Daudi cell line. All mcRFB4 constructs exhibited similar binding to Daudi cells that was comparable to that of cRFB4 (data not shown). In summary, mutations in the FcRn binding site eliminated binding of mcRFB4 to FcRns, but not binding to the CD22 antigen.
PK and stability of mcRFB4 constructs.
A mAb with impaired affinity for FcRn should have a decreased T1/2. All mcRFB4s exhibited shorter T1/2s in Swiss-Webster mice (T1/2β ranged from 39 to 106 h), than cRFB4 (T11/2β = 263.7 ± 17.0 h; ).
To determine whether the decreased T1/2 of mcRFB4 mutants was due to the decreased resistance to proteolysis, the in vitro and in vivo stability of mcRFB4 constructs were analyzed and also compared with the murine RFB4 mAb and with cRFB4. The 125I-labeled mAbs were incubated with pooled mouse serum in vitro for 24 h and then analyzed using autoradiography on SDS gels. A comparison of cRFB4, murine RFB4, and the mutants showed no degradation in serum ().
125I-labeled mAbs were injected into mice to evaluate their in vivo stability. Mouse serum was collected at various time periods (0, 3, 6, 24, 48, 72 and 120 h) post injection and analyzed using size-exclusion chromatography. All samples eluted as single sharp peaks with a similar retention times at 0 and 24 h (, S3 and S4). There were no differences between any constructs at other time periods (data not shown). Neither aggregates nor small fragments were observed with any constructs in the blood.
Preparation of ITs.
Next, we produced ITs with mcRFB4-P247W, mcRFB4-H310A, cRFB4 and compared them with an IT made with murine RFB4. The two mutant mAbs were chosen because they had the longest and shortest T1/2 among the mcRFB4 mAbs. As determined by SDS-PAGE, the ITs contained a major band at 180 kDa and a minor band at 210 kDa, which consisted of one molecule of mAb and either one or two molecules of rRTA respectively ().
Antigen-binding activity and cytotoxicity of ITs.
The antigen-binding activity of cIT and the two mcIT was evaluated by binding to CD22+ Daudi cells using the fluorescent activated cell sorter (FACS). The concentrations at which 50% of the cells were positive (IC50) was determined for each IT and each parental mAb. The IC50 of each IT was compared with its respective parental mAb and with each of the other ITs. The IC50s of mAbs and ITs showed comparable binding activity, with IC50s of approximately 10−9 M ().
Next, the cytotoxic activity of the four ITs was quantitated by assessing their ability to kill CD22+ Daudi cells. As shown in , murine RFB4-rRTA, cRFB4-rRTA and the two mcITs killed the target cells with equal activity. rRTA ITs kill cells via internalization, followed by cleavage between the rRTA and the mAb and then translocation of rRTA to the ribosome which is then inactivated; thus, the highly potent cytotoxicity of the ITs suggests that none of these steps was affected by chimerization or the subsequent mutagenesis.Citation26,Citation27
IT mutants have decreased T1/2s in mice.
Since BALB/c mice were being used to assess PVL induced by ITs, we first measured the PKs of the ITs and their corresponding mAbs in these mice. The PK parameters are summarized in .
The T1/2s of the mcRFB4s were shorter than the T1/2 of cRFB4 in BALB/c mice. Mutant cRFB4-H310A had a T1/2 that was only 18% of the T1/2 of cRFB4. The T1/2s of the mcITs were shorter than the T1/2 of cRFB4-rRTA in BALB/c mice. The T1/2 of mcRFB4-H310A-rRTA was 39% of the T1/2 of cRFB4-rRTA (p < 0.005) (). The percent reduction in the T1/2 in BALB/c mice was similar to the percent reduction in Swiss Webster mice.
Toxicity as evaluated by induction of PVL and weight loss in mice.
Since mcRFB4-H310A-rRTA exhibited a shorter T1/2 than mcRFB4-P247W-rRTA, mcRFB4-H310A-rRTA was chosen for evaluation of induction of PVL in BALB/c mice. Three dose levels were tested. As shown in , the severity of PVL following injection of murine RFB4-rRTA and cRFB4-rRTA increased with each of the two escalating dose levels. However, the severity of PVL induced by mcRFB4-H310A-rRTA did not increase with the same escalating doses. At the highest dose level, the PVL induced by mcRFB4-H310A-rRTA was less severe than PVL induced by murine IT or the cIT and the difference was statistically significant (p < 0.04). In contrast, the difference between the murine IT and cRFB4-rRTA was not statistically significant (p > 0.26). In addition, there was less weight loss at each dose level in mice treated with the mcRFB4-H310A-rRTA than in mice treated with the murine IT or the cRFB4-rRTA ().
Discussion
An IT constructed with the Fab' fragment of the murine mAb, anti-human CD22 mAb, RFB4, exhibited a shorter T1/2 in humans than the same IT prepared with the RFB4 IgG due to the lack of an Fc:FcRn interaction.Citation9,Citation18–Citation21 It also had a higher MTD and gave better clinical responses in patients with B cell lymphoma. However, it was expensive to prepare and yields were low. Our goal in this study was to produce an IT constructed with a short-lived mcRFB4 IgG that would have a decreased T1/2 and reduced toxicity in vivo. Therefore, using site-directed mutagenesis, we constructed five mcmAbs with mutations near the FcRn binding site on the heavy chain of the anti-CD22 cRFB4 mAb. These mcmAbs were then used to produce rRTA-containing ITs. The stability, T1/2, cytotoxicity and systemic toxicity of the mAbs and ITs were evaluated.
The major findings of this study are: (1) a new single amino acid mutation, P247W, plays a role in the FcRn binding; (2) cRFB4 and all the mcRFB4 mutants had comparable stability in vitro in mouse serum and in vivo in mice; (3) single substitutions of Fc residues on the FcRn binding sites were sufficient to eliminate detectable binding to FcRn and to reduce T1/2s in mice. Combinations of these mutations did not further reduce T1/2; (4) mcRFB4-rRTA ITs had shorter T1/2s in mice than the cRFB4-rRTA IT; (5) the in vitro cytotoxicity of mcRFB4-rRTA ITs on CD22+ Daudi cells was not affected by mutations to the FcRn binding sites and (6) compared with murine RFB4-rRTA and cRFB4-rRTA, mcRFB4-H310A-rRTA induced less PVL in mice.
The Fc residues involving the FcRn binding have been well documented.Citation27–Citation29 These include amino acids at positions 253, 310 and 435. Substitution of these residues significantly changed the affinity for FcRn and the half-live of IgG as demonstrated in the present study and other reports.Citation23–Citation25,Citation28–Citation30 Other amino acids around the direct Fc:FcRn interaction sites may also affect the affinity for FcRn and have been identified as candidates for mutagenesis to find IgG molecules with increased affinity for FcRn and prolonged half-life.Citation25,Citation31–Citation35 Our results demonstrated that the substitution of Pro247 with a tryptophan significantly reduced the affinity for FcRn (data not shown) and reduced the T1/2 without affecting the stability. Therefore, Pro247 is a new candidate for mutagenesis to prepare mAbs with altered T1/2.
Our previous study demonstrated that a single substitution of His435 of the cRFB4 mAb with alanine diminished the binding affinity for FcRns and resulted in a shorter T1/2 in mice.Citation22 In this study, we confirmed that mutations that eliminate detectable binding to FcRn result in shorter T1/2s. We have also investigated whether multiple mutations had an additive or synergistic effect by using the mcRFB4-AAA, e.g., mcRFB4-I253A/H310A/H435A, triple mutant. Although the mcRFB4-AAA triple mutant had a shorter T1/2 than that of mcRFB4-I253A and mcRFB4-H435A, its T1/2 was not shorter than the T1/2 of mcRFB4-H310A. It has been reported that a scFv-Fc mAb with a single H310A or H435Q mutation had a decreased T1/2, while the H310A/H435Q double mutant exhibited a synergistic effect.Citation29,Citation30 This might be due to the difference in the molecular mass of the mAb constructs (scFv-Fc vs. whole IgG) and the substitution at position 435 (glutamate vs. alanine). In addition, the mcRFB4-AAA construct showed similar in vivo stability with other constructs, while the stability of the H310A/H435Q was not reported.
The in vivo stabilities of mAbs with mutations to the FcRn binding region have not been documented. In this study, we radiolabeled the mcRFB4 constructs with 125I and evaluated their in vitro stability in mouse serum using radio-autographs and in vivo stability using size-exclusion chromatography. The results demonstrated that the in vitro and in vivo stability of cRFB4 and all mcRFB4 constructs were comparable. Thus, the mutagenesis performed in this study did not affect stability and the mAbs in circulation remained intact.
This study confirmed the results of previous studies that ITs have shorter T1/2s than their parental mAbs.Citation22 The conjugation of rRTA to mAbs using SMPT as a cross-linker modifies their PK in part due to nonspecific interactions of RTA with non-FcRn receptors, such as α2-macroglobulin.Citation36 The shorter T1/2 is not a result of the modification of the mAb molecule by the SMPT cross-linker used to prepare the IT.Citation37 The Fc:FcRn interaction was not affected when rRTA was attached to mAbs as reported previously since the relative affinities for the FcRn of an IT constructed with rRTA and either cRFB4 or a triple mutant of cRFB4, T307A/E380A/N434A, were similar to the affinities of the two parental mAbs.Citation22
Short-lived mAbs have shown advantages in cancer imaging and therapy. Murine mAbs have been used for conjugation to toxins or radioisotopes since they have short T1/2s in humans and thus may cause less damage to normal tissues.Citation6,Citation7,Citation18,Citation19 As another approach to shorten T1/2, ITs were constructed from small fragments of mAbs devoid of the murine Fc.Citation19,Citation38–Citation43 Studies with these ITs in humans demonstrated that their T1/2s were approximately 25% of the T1/2 of ITs made with the full length mAb.Citation41–Citation43 These Fab' ITs were not extensively tested to determine if their higher MTD led to a higher response rate. It is unknown if the tumor-to-background ratio of the Fab' IT was different from those constructed with the whole IgG. Other studies have shown that reduction of T1/2 can lead to a higher tumor-to-background ratio.Citation29,Citation30 Our data showed that the mcRFB4-H310A-rRTA conjugate, which had the shortest T1/2, exhibited reduced toxicity in mice when compared with cRFB4-rRTA IT. This suggests that when this IT is injected into mice xenografted with tumor, the excess non tumor bound IT should be quickly eliminated and a higher tumor-to-background ratio should be achieved.
In summary, this study demonstrates that fully active mcmAbs and mcMab-rRTA ITs with decreased serum T1/2s and toxicity can be constructed by altering residues on or around Fc:FcRn interaction sites. Further studies will be designed to determine the in vivo anti-tumor efficacy of the short-lived mcRFB4 ITs using SCID mice xenografted with CD22+ human B lymphoma cells.
Materials and Methods
Construction, expression and purification of recombinant chimeric mAbs.
We previously chimerized and expressed cRFB4 mAb in murine myeloma Sp2/0 cells.Citation22 To increase the expression level, the VH and VL genes of RFB4 were amplified by polymerase chain reaction (PCR) and inserted sequentially in-frame into pIZDHL, a new vector that expresses chimeric IgG1k at high levels.Citation44 The new cRFB4 expression plasmid was transfected into dihydrofolate reductase-deficient Chinese hamster ovary (CHO/DHFR−) cells (ATCC, Cat#CRL-9096) using Lipofectamine™ LTX reagent (Invitrogen, Cat#15338-100). Stable transfectants were selected in IMDM media (Sigma, Cat#I3390) supplemented with 10% dialyzed FBS (Invitrogen, Cat#26400-036). Supernatants from each cloned cell line were tested for presence of cRFB4 by human Ig specific enzyme linked immunosorbent assays (ELISAs).
To construct mcRFB4 vectors, the gene encoding the heavy chain of cRFB4 was inserted into the pGEM-T-Easy vector (Promega, Cat#A1360). Mutation was performed by PCR using QuikChange II Site-Directed Mutagenesis Kit (Agilent Technologies, Cat#200523) and confirmed by DNA sequencing. The primers used for mutation were as follows: (1) P247W forward, 5′-CTT CCT CTT CCC CCC AAA ATG GAA GGA CAC CCT C-3′; (2) P247W backward, 5′-GAG GGT GTC CTT CCA TTT TGG GGG GAA GAG GAA G-3′; (3) I253A forward, 5′-CAA GGA CAC CCT CAT GGC CTC CCG GAC CCC TGA G-3′; (4) I253A backward, 5′-CTC AGG GGT CCG GGA GGC CAT GAG GGT GTC CTT G-3′; (5) H310A forward, 5′-CCT CAC CGT CCT GGC CCA GGA CTG GCT G-3′; (6) H310A backward, 5′-CAG CCA GTC CTG GGC CAG GAC GGT GAG G-3′; (7) H435A forward, 5′-GCA TGA GGC TCT GCA CAA CGC CTA CAC GCA GAA GAG C-3′; (8) H435Abackward, 5′-GCT CTT CTG CGT GTA GGC GTT GTG CAG AGC CTC ATG C-3′.
Heavy chain mutants were then excised and used to substitute the wild-type heavy chain in the cRFB4 expression plasmid. The resulting plasmids were transfected into CHO/DHFR− cells and cloned as described above.
Supernatants from these clones were purified on Protein G-Sepharose (GE Healthcare, Cat#17-0618-04). After washing with phosphate buffered saline (PBS), pH 7.4, the bound proteins were eluted with 0.1 mol/L glycine-HCl buffer, pH 2.8. The eluted mAbs were dialyzed against PBS, filter-sterilized and stored at 4°C. The purity of mAbs was analyzed by SDS-PAGE and size-exclusion HPLC chromatography.
Preparation of ITs.
Recombinant RTA was prepared as previously described in reference Citation45. cRFB4 or mcRFB4s were chemically conjugated to rRTA using 4-succinimidyl-oxycarbonyl-α-methyl-α-(2-pyridyldithio)-toluene (SMPT; Thermo Scientific, Cat#21558) as a cross-linking reagent and then purified. Briefly, SMPT, dissolved in dimethylformamide (Sigma, Cat#D227056), was added to a solution of antibody (5 mg/mL), to give a final molar ratio of linker:antibody of 5:1. After incubation for 1 h at room temperature, the derivatized protein was purified by passing the solution through a column of Sephadex G-25 (GE Healthcare, Cat#17-0033-01) in PBS. The derivatized protein was then mixed with reduced rRTA and maintained under sterile conditions for 36 h at room temperature. The ITs were purified by affinity chromatography on Blue Sepharose CL-6B (GE Healthcare, Cat#17-0962-25) followed by Superdex 200 (GE Healthcare, Cat#17-1043-02).
Radioiodination.
mAbs and ITs were radiolabeled with Na125I (GE Healthcare, Cat#25004781) using iodination reagents (Thermo Scientific, Cat#28600) as described in reference Citation22. The free 125I was removed by centrifugation on MicroSpin G-25 columns (GE Healthcare, Cat#27-5345-01). The specific radioactivity of the labeled proteins was in the range of 5 × 106 cpm/µg with less than 5% free Na125I.
SDS-PAGE.
The molecular weights and purity of the ITs were determined using 4–15% SDS-PAGE (GE Healthcare, Cat#17-0678-01) under non-reducing conditions. The gel was stained with PhastGel™ Blue R (GE Healthcare, Cat#17-0518-01).
Cells.
CD22+ Daudi cells (ATCC, Cat#CCL-213) were maintained in culture by serial passages in RPMI-1640 (Sigma, Cat#R8758) containing 10% heat-inactivated fetal bovine serum (HyClone, Cat#HY-ME-SH30070-02) and 2 mM L-glutamate (Sigma, Cat#G7513). The cells were grown in a humidified atmosphere of 5% CO2 and air. Cell viability was determined by trypan blue exclusion.
Antigen-binding activity of mAbs and ITs.
The binding of mAbs and ITs to Daudi cells was evaluated using an indirect immunofluorescence assay. 1 × 106 cells/100 µL were incubated with various amounts of mAbs and ITs ranging from 10−8 to 10−12 M, for 30 min at 4°C. After the cells were washed with PBS containing 0.01% sodium azide, they were incubated with FITC-labeled goat anti-human IgG (Fc specific) (Sigma, Cat#F9512) for 30 min at 4°C in the dark (0.25 µg FITC-antibody/100 µL/1 × 106 cells). The samples were analyzed on Becton Dickinson FACS. The percent of fluorescent cells was plotted against the mAb or IT concentration and the concentration at which 50% of the cells were fluorescent was extrapolated from the graph.
[3H]-thymidine incorporation.
The cytotoxicity of ITs against CD22+ cancer cells was determined by [3H]-thymidine incorporation. Daudi cells in complete medium (5 × 104 cells/100 µL) were plated in 96-well plates and incubated for 20 h at 37°C with 100 µL of different concentrations of the ITs ranging from 10−8 to 10−13 M. After incubation, the cells were pulsed for 4 h with 1 µCi [methyl-3H]-thymidine (PerkinElmer, Cat#NET027005MC), harvested and counted in a liquid scintillation spectrometer. [3H]-thymidine incorporation at each IT concentration was compared with a baseline control value and the percent reduction at each concentration of IT was used to quantitate the cytotoxic effect, expressed as the IC50.
Measurement of the relative affinity of mAbs for FcRn.
Recombinant human and rat FcRns were radioiodinated and then incubated with the cRFB4 and mcRFB4 mAbs. The mAb-FcRn mixture was added to human or mouse IgG-Sepharose for separation and quantitation of bound and unbound radioiodinated mAb as described in reference Citation22.
PK analysis.
Six–eight weeks old female BALB/c (Taconic) or Swiss Webster mice (Taconic) were used for PK analysis. Lugol solution (Sigma, Cat#L6146) was added to their drinking water to a concentration of 0.05% from 1 d prior to injection throughout the entire period of the experiment (168 h). Radiolabeled proteins were injected into the tail veins of mice in a volume not larger than 100 µL and whole body radioactivity was measured daily in an AtomLab 100 dose calibrator (Atomic Product Corporation). A computer program kindly provided by Dr. K. Vyas from Merck Sharp and Dohme Research Laboratory was used for the calculation of all pharmacokinetic parameters.
Analysis of the stability of cRFB4 and mcRFB4 mAbs.
The in vitro stability of cRFB4 mAbs was analyzed by autoradiography. Sera from ten female Swiss Webster mice were pooled. Ten microliters of this serum was incubated with 2 µL of radiolabeled mAb at a specific radioactivity of 50,000 cpm/µL for 24 h under sterile conditions at 37°C. After incubation, the samples were boiled under non-reducing conditions at 100°C for 4 min. One microgram samples were loaded on a PhastGel high density gel designed for peptides with molecular weight between 1,000 and 20,000 (GE Healthcare, Cat#17-0679-01). The gel was electrophoresed using PhastSystem apparatus using the program designed for the PhastGel high density gel. The gel was stained with PhastGel Blue R. Autoradiography was performed by exposing blue basic autoradiographic film (BioExpress, Cat#F-9023) to the gel.
To test in vivo stability, radiolabeled mAbs in a volume of 100 µL were injected into the tail veins of 6–8 week old female Swiss Webster mice (Taconic). Mice were bled via their tail veins using heparinized capillary tubes at time 0 (1 min) and at 3, 6, 24, 48, 72 and 120 h after injection. A volume of 50 µL blood was diluted in 250 µL PBS and centrifuged at 4,000 RPM for 10 min at RT. Two hundred microliters of diluted sera was removed and analyzed by a high performance liquid chromatography (HPLC) using a TSK guard column followed by a preparative TSK-G3000SW equilibrated with PBS at pH 6.85 (Tosoh Bioscience LLC). One milliliter samples were collected and the absorbance at 280 nm and radioactivity of the samples were measured. The serum IgM and albumin peaks were identified by comparison of elution times of the samples with elution times of proteins of known molecular weights (MWs) ranging from 900-12 kDa (Sigma, Cat#MS170 and MWGF200). The corresponding radioactive counts were overlaid on the graphs of the OD280 measurements to estimate to MWs of the iodinated protein peaks.
PVL assay in mice.
Six-to-eight weeks old female BALB/c mice (Charles Rivers Labs) were used for PVL assays. Lugol solution was added to their drinking water to a concentration of 0.05% from 1 d prior to injection and throughout the entire period of the experiment (168 h). ITs at the doses of 2.5, 5.0 and 7.5 µg/g body weight/day were injected i.p daily at a constant volume of 480 µL from day one through day three. On day 3, 125I-albumin was injected i.v. and whole body radiation was immediately measured. After 24 h, whole body radioactivity was measured and mice were sacrificed. Left lungs were harvested, weighed and the radioactivity of the fluid in the left lung was measured as described previously in reference Citation17.
Statistical tests.
Two tailed t tests were used to evaluate the statistical significances of differences found in the comparisons described above. Values <0.05 were considered statistically significant.
Abbreviations
| ALL | = | acute lymphoblastic leukemia |
| AUC | = | area under the curve |
| CH2 | = | heavy chain constant domain-2 |
| CH3 | = | heavy chain constant domain-3 |
| cRFB4 | = | chimeric RFB4 |
| dgRTA | = | deglycosylated ricin toxin A chain |
| DLT | = | dose limiting toxicity |
| FCR | = | fractional catabolic rate |
| FcRn | = | neonatal Fc receptor |
| IT | = | immunotoxin |
| mAb | = | monoclonal antibody |
| mcRFB4 | = | mutant chimeric RFB4 |
| mcIT | = | mutant chimeric immunotoxin |
| MRT | = | mean residence time |
| MTD | = | maximum tolerant dose |
| NHL | = | non-Hodgkin's lymphoma |
| PBS | = | phosphate buffer saline |
| PVL | = | pulmonary vascular leak |
| rRTA | = | recombinant ricin toxin A chain |
| RTA | = | ricin toxin A chain |
| SDS-PAGE | = | sodium dodecyl sulfate polyacrylamide gel electrophoresis |
| SMPT | = | 4-succinimidyl-oxycarbonyl-α-methyl-α-(2-pyridyldithio)-toluene |
| T1/2 | = | half life |
| VLS | = | vascular leak syndrome |
Figures and Tables
Figure 1 4–15% SDS-PAGE of murine and cRFB4 constructs under non-reducing (A) and reducing (B) conditions. Lane 1, cRFB4; lane 2, mcRFB4-P247W; lane 3, mcRFB4-I253A; lane 4, mcRFB4-H310A; lane 5, mcRFB4-H435A; lane 6, mcRFB4-AAA; lane 7, murine RFB4. This is one of three experiments.
Figure 2 Analysis of purified wild-type cRFB4 and His310Ala constructs using size-exclusion chromatography. (A) cRFB4; (B) mcRFB4-H310A. This is one of three experiments.
Figure 3 Autoradiograph of murine and chimeric RFB4 constructs on SDS gels. 125I-labeled mAbs were incubated for 24 h with mouse serum at 37°C and then 4–15% SDS-PAGE was performed under non-reducing (A) or reducing (B) conditions. Lane 1, murine RFB4; lane 2, cRFB4; lane 3, mcRFB4-P247W; lane 4, mcRFB4-I253A; lane 5, mcRFB4-H310A; lane 6, mcRFB4-H435A; lane 7, mcRFB4-AAA. This is one of three experiments.
Figure 4 Analysis of the stability of cRFB4 constructs in mouse serum in vivo. (A) cRFB4: time 0 h; (B) cRFB4: time 24 h; (C) mcRFB4-H310A: time 0 h. (D) mcRFB4-H310A: time 24 h. Black line: measurement of radioactivity in cpm.; red line: measurement of the absorbance at 280 nm. IgM, immunoglobulin M; IgG, immunoglobulin G; Alb, albumin. This is one of two experiments.
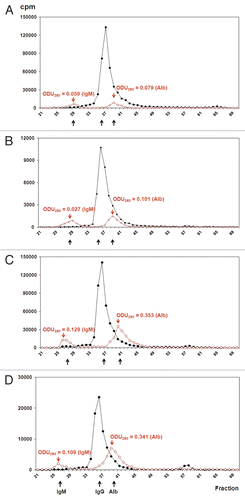
Figure 5 4–15% SDS-PAGE of murine and chimeric RFB4 ITs under non-reducing conditions. Lane 1, RFB4-rRTA; lane 2, cRFB4-rRTA; lane 3, mcRFB4-P247W-rRTA; lane 4, mcRFB4-H310A-rRTA. This is one of three experiments.
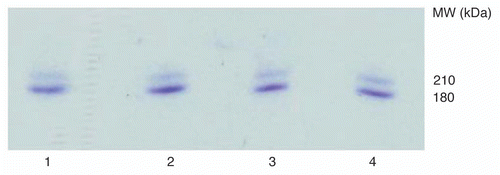
Figure 6 Antigen-binding activity of the ITs vs. the corresponding cmAbs as determined by FACS. (A) (▴) cRFB4; (●) cRFB4-rRTA; (B) (▴) mcRFB4-P247W; (●) mcRFB4-P247W-rRTA; (C) (▴) mcRFB4-H310A; (●) mcRFB4-H310A-rRTA. This is one of three experiments.
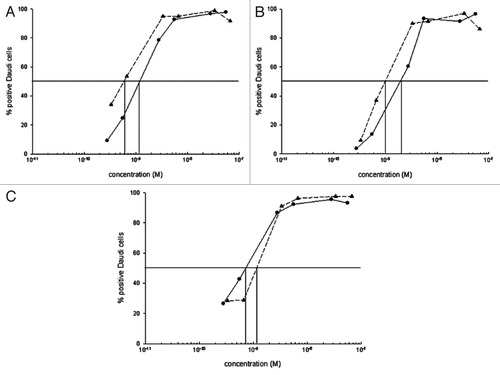
Figure 7 PVL of anti-CD22 ITs in mice. Open column, RFB4-rRTA; Gray column, cRFB4-rRTA; Black column, mcRFB4-H310A-rRTA. Murine RFB4-rRTA and cRFB4-rRTA ITs showed similar toxicity regardless the doses (p > 0.26), while at the dose of 7.5 mg/kg, mcRFB4-H310A-rRTA is significantly less toxic as compared with both murine and chimeric RFB4 ITs (p < 0.04). This is one of two experiments.
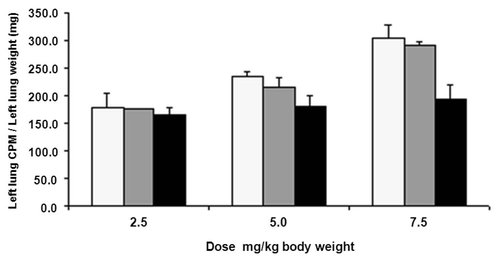
Figure 8 Changes in the body weight of BALB/c mice after treatment with various doses of ITs. (A) 2.5 mg/kg; (B) 5 mg/kg; (C) 7.5 mg/kg; (■) RFB4-rRTA; (□) cRFB4-rRTA; (▴) mcRFB4-H310A-rRTA. This is one of two experiments.
Table 1 Pharmacokinetics of cRFB4 constructs in Swiss Webster miceTable Footnotea
Table 2 Cytotoxicity of ITs on CD22+ Daudi tumor cellsTable Footnotea
Table 3 Pharmacokinetics of ITs and their corresponding mAbs in BALB/c miceTable Footnotea
Additional material
Download Zip (370.8 KB)Acknowledgments
We are grateful to Dr. Pamela Bjorkman for the generous gift of human and rat FcRns. We gratefully acknowledge Dr. Victor Ghetie for invaluable comments and discussions in the preparation of the manuscript. We thank Kelly Mapes and Xiang Gu for the technical help and Linda Berry for the assistance in preparing the manuscript. These studies were supported by the Cancer Immunobiology Center.
References
- Pastan I, Hassan R, FitzGerald DJ, Kreitman RJ. Immunotoxin treatment of cancer. Annu Rev Med 2007; 58:221 - 237; PMID: 17059365; http://dx.doi.org/10.1146/annurev.med.58.070605.115320
- Fodstad O, Kvalheim G, Godal A, Lotsberg J, Aamdal S, Høst H, et al. Phase I study of the plant protein ricin. Cancer Res 1984; 44:862 - 865; PMID: 6692385
- Kreitman RJ, Wilson WH, White JD, Stetler-Stevenson M, Jaffe ES, Giardina S, et al. Phase I trial of recombinant immunotoxin anti-Tac(Fv)-PE38 (LMB-2) in patients with hematologic malignancies. J Clin Oncol 2000; 18:1622 - 1636; PMID: 10764422
- Thorpe PE, Wallace PM, Knowles PP, Relf MG, Brown AN, Watson GJ, et al. Improved antitumor effects of immunotoxins prepared with deglycosylated ricin A-chain and hindered disulfide linkages. Cancer Res 1988; 48:6396 - 6403; PMID: 3263186
- Herrera L, Bostrom B, Gore L, Sandler E, Lew G, Schlegel PG, et al. A phase 1 study of Combotox in pediatric patients with refractory B-lineage acute lymphoblastic leukemia. J Pediatr Hematol Oncol 2009; 31:936 - 941; PMID: 19875969; http://dx.doi.org/10.1097/MPH.0b013e3181bdf211
- Schnell R, Staak O, Borchmann P, Schwartz C, Matthey B, Hansen H, et al. A Phase I study with an anti-CD30 ricin A-chain immunotoxin (Ki-4.dgA) in patients with refractory CD30+ Hodgkin's and non-Hodgkin's lymphoma. Clin Cancer Res 2002; 8:1779 - 1786; PMID: 12060617
- Schnell R, Vitetta E, Schindler J, Borchmann P, Barth S, Ghetie V, et al. Treatment of refractory Hodgkin's lymphoma patients with an anti-CD25 ricin A-chain immunotoxin. Leukemia 2000; 14:129 - 135; PMID: 10637488; http://dx.doi.org/10.1038/sj.leu.2401626
- Lynch TJ Jr, Lambert JM, Coral F, Shefner J, Wen P, Blattler WA, et al. Immunotoxin therapy of small-cell lung cancer: a phase I study of N901-blocked ricin. J Clin Oncol 1997; 15:723 - 734; PMID: 9053498
- Amlot PL, Stone MJ, Cunningham D, Fay J, Newman J, Collins R, et al. A phase I study of an anti-CD22-deglycosylated ricin A chain immunotoxin in the treatment of B-cell lymphomas resistant to conventional therapy. Blood 1993; 82:2624 - 2633; PMID: 8219217
- Avarbock AB, Loren AW, Park JY, Junkins-Hopkins JM, Choi J, Litzky LA, et al. Lethal vascular leak syndrome after denileukin diftitox administration to a patient with cutaneous gamma/delta T-cell lymphoma and occult cirrhosis. Am J Hematol 2008; 83:593 - 595; PMID: 18335564; http://dx.doi.org/10.1002/ajh.21180
- Posey JA, Khazaeli MB, Bookman MA, Nowrouzi A, Grizzle WE, Thornton J, et al. A phase I trial of the single-chain immunotoxin SGN-10 (BR96 sFv-PE40) in patients with advanced solid tumors. Clin Cancer Res 2002; 8:3092 - 3099; PMID: 12374676
- Schindler J, Sausville E, Messmann R, Uhr JW, Vitetta ES. The toxicity of deglycosylated ricin A chain-containing immunotoxins in patients with non-Hodgkin's lymphoma is exacerbated by prior radiotherapy: a retrospective analysis of patients in five clinical trials. Clin Cancer Res 2001; 7:255 - 258; PMID: 11234876
- Baluna R, Rizo J, Gordon BE, Ghetie V, Vitetta ES. Evidence for a structural motif in toxins and interleukin-2 that may be responsible for binding to endothelial cells and initiating vascular leak syndrome. Proc Natl Acad Sci USA 1999; 96:3957 - 3962; PMID: 10097145; http://dx.doi.org/10.1073/pnas.96.7.3957
- Lindstrom AL, Erlandsen SL, Kersey JH, Pennell CA. An in vitro model for toxin-mediated vascular leak syndrome: ricin toxin A chain increases the permeability of human endothelial cell monolayers. Blood 1997; 90:2323 - 2334; PMID: 9310483
- Siegall CB, Liggitt D, Chace D, Tepper MA, Fell HP. Prevention of immunotoxin-mediated vascular leak syndrome in rats with retention of antitumor activity. Proc Natl Acad Sci USA 1994; 91:9514 - 9518; PMID: 7937798; http://dx.doi.org/10.1073/pnas.91.20.9514
- Baluna R, Coleman E, Jones C, Ghetie V, Vitetta ES. The effect of a monoclonal antibody coupled to ricin A chain-derived peptides on endothelial cells in vitro: insights into toxin-mediated vascular damage. Exp Cell Res 2000; 258:417 - 424; PMID: 10896793; http://dx.doi.org/10.1006/excr.2000.4954
- Smallshaw JE, Ghetie V, Rizo J, Fulmer JR, Trahan LL, Ghetie MA, et al. Genetic engineering of an immunotoxin to eliminate pulmonary vascular leak in mice. Nat Biotechnol 2003; 21:387 - 391; PMID: 12627168; http://dx.doi.org/10.1038/nbt800
- Sharkey RM, Press OW, Goldenberg DM. A re-examination of radioimmunotherapy in the treatment of non-Hodgkin lymphoma: prospects for dual-targeted antibody/radioantibody therapy. Blood 2009; 113:3891 - 3895; PMID: 19182204; http://dx.doi.org/10.1182/blood-2008-11-188896
- Ghetie MA, Richardson J, Tucker T, Jones D, Uhr JW, Vitetta ES. Antitumor activity of Fab' and IgG-anti-CD22 immunotoxins in disseminated human B lymphomas grown in mice with severe combined immunodeficiency disease: effect on tumor cells in extranodal sites. Cancer Res 1991; 51:5876 - 5880; PMID: 1933855
- Borvak J, Richardson J, Medesan C, Antohe F, Radu C, Simionescu M, et al. Functional expression of the MHC class I-related receptor, FcRn, in the endothelial cells of mice. Int Immunol 1998; 10:1289 - 1298; PMID: 9786428; http://dx.doi.org/10.1093/intimm/10.9.1289
- Ghetie V, Ward ES. Multiple roles for the major histocompatibility complex class I-related receptor FcRn. Annu Rev Immunol 2000; 18:739 - 766; PMID: 10837074; http://dx.doi.org/10.1146/annurev.immunol.18.1.739
- Pop LM, Liu X, Ghetie V, Vitetta ES. The generation of immunotoxins using chimeric anti-CD22 antibodies containing mutations which alter their serum half-life. Int Immunopharmacol 2005; 5:1279 - 1290; PMID: 15914332; http://dx.doi.org/10.1016/j.intimp.2005.03.013
- Medesan C, Matesoi D, Radu C, Ghetie V, Ward ES. Delineation of the amino acid residues involved in transcytosis and catabolism of mouse IgG1. J Immunol 1997; 158:2211 - 2217; PMID: 9036967
- Kim JK, Firan M, Caius RG, Kin CH, Ghetie V, Ward ES. Mapping the site on human IgG for binding of the MHC class I-related receptor, FcRn. Eur J Immunol 1999; 29:2819 - 2825; PMID: 10508256; http://dx.doi.org/10.1002/(SICI)1521-4141(199909)29:09<2819::AID-IMMU2819>3.0.CO;2-6
- Martin WL, West AP Jr, Gan L, Bjorkman PJ. Crystal structure at 2.8 A of an FcRn/heterodimeric Fc complex: mechanism of pH-dependent binding. Mol Cell 2001; 7:867 - 877; PMID: 11336709; http://dx.doi.org/10.1016/S1097-2765(01)00230-1
- van Horssen PJ, van Oosterhout YV, de Witte T, Preijers FW. Cytotoxic potency of CD22-ricin A depends on intracellular routing rather than on the number of internalized molecules. Scand J Immunol 1995; 41:563 - 569; PMID: 7770726; http://dx.doi.org/10.1111/j.1365-3083.1995.tb03608.x
- van Oosterhout YV, van den Herik-Oudijk IE, Wessels HM, de Witte T, van de Winkel JG, Preijers FW. Effect of isotype on internalization and cytotoxicity of CD19-ricin A immunotoxins. Cancer Res 1994; 54:3527 - 3532; PMID: 7516821
- Firan M, Bawdon R, Radu C, Ober RJ, Eaken D, Antohe F, et al. The MHC class I-related receptor, FcRn, plays an essential role in the maternofetal transfer of gamma-globulin in humans. Int Immunol 2001; 13:993 - 1002; PMID: 11470769; http://dx.doi.org/10.1093/intimm/13.8.993
- Kenanova V, Olafsen T, Crow DM, Sundaresan G, Subbarayan M, Carter NH, et al. Tailoring the pharmacokinetics and positron emission tomography imaging properties of anti-carcinoembryonic antigen single-chain Fv-Fc antibody fragments. Cancer Res 2005; 65:622 - 631; PMID: 15695407
- Kenanova V, Olafsen T, Williams LE, Ruel NH, Longmate J, Yazaki PJ, et al. Radioiodinated versus radiometal-labeled anti-carcinoembryonic antigen single-chain Fv-Fc antibody fragments: optimal pharmacokinetics for therapy. Cancer Res 2007; 67:718 - 726; PMID: 17234783; http://dx.doi.org/10.1158/0008-5472.CAN-06-0454
- Hinton PR, Johlfs MG, Xiong JM, Hanestad K, Ong KC, Bullock C, et al. Engineered human IgG antibodies with longer serum half-lives in primates. J Biol Chem 2004; 279:6213 - 6216; PMID: 14699147; http://dx.doi.org/10.1074/jbc.C300470200
- Dall'Acqua WF, Kiener PA, Wu H. Properties of human IgG1s engineered for enhanced binding to the neonatal Fc receptor (FcRn). J Biol Chem 2006; 281:23514 - 23524; PMID: 16793771; http://dx.doi.org/10.1074/jbc.M604292200
- Yeung YA, Leabman MK, Marvin JS, Qiu J, Adams CW, Lien S, et al. Engineering human IgG1 affinity to human neonatal Fc receptor: impact of affinity improvement on pharmacokinetics in primates. J Immunol 2009; 182:7663 - 7671; PMID: 19494290; http://dx.doi.org/10.4049/jimmunol.0804182
- Gillies SD, Lo KM, Burger C, Lan Y, Dahl T, Wong WK. Improved circulating half-life and efficacy of an antibody-interleukin 2 immunocytokine based on reduced intracellular proteolysis. Clin Cancer Res 2002; 8:210 - 216; PMID: 11801561
- Kim JK, Tsen MF, Ghetie V, Ward ES. Evidence that the hinge region plays a role in maintaining serum levels of the murine IgG1 molecule. Mol Immunol 1995; 32:467 - 475; PMID: 7783750; http://dx.doi.org/10.1016/0161-5890(95)00019-B
- Ghetie MA, Uhr J, Vitetta ES. Covalent binding of human a2-macroglobulin to deglycosylated ricin A chain and its immunotoxins. Cancer Res 1991; 51:1482 - 1487; PMID: 1705175
- Ghetie V, Ward ES, Vitetta ES. Figg WD, MsLeod H. Pharmacokinetics of antibodies and immunotoxins in mice and humans. Handbook of anticancer pharmacokinetics and pharmacodynamics 2004; Humana Press 475 - 498
- Weldon JE, Xiang L, Chertov O, Margulies I, Kreitman RJ, FitzGerald DJ, et al. A protease-resistant immunotoxin against CD22 with greatly increased activity against CLL and diminished animal toxicity. Blood 2009; 113:3792 - 3800; PMID: 18988862; http://dx.doi.org/10.1182/blood-2008-08-173195
- Rosenblum MG, Cheung LH, Liu Y, Marks JW 3rd. Design, expression, purification and characterization, in vitro and in vivo, of an antimelanoma single-chain Fv antibody fused to the toxin gelonin. Cancer Res 2003; 63:3995 - 4002; PMID: 12873997
- Woo JH, Lee YJ, Neville DM, Frankel AE. Pharmacology of anti-CD3 diphtheria immunotoxin in CD3 positive T-cell lymphoma trials. Methods Mol Biol 2010; 651:157 - 175; PMID: 20686966; http://dx.doi.org/10.1007/978-1-60761-786-0_10
- Tazzari PL, Polito L, Bolognesi A, Pistillo MP, Capanni P, Palmisano GL, et al. Immunotoxins containing recombinant anti-CTLA-4 single-chain fragment variable antibodies and saporin: in vitro results and in vivo effects in an acute rejection model. J Immunol 2001; 167:4222 - 4229; PMID: 11591743
- Alderson RF, Kreitman RJ, Chen T, Yeung P, Herbst R, Fox JA, et al. CAT-8015: a second-generation pseudomonas exotoxin A-based immunotherapy targeting CD22-expressing hematologic malignancies. Clin Cancer Res 2009; 15:832 - 839; PMID: 19188153; http://dx.doi.org/10.1158/1078-0432.CCR-08-1456
- Liu Y, Zhang W, Cheung LH, Niu T, Wu Q, Li C, et al. The antimelanoma immunocytokine scFvMEL/TNF shows reduced toxicity and potent antitumor activity against human tumor xenografts. Neoplasia 2006; 8:384 - 393; PMID: 16790087; http://dx.doi.org/10.1593/neo.06121
- Liu XY, Pop LM, Tsai L, Pop IV, Vitetta ES. Chimeric, divalent and tetravalent anti-CD19 monoclonal antibodies with potent in vitro and in vivo antitumor activity against human B-cell lymphoma and pre-B acute lymphoblastic leukemia cell lines. Int J Cancer 2011; 129:497 - 506; PMID: 20878959; http://dx.doi.org/10.1002/ijc.25695
- O'Hare M, Roberts LM, Thorpe PE, Watson GJ, Prior B, Lord JM. Expression of ricin A chain in Escherichia coli. FEBS Lett 1987; 216:73 - 78; PMID: 3556218; http://dx.doi.org/10.1016/0014-5793(87)80759-7