Abstract
To test the hypothesis that dual-targeting confers the novel ability of selective binding to antigen double-positive over antigen single-positive cells, a single-chain triplebody (sctb), HLA-ds16-hu19, was produced and characterized. The molecule carries three single-chain Fv (scFv) antibody fragments in a single polypeptide chain, the two distal ones specific for the human histocompatibility protein HLA-DR and the B-lymphoid cell surface protein CD19, the central one for CD16, the human low affinity Fc-receptor FcγRIII. For comparison, the bispecific scFvs (bsscFv) hu19-ds16 and HLA-ds16 were also produced. All CD16 binding modules are disulfide-stabilized (ds). The sctb bound simultaneously to both CD19 and HLA-DR on the same cancer cell and, thus, showed functional dual-targeting. In a mixing-experiment with HLA-DR single-positive HUT-78 cells and (HLA-DR plus CD19) double-positive SEM cells, the triplebody showed preferential binding to the double-positive cells, even when the single-positive cells were present in a numerical excess of up to 20-fold. In antibody-dependent cellular cytotoxicity experiments with mononuclear cells as effector cells, the sctb promoted equal lysis of Raji cells, an antigen double-positive cell line, at 130-fold lower concentrations than the bsscFv hu19-ds16, indicating that both distal scFvs of the sctb contributed to tumor cell lysis. A panel of stably-transfected HEK293 cell lines was generated that included CD19- and HLA-DR single-positive and (HLA-DR plus CD19) double-positive lines with antigen-surface densities varying over a broad range. Using a pair of cell lines with matching densities, the sctb eliminated double-positive target cells preferentially single-positive cells. This ability of preferential or selective targeting of antigen double-positive over single-positive cells opens attractive new perspectives for the use of dual-targeting sctbs in cancer therapy.
Introduction
Antibodies have become an integral part of therapeutic protocols for a number of malignancies, including both hematologic diseases and solid tumors such as breast and colon cancer.Citation1–Citation3 So far, 11 antibodies or antibody-derived agents have received drug approval. Although monoclonal antibodies clearly improved the treatment outcome, antibodies do not cure patients as single agents, response rates remain unsatisfactory and relapse still presents a serious problem. A strong imbalance strikes the attention; while hematologic malignancies represent less than one-tenth of all human cancers, 6 of the 11 (55%) approved antibody-therapeutics target hematologic diseases. An intense search has been conducted to develop similarly successful therapeutic antibodies for solid tumors, and more than 100 antibodies for use against solid tumors are in clinical trials. However, the imbalance persists. To date, solid tumors are still more difficult to treat with intact immunoglobulins (Igs) than hematologic cancers. This could be due at least in part to the fact that hematologic malignancies are more accessible to antibodies than solid tumors. Another relevant difference may be that a number of surface antigens that are uniquely present on hematologic cells have been discovered, whereas relevant antigens such as the EGF receptor (EGFR), epithelial cell adhesion molecule (EpCAM), melanoma-associated chondroitin sulfate proteoglycan, prostate specific membrane antigen or GD2, which are attractive targets for the treatment of solid cancers, often have broader tissue distribution and may, therefore, cause undesirable side effects when targeted by therapeutic antibodies.Citation4–Citation10
To develop more successful antibody-derived agents also for solid tumors, one possible solution may be to go beyond the format of intact Igs. To this effect, a number of recombinant antibody-derived formats have been developed including immunotoxins, radio-immunoconjugates, bispecific Igs and bispecific single-chain Fv antibody-derivatives, so-called bsscFvs. A recently approved bispecific antibody is catumaxomab (Removab®), a hybrid Ig with one antigen-binding site each for EpCAM and CD3, which was approved for the treatment of malignant ascites.Citation7 An example of the bsscFv format is blinatumomab, a recombinant fusion protein comprising scFvs specific for CD19 on malignant B-lymphoid cells and CD3 on effector T lymphocytes.Citation11 This molecule produced encouraging clinical responses against non-Hodgkin lymphoma (NHL) and pediatric acute lymphoblastic leukemia (ALL).Citation4,Citation9 Additional bsscFvs are under development.Citation12
Despite blinatumomab's success, the agent has deficits, as one would expect for a prototype of a new class of agents. It has a short plasma retention time (plasma half-life) and thus requires sophisticated administration as a 4-week continuous infusion, which is inconvenient for the patient and adds costs for hospitalization. Furthermore, this therapy is associated with side effects for the majority of patients, including grade 3 and 4 adverse events.Citation11 To further improve the format of tandem scFvs, our group recently developed a new format of recombinant antibody-derivatives, single-chain triplebodies (sctbs). These are fusion proteins of three scFv modules connected in tandem with flexible linkers into a single polypeptide chain.Citation13–Citation16 The two distal scFvs are directed against tumor antigens and the central scFv against the trigger molecule on the effector cell. Sctbs have a larger molecular mass than bsscFvs and therefore have a longer plasma half-life in mice, with anticipated but still unproven favorable effects on the pharmacokinetics in humans.Citation13 The presence of the third scFv component also adds a major new quality, i.e., the capacity for dual-targeting. The two distal scFvs in sctbs can be directed either against the same tumor antigen or against two different antigens. Provided the surface architecture of the tumor cell permits it, this allows a single sctb molecule to bind simultaneously to two different antigens on the same tumor cell and to the trigger molecule on the effector cell, thereby inducing effector cell functions, such as cytolysis by antibody-dependent cellular cytotoxicity (ADCC) or phagocytosis. Combinations of tumor antigens and trigger molecules have been identified that permit such three-point connections, the assembly of a productive immunological synapse and efficient tumor cell lysis. Examples are triplebodies ds19-ds16-ds19, connecting CD19 on malignant B-lymphoid cells with CD16, the low affinity Fc-receptor for Igs, FcγRIII, on natural killer (NK) cells and macrophages;Citation13 33-ds16–33 and 123-ds16–33, connecting either two molecules of CD33 or one molecule of CD33 with one of CD123, respectively, on the surface of AML cells with CD16 on effector cells;Citation14,Citation16 and 33-ds16-ds19, connecting one CD33 and one CD19 molecule on the surface of mixed lineage leukemia cells with CD16 on effectors.Citation15 In two of these cases, evidence supporting the concept of dual-targeting was obtained, and it was clear that the overall cytolytic effects of these agents were not due to the combined effects of two subsets of the sctb each contacting the tumor cell by monovalent-targeting with only one of its two scFv components. Therefore, in favorable instances a judicious choice of target antigens, trigger molecules and effector cells was possible, which permitted elimination of the tumor cell with high efficiency, enhanced by dual-targeting.
This new quality offers another major advantage over the use of bsscFvs. The triplebodies bind with higher avidity to the cancer cell, due to simultaneous binding with both scFv components. This effect is more than additive, i.e., the triplebody binds more strongly than with the sum of the monovalent affinities of the two scFv components directed against the tumor antigens. For two of the examples noted, the avidity of the sctb for double-positive cells was ∼3-fold larger than the monovalent affinity for single-positive cells. Surprisingly, this 3-fold gain in binding strength led to a gain in specific cytolytic activity of ∼20- to 30-fold and, in some cases, up to more than 100-fold.Citation13,Citation16 Other still unknown variables must contribute even more strongly to the increased specific cytolytic potential of an sctb over the corresponding bsscFv.Citation13–Citation16
The objective in designing dual-targeting sctbs such as the 33-ds16-ds19Citation15 was to discriminate better between antigen double-positive cancer cells and normal cells, which display only one of these antigens on their surface. This should result in preferential elimination of the antigen double-positive cancer cell over single-positive healthy cells. This sctb was indeed capable of simultaneous binding to one molecule each of CD19 and CD33 on the same cancer cell and of eliminating double-positive mixed lineage leukemic (MLL) cells with greater efficacy than the corresponding bsscFvs 33-ds16 and ds19-ds16, which served as controls in ADCC experiments.Citation15 However, these data did not include a test of the hypothesis that a dual-targeting sctb may be capable of eliminating double-positive MLL blasts with preference over single-positive healthy cells.
The present study was designed to test this hypothesis. Here, however, we chose not to work with MLL-derived cells because these cells usually have a far greater surface density of CD19 than of CD33, which makes a quantitative interpretation of the results difficult. A quantitative interpretation of the results would be facilitated by the use of double-positive cell lines with comparable numbers of both antigens. Therefore, in the present study a better suited model-system based on double-positive cell lines with surface densities of the relevant target antigens comparable to those on corresponding single-positive lines was created to test the hypothesis. Panels of stably transfected cell lines that were either single-transfected with one each of the target antigens or double-transfected with both were produced. The target antigens used were HLA-DR and CD19; the transfected cells were HEK 293 cells, which are not of hematopoietic descent and express no endogenous HLA-DR and CD19. A panel of 23 transfected cell lines was established and characterized, and then cell lines with suitable surface densities of either HLA-DR alone or the combination of both HLA-DR and CD19 were chosen for ADCC experiments with the triplebody HLA-ds16-hu19 and human mononuclear cells (MNCs) as a source of NK cells. Preferential elimination of double-positive over single-positive cells was obtained in this model. If this result can be extended from transfected cells in culture to cancer cells in vivo, this will open the possibility for a broad range of applications for dual targeting sctbs in cancer treatment.
Dual-targeting, as demonstrated here, will further increase drug specificity and tumor selectivity. On this basis, it should be possible to address cancer cells in a more exclusive way, which, on the one hand will enhance better response rates in terms of long-lasting complete clinical and molecular remission rates as the fundamental prerequisite for permanent cure of cancer, and on the other hand, will provide better drug safety and tolerability to improve the patient's quality of life.
Results
Construction, expression and enrichment of the dual targeting triplebody HLA-ds16-hu19 and control proteins.
The sctb HLA-ds16-hu19 was constructed from previously published scFv components with specificities for CD19, CD16 or HLA-DR ().Citation17,Citation18 The sctb, the bsscFvs hu19-ds16 and HLA-ds16, and the monospecific triplebody HLA-ds16-HLA were produced in transiently transfected HEK 293T cells, and enriched from cell culture supernatants by affinity chromatography. Yields of the enriched proteins ranged from 0.8–1.2 mg per liter of culture medium in different production runs. The sctb had an electrophoretic mobility in SDS-PAGE experiments corresponding to a Mr of ∼90 kDa, in close agreement with the value of 89 kDa predicted from its sequence (). The 50 kDa band is probably bovine serum albumin, a contamination resulting from the cell culture media that in our controls migrate at this mobility. No immunoreactive degradation products were observed in protein gel blot experiments ( and D).
Binding characteristics of the dual-targeting triplebody HLA-ds16-hu19.
The sctb reacted with HLA-DR, CD16 and CD19 on separate, stably transfected, antigen single-positive cells (), and no binding to the untransfected cells () was observed. To assess whether incorporation of a second scFv-component with specificity for a tumor-cell antigen into the sctb resulted in a gain of avidity for CD19 and HLA-DR double-positive cells, the avidity (equilibrium binding constant KD) of the sctb was determined. Equilibrium binding curves were recorded by calibrated flow cytometry, and KD values were determined (). The sctb had an overall KD of 37 ± 4 nM for antigen double-positive cells, while the affinity of the control bsscFvs was ∼1.6-fold lower for CD19 (KD = 61 ± 13 nM) and 2-fold lower for HLA-DR (KD = 71 ± 6 nM). These data indicate that both scFv components in the sctb were functional and contributed to the overall avidity of its binding to antigen double-positive cells. In contrast, the affinity of the CD16-specific scFv component remained almost unchanged, regardless of whether this component was carried in the bsscFv, where only its N-terminus was engaged, or in the sctb, where it was engaged at both ends by scFvs. The KD value for binding to CD16 in the sctb was 31 ± 2 nM, and for the CD16-specific scFv-component contained in the bsscFvs HLA-ds16 and hu19-ds16 the values were 39 ± 3 and 40 ± 4 nM, respectively (). This experiment provides strong support for the conclusion that both scFv components contributed to the overall avidity of the sctb for antigen double-positive cells.
Serum-stability of the triplebody and control proteins 37°C in vitro.
An important factor affecting the therapeutic efficacy of antibody-derived proteins is their stability in human serum. To measure this property, which reflects the combined stability against proteolytic degradation and denaturation of a protein, the enriched protein was incubated in human serum at 37°C in vitro. After different time intervals, aliquots were removed and residual binding to CD19, HLA-DR, CD16 and combinations of these antigens, respectively, on antigen positive cells was measured by FACS-analysis. Numerical values for half-life (t1/2) were determined (). The individual scFv components specific for the three different antigens showed individual sequence-dependent stabilities, but the entire sctb still retained 71% of its initial binding capacity for double-positive cancer cells after an incubation for five days (Fig. S1). Therefore, in comparison to other scFv fusion proteins,Citation13,Citation16–Citation18 this sctb had an elevated serum-stability.
Selectivity of binding to antigen double-positive cells mediated by dual-targeting.
Our working hypothesis was that the capacity for dual-targeting should endow the sctb with the ability to bind antigen double-positive cells with a higher degree of selectivity over antigen single-positive cells. This prediction was based on the consideration that, according to chemical reaction kinetics, a dual-targeting sctb should have a probability for simultaneous binding to two different target antigens on the same double-positive cell proportional to the sum of the respective surface antigen densities. In contrast, the probability for binding of this sctb to antigen single-positive cells should be proportional to the density of the single antigen only. To test this hypothesis, binding of the dual-targeting sctb HLA-ds16-hu19 to antigen double-positive SEM cells and antigen single-positive HUT-78 cells was measured using FACS assays. Human leukemia-derived SEM cells carried ∼61,000 molecules of HLA-DR per cell and 90,000 molecules of CD19. The HUT-78 cells, a human T-cell leukemia-derived cell line, carried ∼72,000 molecules of HLA-DR per cell and lacked detectable CD19. The SEM cells were labeled with the fluorescent dye calcein-AM and were then mixed with unlabeled HUT-78 cells in various differing proportions, ranging from 1:1 to 1:20. The two component populations of the mixture were clearly distinguished by FACS analysis, reflecting the 1:1 input ratio (). The mixture was then incubated with the sctb and with a second antibody specific for the His-tag contained in the sctb plus a tertiary phycoerythrin (PE) conjugated Fab-fragment to detect the bound anti-his-tag reagent.
After incubation with 55 nM of the sctb, double-positive SEM cells were shifted to the right (red/green fluorescent) quadrant (), whereas less than half of the antigen single-positive HUT-78 cells were shifted. For and D, mixing ratios of SEM:HUT cells of 1:10 and 1:20 were used and, even in these cases, all SEM cells were shifted into the right quadrant, but only about half of the HUT-78 cells ( and D). We conclude that the sctb showed strong preferential binding to the antigen double-positive SEM cells over the antigen single-positive HUT-78 cells, when both were simultaneously present in the same reaction mixture, and that this near-total selectivity occurred even when the single-positive cells were present in an up to 20-fold numerical excess. These data therefore provide strong support for our working hypothesis.
ADCC of leukemia-derived cell lines mediated by the sctb and the bsscFvs.
The ability of the sctb to mediate ADCC in combination with effector cells from healthy human donors was measured for the CD19- and HLA-DR-double-positive Burkitt lymphoma-derived cell line Raji. ADCC reactions were performed with freshly prepared unstimulated effector cells (MNCs or PMNs). Tumor cell lysis was antigen-specific, and the sctb required specific interaction with both the target antigens on the tumor cells and the trigger molecule on the effector cells to achieve its lytic effect. Moreover, the sctb induced target cell lysis by ADCC over a broad range of effector-to-target cell (E:T) ratios, ranging from 80:1 to 1:1 (). The extent of lysis increased with increasing E:T ratios, demonstrating a clear dependence on the amount of effector cells for tumor cell lysis. The cytolytic effect was antigen-specific because the similarly constructed control sctb with specificity for CD7 produced no significant lysis of the CD7-negative Raji cells, not even at high E:T ratios. To narrow down the fraction of leukocytes that contained the relevant effector cells, whole blood from healthy donors was fractionated into mononuclear (MNC) and granulocytic polymorphonuclear (PMN) sub-fractions. The different sub-fractions were then employed in 51Cr-release assays with Raji cell targets (). The sctb triggered significant tumor cell lysis with MNCs, but not with PMNs. Similar experiments had earlier been conducted by our team for other sctbs, which carried a scFv for CD16 as the trigger. In those experiments purified NK cells were also employed, and the result was, within the MNC fraction, NK cells were the relevant effector cells. No contribution from T cells, NKT-cells, monocytes or mast cells was observed,Citation16 and by analogy we presume the same to be true for the sctb studied here. To test whether target cell lysis was antigen-specific, competition ADCC experiments were performed with the sctb HLA-ds16-hu19 at a concentration of 1 nM (). Co-incubation with a 500-fold molar excess of both CD19- and HLA-DR-specific scFvs blocked lysis of Raji cells, whereas competition with either scFv alone reduced target cell lysis only to about half-maximum values. Therefore, both scFvs specific for CD19 and HLA-DR contained in the triplebody must have contributed to the ADCC reaction. Co-incubation with a 125-fold molar excess of the parental CD16-specific antibody 3G8, but not with an IgG1 isotype control antibody (anti-CD7 TH69),Citation19 significantly reduced lysis of Raji cells, demonstrating the specificity of the reaction for CD16-positive effector cells.
To investigate whether the increased binding avidity of the sctb over the bsscFvs specific for CD19 and HLA-DR would be associated with enhanced cytotoxic potential, the ability of all three molecules to mediate ADCC was measured in parallel for Raji cells. The cell line was efficiently eliminated by the sctb and the bsscFvs in a concentration-dependent manner, whereas a control sctb with specificities for CD7 and CD16 was ineffective (). EC50 values (half maximum effector concentrations) are shown in . The EC50 value for the sctb was 8 pM, and for the bsscFvs hu19-ds16 and HLA-ds16, the values were 1,002 and 25 pM, respectively. Therefore, the sctb achieved an equal degree of lysis at a 136-fold lower concentration than the bsscFv hu19-ds16 and at a 3-fold lower concentration than the bsscFv HLA-ds16. The difference between the two bsscFvs is probably due in part to the fact that Raji cells carried approximately 10-fold greater cell surface density of HLA-DR than of CD19 (79,400 HLA-DR and 7,800 CD19 molecules per cell). The differences in the dose-response curves between the triplebody and the bsscFv hu19-ds16 were statistically significant. The sctb had a comparatively high specific cytolytic activity, because an EC50 value in the low picomolar range is remarkable. The comparison between the sctb and the bsscFv hu19-ds16 shows that the addition of the third scFv-component specific for HLA-DR resulted not only in a gain of binding avidity, but also in a more than proportional gain in cytolytic activity, indicating that both scFv components specific for CD19 and HLA-DR of one single sctb molecule were capable of simultaneous binding to the cancer cell and of contributing to the ADCC activity. These results allow us to rule out the alternative that the overall degree of lysis achieved was simply the sum of two components, one due to monovalent binding of the sctb to HLA-DR and the other to monovalent binding to CD19. Had this been the case, then no change in avidity of the sctb over the bsscFvs should have been observed, and the dose-response curve for the sctb in should have shown only a minimal shift to the left, but not the substantial shift actually observed.
Preferential ADCC lysis of double-positive over single-positive target cells by dual targeting.
To assess whether dual-targeting can lead to preferential lysis of antigen double-positive over single-positive cells, a panel of 23 stably transfected HEK 293 cells was established (). Six CD19 single-positive, seven HLA-DR single-positive and nine double-positive lines were obtained. The lines retained stable antigen densities over an extended number of passages, as shown for three consecutive passages of the line EZK20 as a representative example (). The antigen densities for all CD19-positive lines exceeded 100,000 antigen molecules per cell, ranging up to approximately half a million molecules per cell, indicating an effective transfection procedure and very efficient expression of CD19. On the doubly-transfected cells, HLA-DR surface density varied over a wide range from 160–260,000 molecules/cell and similarly the CD19 densities of these clones varied between 1,700 and 670,000 molecules/cell. On the seven single-positive lines, the density of HLA-DR varied between 2,000 and 44,000 molecules/cell.
To assess the possibility of preferential elimination of double- over single-positive target cells by the dual-targeting sctb, the experiment depicted in was performed. In one arm of the experiment, double-positive EZK20 cells with ∼2,000 molecules each of CD19 and HLA-DR on their surface () were labeled with 51Cr, and single-positive EZK-HLA 16 cells with comparable density of HLA-DR (∼1,800 molecules per cell; ) were left unlabeled. Equal numbers of both cell lines were mixed and placed into a well of a microtiter plate. In the other arm, HLA-DR single-positive EZK-HLA 16 cells were labeled with 51Cr and mixed with an equal number of unlabeled, double-positive EZK20 cells. The first group was then treated with the dual-targeting sctb HLA-ds16-hu19 at 1 nM concentration in the reaction, a saturating concentration according to the dose-response curve (). MNCs were added and ADCC reactions were performed with an E:T ratio of 40:1, sufficient for maximum lysis (). The same reagent was added to the second arm of the experiment performed in the same microtiter plate, with all other experimental conditions identical for both arms. As a result, in the first arm ( and left bar) the dual-targeting sctb achieved a significantly higher degree of lysis of the double-positive (labeled) cells than a monospecific sctb directed against HLA-DR and CD16 (data not shown). In the second arm ( and right bar), the sctb achieved a lower degree of lysis of the single-positive (labeled) cells. This experiment was performed 4 times, and statistical significance for the difference in lysis of the two cell populations (* in ) was achieved. We interpret these results to indicate that the dual-targeting sctb preferentially lysed the double-positive target cells over the single-positive ones when the target HLA-DR was present in equal numbers on both cell lines.
Discussion
Antibodies evolved primarily as tools for the defense against infectious agents and foreign invaders, such as bacteria, viruses, fungi, parasites and worms. Apparently, there has been no selective pressure favoring the development of antibodies as tools for the defense against spontaneously arising human tumors. In general, human tumor patients carry no antibodies and reactive lymphocytes against their own tumor antigens, although tolerance is occasionally broken and autologous anti-tumor responses have been reported in reference Citation20 and Citation21. However, these responses in general are not strong enough to prevent the outgrowth of tumors and deliberate attempts to break tolerance by tumor vaccination in humans have only shown modest success.Citation22 Most often these invaders carry multiple copies of the same target antigen on their surface, and antibodies can use both antigen-binding sites for simultaneous binding to two copies of the same antigen on the same invader particle, thus leading to enhanced binding by an avidity effect. For example, major neutralizing human antibodies are directed against the glycoprotein N of the human cytomegalovirus.Citation23 This antigen is present in several hundred copies per virus particle,Citation24 and a rough calculation demonstrates that the average spacing between two such antigens is on the order of less than ≤20 nm. Therefore, a typical IgG molecule, with spacing between its two identical antigen binding sites of ∼15 nm, will be suited for simultaneous high avidity binding to two copies of the same antigen on the same viral particle, mediating its efficient elimination by Fc-receptor carrying phagocytes. Moreover, it can trigger complement activation, and viruses opsonized with complement fragments such as C3b are eliminated with enhanced efficacy by phagocytes double-positive for C3b- and Fc-receptors. In this instance, the innate immune defense greatly benefits from employing the principle of dual-targeting. Our design was inspired by this important precedent case, without which human evolution would likely have taken a different course.Citation25
In contrast, on most spontaneously arising human cancer cells, the surface density of suitable target antigens is far lower than on the infectious agents just discussed. Although some antigens are present in excess of 100,000 copies per cancer cell, many others are present in far lower densities in the range of several thousand to 10,000 copies per cell. The highly successful antibodies in today's cancer therapies, such as rituximab (Rituxan®) and trastuzumab (Herceptin®), are directed against antigens present in high density, such as CD20, which is present on most lymphoma cells in excess of 200,000 copies per cell, and Her2/neu, which is present on breast cancer cells, in densities often exceeding 1 million copies per cell. At these densities, the average spacing between two copies of the target antigen is again in the range of ≤20 nm, assuming free lateral diffusion of the antigen in the tumor cell membrane. Therefore, the therapeutically successful antibodies available today most likely achieve their effect via simultaneous, high avidity binding with both their antigen combining sites to two copies of the same antigen on the same cancer cell.
By contrast, many surface antigens that are attractive on the ground of theoretical considerations, such as a highly restricted tissue distribution, are present in far lower densities on cancer cells. CD19 for example, is an attractive target for leukemia therapy, but on most human leukemia cells it is present in densities of only about ≤25,000 copies per cell.Citation26 A rough calculation demonstrates that at this antigen density, the average spacing between two copies of CD19 on the same cell is about 200 nm, assuming random spacing in the membrane and no clustering in protein islands.Citation27 Therefore, a conventional IgG antibody has a far lower probability for simultaneous, high avidity binding with both of its antigen binding sites to the same cell for CD19 than for CD20, Her2 or EGFR, and this presumably is one of the main reasons why conventional CD19 antibodies were not successful in clinical trials for human leukemias and B-lineage lymphomas.
In the case of antigens present at intermediate densities, it therefore seems necessary to adjust the antibody format to these densities, provided the fluid membrane model of human tumor cells adequately reflects reality. Since, for most human tumor cells, the precise surface architecture and the lateral mobility of antigens are still unknown, we assume unrestricted, random lateral diffusion of the antigens within the membrane.
To enhance the probability of the high avidity binding of the therapeutic protein to tumor cells, our group therefore developed the dual-targeting sctb format.Citation14,Citation15 These molecules carry two binding sites for target antigens on the cancer cell with a spacing of ≤20 nm, thus greater than the maximum spacing of the two binding sites of a conventional IgG.Citation16 For two dual-targeting sctbs, 123-ds16–33 and 33-ds16–19, we previously demonstrated that they can indeed simultaneously bind to one copy each of the two different antigens on the same cancer cell, and that binding to both contributes significantly to both overall binding avidity of the molecule and biological activity in ADCC assays.Citation14,Citation15 A so far untested hypothesis is that dual-targeting should lead to preferential or more selective binding of a sctb to antigen double-positive target cells over antigen single-positive cells when both cells carry the antigen in similar density and are simultaneously present in the same reaction compartment in comparable numbers. The present study was designed to test this hypothesis in a suitable model situation. Stable human tumor-derived cell lines carrying the relevant antigens in the required densities needed to be established first by stable transfection and long-term culture monitoring the stable presence of the antigens.
The three central new findings of this study were: (1) The dual-targeting sctb HLA-ds16-hu19 was capable of binding to antigen double-positive cells with both of its scFv components simultaneously and of achieving an enhanced ADCC effect. The enhancement was due to the presence of the additional scFv component compared with the corresponding bsscFvs hu19-ds16 and HLA-ds16, each capable of monovalent targeting only; (2) The dual-targeting agent was capable of preferential binding to antigen double-positive cells over antigen single-positive cells carrying the target antigen HLA-DR at similar densities, when both were present in the same reaction mixture, even when the single-positive cells were present in an up to 20-fold numerical excess. Thus, selective binding can be achieved by dual targeting, confirming the working hypothesis. And (3), The dual-targeting sctb was capable of achieving a degree of preferential elimination of antigen double-positive over single-positive cells in ADCC reactions, when both cell types were present in comparable numbers and carried comparable antigen-densities on their surface.
To better demonstrate the preferential or selective lysis, an experimental model was set up, using stably transfected target cell lines with carefully selected densities of the relevant surface antigens, rather than using existing leukemia-derived cell lines as targets. We chose this approach, because the densities of two target antigens on existing leukemia lines were rarely within 10% of each other, but rather differed by an order of magnitude or more, such as HLA-DR and CD19 on Raji cells, rendering the interpretation of ADCC results mediated by dual-targeting sctbs difficult.
Critics may object that ADCC lysis of stably transfected HEK 293 cell lines by NK cells mediated by our fusion proteins is an artificial model situation that is not representative of lysis of primary cancer cells in vivo because primary cancer cells probably have cell-type specific sensitivity or resistance to the lytic mechanisms imposed by NK cells differing from those of HEK 293 cells. While this argument is reasonable, it does not invalidate the conclusions drawn from the present study because the main purpose of this study was not to develop a new therapeutic agent with optimized in vivo anti-cancer activity, but rather to investigate in a fundamental manner whether it was possible to achieve preferential lysis of double-positive over single-positive cells with the help of dual-targeting agents. This approach is new and has only recently arisen due to the development of dual-targeting sctbs. It has therefore not been studied for other available antibody-derived therapeutics, with one possible exception.Citation28 In this case, a dual-targeting ADC had been designed, DT2219, a single-chain fusion polypeptide consisting of a fragment of diphtheria toxin (DT) fused to scFv-components specific for CD22 and CD19 in tandem. This agent resulted in stronger elimination of CD19- plus CD22-double-positive lymphoma cells than the monospecific control molecules DT1919 and DT2222, carrying two copies of the same scFvs fused in tandem. However, preferential elimination of antigen double-positive over simultaneously present single-positive cells by this agent has not been shown.
The main result supporting our finding (1) described above was the shift of the dose-response curve toward lower concentrations in the ADCC experiments with double-positive Raji cells (). The conclusion is further supported by the 136-fold drop in the EC50 value for the sctb relative to the bsscFv huCD19-dsCD16 (). The experimental result supporting our finding (3) was the outcome of the ADCC experiments given in . For a mixture of the same number of antigen double-positive EZK20 cells and single-positive EZK-HLA 16 cells carrying comparable numbers of HLA-DR molecules per cell, the dual targeting sctb mediated significantly stronger lysis of the double-positive cells. Together, these two results permit the conclusion that preferential lysis of the double-positive over single-positive cells has been achieved by the dual-targeting agent.
In the future, another desirable experiment would be to label both antigen single- and double-positive cells with different labels and to xenotransplant them into suitable immunocompromised mice and measure the differential effect of the dual-targeting sctb on the evolution of both populations. Suitable mice for this purpose are not yet available, but their generation is ongoing.
To test the dual-targeting of the sctb HLA-ds16-hu19 in a more natural situation, a FACS-based binding study was performed. The pro-B-ALL derived SEM cells, double-positive for HLA-DR and CD19, were stained with calcein-AM and mixed with unlabeled T-cell derived, HLA-DR single-positive HUT-78 cells (). The dual-targeting sctb was able to bind all SEM cells, even when the single-positive cells were present in a 20-fold numerical excess (). These data demonstrate a clear benefit of dual-targeting and establish that molecules designed for this ability are able to bind a minority of double-positive cells in the presence of single-positive cells. Comparable data for selectivity of binding, but not for selectivity of ADCC lysis by dual-targeting agents have previously been published by other authors using a bsscFv directed against ErbB2 and ErbB3.Citation29
The preferential lysis of double- over single-positive cells reported here and the clear benefit of the dual-targeting approach opens attractive new perspectives for future applications of such sctbs. One important goal for the development of new anti-cancer agents is to eliminate cancer stem cells (CSCs). This is necessary to prevent relapse from so-called minimal residual disease and to achieve long-lasting therapeutic effects.Citation30 However, CSCs, and in particular leukemia stem cells (LSCs), have properties close to hematopoietic (HSCs) or healthy tissue stem cells. Therefore, the new agents should ideally be capable of preferential targeting of LSCs, discriminating them from healthy HSCs. This would leave sufficient numbers of HSCs to allow hematopoietic reconstitution after the end of therapy without the need for an allogeneic or autologous stem cell transplant. The preferential targeting of double- over single-positive cells achieved by dual-targeting sctbs may conceivably help in the future to reach this goal, at least in part, provided pairs of surface antigens can be identified that discriminate sufficiently between LSCs and healthy HSCs. For several types of leukemias, the immunophenotype of LSCs is under intense study and first antigens that are preferentially present on LSCs over healthy HSCs, and which may become useful for this purpose, have been discovered.Citation30 Examples for certain subtypes of AML are CD123, CD96, CLL-1 and CD47.Citation31–Citation35 CD33 has also been reported to be expressed on a subset of AML-LSCs,Citation36 and a dual targeting sctb 123-ds16–33 has been recently produced in reference Citation14. Based on the results of the present study, we expect many new and highly-specific anticancer agents to be designed in the future. These new agents will provide a new era of targeted-therapy on the basis of selectivity and specificity resulting in a better drug tolerability and safety with thus preserving and/or improving the cancer patient's quality of life, and in increasing long-lasting response rates with the potential of curing the cancer patient from his/her life-threatening disease.
Materials and Methods
Cell lines and hybridomas.
Chinese hamster ovary (CHO) cells, stably transfected with a cDNA expression construct for human FcγRIIIa, were from Dr. J. van de Winkel (University Medical Centre; Utrecht, The Netherlands). L66 murine fibroblasts, stably transfected with two cDNA expression constructs coding for the α- and β-chains of a particular variant of human HLA-DR derived from the hybridoma L243 provided from Dr. B. Stockmeyer. The hybridoma 4G7 CD19, mIgG1 Citation37 was from Dr. R. Levy (Stanford University; Palo Alto, CA). Hybridoma L 243 (HLA-DR, mIgG2a) and Hybridoma 3G8 FcgRIII, CD16, mIgG1 Citation38 were from the American Type Cell Culture Collection (ATCC; Manassas, VA). CHO cells, the hybridomas, L66 cells, the Burkitt lymphoma-derived cell line Raji (German Collection of Microorganisms and Cell Cultures, DSMZ, Braunschweig, Germany), the pro-B-ALL derived cell line SEMCitation39 (ATCC) and the T-cell line HuT-78 (ATCC) were cultured in Roswell Park Memorial Institute 1640 Glutamax-I medium (Invitrogen; Karlsruhe, Germany) containing 10% fetal calf serum (FCS; Invitrogen), 100 units/ml penicillin (Invitrogen) and 100 µg/ml streptomycin (Invitrogen). Human embryonic kidney (HEK) 293 and 293 T cells (ATCC) were maintained in DMEM Glutamax-I medium (Invitrogen) supplemented with 10% FCS, and penicillin and streptomycin at 100 units/ml and 100 µg/ml, respectively.
Bacterial strains and plasmids.
Escherichia coli strain XL-1 blue (Stratagene; Amsterdam, The Netherlands) was used as host for the amplification of the plasmids and for cloning. For construction and eukaryotic expression, the vector pSecTag2HygroC (Invitrogen) was employed.
Construction of recombinant scFv fusion proteins.
To generate the expression plasmid for the sctb HLA-ds16-hu19, the cDNA sequence coding for the scFv of the L243 variant of HLA-DR was amplified by PCR from the cDNA coding for the monoclonal antibody produced by hybridoma L243 and cloned as an SfiI cassette into the vector pSecTag2Hy-groC-Strep-ds19-ds16-ds19,Citation13 generating the plasmid pSec-Tag2HygroC-HLA-dsCD16-dsCD19. The coding sequence for the CDR loop-grafted humanized CD19-specific scFv was amplified by PCR from plasmid pAK400-4G7GRAFTCitation18 and cloned using the restriction enzymes XhoI/AgeI into the correspondingly linearized plasmid, to achieve the plasmid pSecTag2HygroC-HLA-dsCD16-huCD19. The plasmid pSecTag2HygroC-HLA-dsCD16-HLA was obtained by replacing the sequences for 4G7GRAFT by those encoding scFv L243 using XhoI/AgeI restriction sites. The final constructs were sequencedCitation40 on an Applied Biosystems automated DNA sequencer (ABI Prism 310 Genetic Analyzer; Perkin-Elmer, Ueberlingen, Germany).
Generation of stably transfected HEK 293 cells.
HEK 293 cells were transfected either separately or simultaneously with the plasmids pcDNA3.1-CD19 Citation41 and pBud CE4./AmpR-HLA-DR-α&β-HygroC, coding for CD19 and HLA-DR, respectively. To obtain the plasmid pBud CE4./AmpR-HLA-DR-α&β-HygroC, the coding sequences of the α- and β-chains of HLA-DR were amplified via PCR from the plasmids pCR/CMV-HLA-DRα and pCR/CMV-HLA-DRβ (provided by B. Stockmeyer) and cloned in the vector pBud CE4 (Invitrogen) using the restriction enzymes SacI/EcoRV and NotI/PmeI, respectively. The plasmids coded for the full-length open reading frames of both proteins, including their cytoplasmic domains. For transfection, 20 µg of linearized plasmid DNA was added to HEK 293 cells in a 10 cm culture dish at 80% confluence, containing ∼7 million cells. Calcium phosphate transfection was performed according to standard procedures. Single-positive clones for HLA-DR were selected in the presence of 200 µg/ml Hygromycin B (Roth; Karlsruhe, Germany), double-positive clones in the presence of 200 µg/ml Hygromycin B plus 400 µg/ml Geneticin (Invitrogen), and single cell clones were isolated by limited dilution into microwell dishes. Cells were analyzed for the expression of the antigen by flow cytometry. Antigen densities were determined in 3–8 separate experiments with a commercial kit based on calibration with fluorescent microspheres (QifiKit®, Dako Diagnostica; Hamburg, Germany) following manufacturer's instructions.
Expression and purification of recombinant scFv fusion-proteins.
For expression of bsscFvs HLA-ds16, hu19-ds16, the sctb HLA-ds16-hu19, and the corresponding CD7-specific control sctb 7-ds16–7 and bsscFv 7-ds16,Citation13 HEK 293T cells were transiently transfected with the expression plasmids using the calcium phosphate technique including chloroquine.Citation40 Supernatants containing the secreted proteins were collected 5 times over a period of one week and dialyzed at 4°C against a buffer containing 50 mM NaH2PO4, 300 mM NaCl, and 10 mM imidazole at pH 8.0. The recombinant His-tagged proteins were enriched by affinity chromatography with nickel-nitrilotriacetic acid (Ni-NTA) agarose beads (Qiagen; Hilden, Germany) and dialyzed against phosphate buffered saline (PBS). ScFvs were expressed in E. coli and purified as described in reference Citation42 and Citation43.
Sodium dodecyl sulfate (SDS)-PAGE and protein gel blot analysis.
Reducing SDS-PAGE was performed by standard procedures.Citation40 In protein gel blot experiments, the recombinant proteins were detected either with a horseradish peroxidase (HRP)-conjugated antibody specific for the Strep-tag (IBA, Goettingen, Germany), or with an unconjugated penta-His antibody (Qiagen), and a secondary HRP-coupled goat anti-mouse IgG (Dianova, Hamburg, Germany). Protein gel blots were developed using enhanced chemiluminescence reagents (Amersham Pharmacia Biotech, Freiburg, Germany).
Flow cytometric analysis.
Immunofluorescence analysis was performed on a FACS Calibur instrument using CellQuest software (Becton Dickinson, Heidelberg, Germany) as described in reference Citation44. For each sample 1 × 104 events were collected, and whole cells were analyzed using appropriate scatter gates to exclude cellular debris and aggregates. The recombinant fusion proteins were detected using a penta-His antibody and a phycoerythrin (PE)-conjugated goat anti-mouse IgG (Dako), unless otherwise stated.
Determination of equilibrium binding constants (KD).
Equilibrium binding constants (KD) for binding to viable human cancer cells were determined using calibrated flow cytometric methods, as described in reference Citation44 and Citation45. The highest mean fluorescence value was set to 100% and all data points were normalized to this value. The experiments were repeated 5 times and mean values are reported. KD values were calculated using a nonlinear regression curve fit.
Measurement of in vitro stability in human serum.
The sctb HLA-ds16-hu19 was incubated in human serum at sub-saturating concentrations of 2.5 µg/ml in a volume of 350 µl at 37°C. The stabilities in human serum of the individual binding sites with specificity for HLA-DR and CD19 were determined in the same manner, when they were carried in the corresponding bsscFvs with monovalent binding to the target cell. Residual binding activity to viable HLA- and CD19 double positive Raji cells was determined at various time points by flow cytometry. Time point t0 was set to 100%, and all data were normalized to this value. Experiments were performed 3 times and mean values are reported. Retention half-life for each binding site were calculated from a one-phase exponential decay curve fit.
Isolation of mononuclear cells (MNCs) and polymorphnuclear cells (PMNs) from healthy human donors.
Citrate-buffered peripheral blood samples were obtained from healthy volunteers, after receiving informed consent and with the approval of the Ethics Committee of the University of Erlangen-Nuremberg. MNCs and PMNs were enriched by Lymphoflot (Biotest, Dreieich, Germany) Ficoll density centrifugation in Leukosep tubes (Greiner, Frickenhausen, Germany) according to manufacturers' instructions. Cells were resuspended in Roswell Park Memorial Institute 1640 Glutamax-I medium containing 10% FCS and penicillin and streptomycin at 100 units/ml and 100 µg/ml, respectively. Viability was verified by trypan blue exclusion and exceeded 95%.
ADCC reactions.
ADCC assays, using MNCs from healthy donors as effector cells at different or constant effector-to-target cell ratios (E:T ratio), were performed in triplicates using a 3 h 51Cr release assay as described in reference Citation46. Dose-response curves were recorded using several equimolar 5-fold or 25-fold serial dilutions of the respective fusion proteins at a constant E:T ratio of 40:1. Extent of lysis as a function of E:T ratio was determined at a constant concentration of 1 nM of the fusion proteins. Background lysis induced by MNCs alone was subtracted from each data point for the dose-response curves, and EC50 values (concentration of fusion protein producing 50% of maximum specific lysis) were calculated using a nonlinear regression curve fit (variable slope). For the dual-targeting ADCC experiments, a HLA-DR- and CD19-double-positive and a HLA-DR single-positive stably transfected HEK 293 cell clone were each separately labeled with 51Cr as described in reference Citation46. The double-positive clone was then mixed with the same amount of cells of the unlabelled single-positive clone. Conversely, in the parallel experiment the labeled single-positive clone was mixed at equal proportions with the unlabelled double-positive clone. Experiments were repeated 4–8 times and mean values are reported.
Selective binding by dual-targeting.
SEM cells (CD19 and HLA-DR positive) were stained with 1 nM Calcein-AM (Invitrogen) for 30 min and HUT-78 cells (CD19 negative, HLA-DR positive) were left unstained. Both cells were mixed at ratios 1:1, 1:10 and 1:20 (SEM:HUT-78) with a final cell count of 5 × 105, incubated with 55 nM sctb HLA-ds16-hu19 and binding of the sctb was analyzed by flow cytometry as described above.
Graphical and statistical analysis.
Graphical and statistical analyses were performed using Graph Pad Prism Software (Graph Pad Software Inc.; San Diego, CA). Group data are reported as means ± standard error of the mean. Differences between groups were analyzed using unpaired or, where appropriate, paired Student's t-test. p values ≤ 0.05 were considered statistically significant.
Financial Support
This research was supported by grants from the DFG (Deutsche Forschungsgemeinschaft; German Research Community) to G.H.F. and the late Wolfgang Hillen (SFB643/C3), a student fellowship from the Bayerische Eliteförderung (Bavarian Scholarship Foundation) to C.S., postdoctoral/Ph.D., fellowships from the German Jose-Carreras Leukemia-Foundation to M.S. and I.S., a research grant No. 2007.049.1 from the Wilhelm Sander Foundation, Neustadt, Germany to G.H.F. and B.S., and support from the Stiftung Deutsche Krebshilfe, the Beitlich Foundation, Tübingen and the Association “Kaminkehrer helfen krebskranken Kindern” (Chimney Sweeps support children with cancer) to G.H.F. Part of this work was funded by an intramural grant from the ELAN fond and the Training Grant GK592 from the German Research Community (DFG).
Figures and Tables
Figure 1 Design, purification and antigen binding of sctb HLA-ds16-hu19. (A) Block-structure of expression construct. I, secretion leader sequence from the murine Igkappa L chain; VL, VH, cDNA sequences coding for the V regions of Ig L-or H-chains; L, cDNA coding for a 20 amino acid flexible linker (Gly4Ser)4; S, m, H, cDNA coding for a strep-, c-myc- or a hexahistidine tag; S-S, stabilizing disulfide bond. (B) Integrity and purity of the sctb HLA-ds16-hu19 after affinity chromatography with Strep-tactin agarose beads as evaluated by reducing SDS-PAGE and staining with Coomassie blue. (C) Protein gel blot analysis using anti-his or (D) anti-strep antibodies for detection.
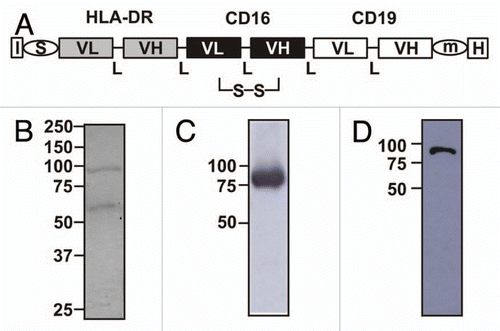
Figure 2 Specific antigen binding of sctb HLA-ds16-hu19. (A) FACS analysis of specific binding of sctb to CD19-transfected HEK 293 cells (i), untransfected HEK 293 cells (ii). (B) Specific binding to CD16-transfected CHO cells (i), an incubation with untransfected CHO cells showed no binding (ii). (C) Incubation of sctb with HLA-DR-transfected L66 cells resulted in binding (i), whereas incubation with untransfected L66 cells produced no binding (ii). Dark gray peak, signal from sctb; light gray peak, signal from control sctb.
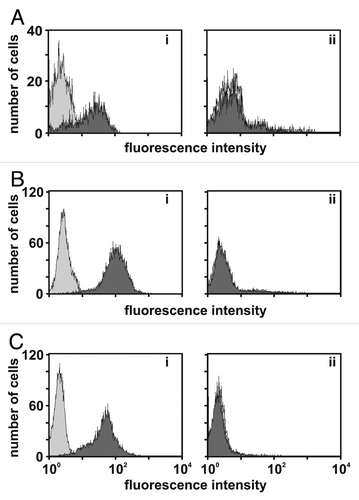
Figure 3 HLA-ds16-hu19 selectively binds double-positive cells by dual-targeting. Stained SEM cells (CD19- and HLA-DR-positive) were mixed with unlabeled HUT-78 cells (HLA-DR positive) in an equal amount (A and B), and with a ratio of 1:10 (C) or 1:20 (D). Mixtures were incubated with PBA (A) or the sctb HLA-ds16-hu19 (B–D). Bound molecules were detected by flow cytometry (10,000 events collected) with an anti-His-antibody and a PE-conjugated Fab-fragment. SEM cells were detected in the upper left quadrant, the unstained HUT-78 cells in the lower left quadrant. Cells bound by the sctb shifted to the right quadrants. Numbers represent the percentage of the cells in the respective quadrants.
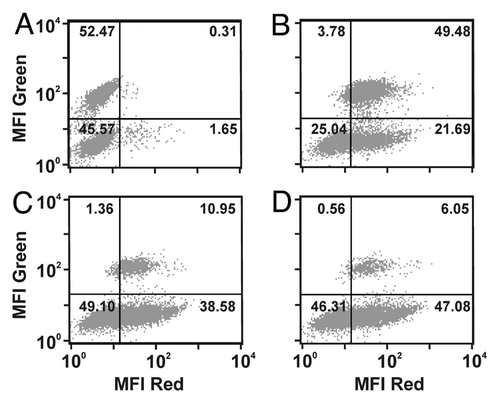
Figure 4 The dual-targeting sctb HLA-ds16-hu19 mediates potent lysis of CD19- and HLA-DR-positive Raji cells with isolated MNCs from healthy donors as effector cells. (A) At the concentration of 1 nM the sctb induced significant ADCC over a broad range of E:T ratios and the extent of specific lysis increased with increasing E:T ratios (black bars: sctb; gray bars: control sctb, white bars: no fusion protein). Data are presented as mean percentage lysis ± standard error of the mean (SEM) obtained with MNCs from four different healthy donors. *Statistically significant values, p ≤ 0.05. (B) Whole blood was fractionated into PMN and MNC to identify the relevant effector population for the sctb. Bars indicate sctb at a concentration of 1 nM. *Statistically significant (p ≤ 0.05) lysis. Data are acquired for four different donors. (C) Ag-specific induction of ADCC in the double-positive cell-line Raji by sctb HLA-ds16-hu19. The sctb induced potent lysis of Raji cells at a concentration of 1 nM. Simultaneous addition of a 500-fold molar excess of CD19- and HLA-DR-specific scFvs significantly blocked the ADCC reaction, but not the addition of a control scFv. Simultaneous incubation with a 125-fold molar excess of the CD16-specific monoclonal antibody 3G8, but not with a control IgG1, significantly reduced ADCC. Data points represent mean percent of specific lysis obtained with isolated MNCs from four different healthy donors at an E:T ratio of 40:1. Specific lysis measured for sctb HLA-ds16-hu19 was defined as 100%. Specific lysis is total lysis minus spontaneous lysis. *Statistically significant reduced (p ≤ 0.05) lysis.
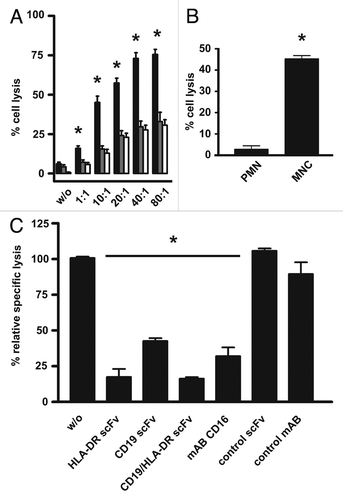
Figure 5 Dose dependent induction of ADCC of the Raji tumor cell line by the sctb and the bsscFvs and test for selective lysis. The CD19- and HLA-DR-double-positive tumor cell line Raji (A) was used as target to compare efficacy of the fusion proteins at a constant E:T ratio of 40:1. The sctb (filled squares), the bsscFv hu19-ds16 (open triangles) and the bsscFv HLA-ds16 (filled inverted triangles) triggered ADCC in a dose-dependent manner. The non-relevant control bsscFv (filled diamonds) induced no significant killing. Data points represent mean percentage of lysis ± SEM obtained with isolated MNCs from 8 different healthy donors. *Statistically significant differences (p ≤ 0.05) between lysis induced by sctb and the bsscFvs. (B) For the first arm of the experiment (left bar), ag double-positive EZK 20 cells were labeled with 51Cr and mixed with an equal number of unlabeled HLA-DR single-positive EZK-HLA 16 cells (). For the second arm (right bar), single-positive EZK HLA 16 cells were labeled with 51Cr and mixed with an equal number of unlabeled double-positive EZK 20 cells. Sctb HLA-ds16-hu19 was added to a final concentration of 1 nM and MNCs were added to reach an E:T ratio of 40:1. The dual-targeting sctb (gray bar) induced statistically significant greater lysis of the double-positive cells in comparison to the lysis of the single-positive cells. Data points represent mean percentage of lysis ± SEM obtained with isolated MNCs from 4 different healthy donors. *Statistically significant (p ≤ 0.05) differences of lysis induced by the dual-targeting sctb.
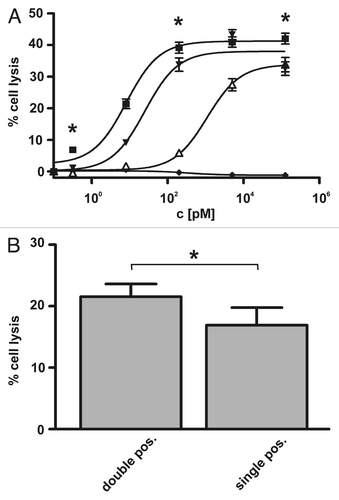
Table 1 KD-values and stability in human serum for sctb HLA-ds16-hu19 and bsscFvs hu19-ds16 and HLA-ds16
Table 2 EC50 for ADCC by sctb HLA-ds16-hu19 and bsscFvs hu19-ds16, HLA-ds16Table Footnotea
Table 3 Ag-density of stably transfected HEK 293 clone EZK20 over 3 consecutive passages
Table 4 Antigen-density of the double- and single-positive single cell clonesTable Footnotea
Additional material
Download Zip (221.3 KB)Acknowledgments
We thank Dr. R. Levy for the 4G7 hybridoma, Dr. J.G. van de Winkel for the CD16 transfected CHO cells, T. Mentz and B. Bock for excellent technical assistance and for support in the laboratory. Th. Lange is gratefully acknowledged for administrative assistance. The German José-Carreras Leukemia-Foundation is acknowledged for financial support, through grant No. DJCLS F07/03.
References
- Adams GP, Weiner LM. Monoclonal antibody therapy of cancer. Nat Biotechnol 2005; 23:1147 - 1157; PMID: 16151408; http://dx.doi.org/10.1038/nbt1137
- Duebel S. Handbook of Therapeutic Antibodies 2007 Weinheim WILEY-VCH
- Weiner LM, Dhodapkar MV, Ferrone S. Monoclonal antibodies for cancer immunotherapy. Lancet 2009; 373:1033 - 1040; PMID: 19304016; http://dx.doi.org/10.1016/S0140-6736(09)60251-8
- Bargou R, Leo E, Zugmaier G, Klinger M, Goebeler M, Knop S, et al. Tumor regression in cancer patients by very low doses of a T cell-engaging antibody. Science 2008; 321:974 - 977; PMID: 18703743; http://dx.doi.org/10.1126/science.1158545
- Clynes R. Antitumor antibodies in the treatment of cancer: Fc receptors link opsonic antibody with cellular immunity. Hematol Oncol Clin North Am 2006; 20:585 - 612; PMID: 16762726; http://dx.doi.org/10.1016/j.hoc.2006.02.010
- de Haij S, Jansen JH, Boross P, Beurskens FJ, Bakema JE, Bos DL, et al. In vivo cytotoxicity of type I CD20 antibodies critically depends on Fc receptor ITAM signaling. Cancer Res 2010; 70:3209 - 3217; PMID: 20354182; http://dx.doi.org/10.1158/0008-5472.CAN-09-4109
- Heiss MM, Murawa P, Koralewski P, Kutarska E, Kolesnik OO, Ivanchenko VV, et al. The trifunctional antibody catumaxomab for the treatment of malignant ascites due to epithelial cancer: Results of a prospective randomized phase II/III trial. Int J Cancer 2010; 127:2209 - 2221
- Musolino A, Naldi N, Bortesi B, Pezzuolo D, Capelletti M, Missale G, et al. Immunoglobulin G fragment C receptor polymorphisms and clinical efficacy of trastuzumab-based therapy in patients with HER-2/neu-positive metastatic breast cancer. J Clin Oncol 2008; 26:1789 - 1796; PMID: 18347005; http://dx.doi.org/10.1200/JCO.2007.14.8957
- Topp MGN, Kufer P, Zugmaier G, Degenhard E, Neumann S, Horst HA, et al. Treatment with anti-CD19 BiTE antibody blinatumomab (MT103/MEDI-538) is able to eliminate minimal residual disease (MRD) in patients with B-precursor acute lmphoblastic leukemia (ALL): first results of an ongoing phase II study (ASH Annual Meeting Abstract). Blood 2008; 112:1926
- Zhang W, Gordon M, Schultheis AM, Yang DY, Nagashima F, Azuma M, et al. FCGR2A and FCGR3A polymorphisms associated with clinical outcome of epidermal growth factor receptor expressing metastatic colorectal cancer patients treated with single-agent cetuximab. J Clin Oncol 2007; 25:3712 - 3718; PMID: 17704420; http://dx.doi.org/10.1200/JCO.2006.08.8021
- Topp MS, Kufer P, Gokbuget N, Goebeler M, Klinger M, Neumann S, et al. Targeted therapy with the T-cell-engaging antibody blinatumomab of chemotherapy-refractory minimal residual disease in B-lineage acute lymphoblastic leukemia patients results in high response rate and prolonged leukemia-free survival. J Clin Oncol 2011; 29:2493 - 2498; PMID: 21576633; http://dx.doi.org/10.1200/JCO.2010.32.7270
- Bluemel C, Hausmann S, Fluhr P, Sriskandarajah M, Stallcup WB, Baeuerle PA, et al. Epitope distance to the target cell membrane and antigen size determine the potency of T cell-mediated lysis by BiTE antibodies specific for a large melanoma surface antigen. Cancer Immunol Immunother 2010; 59:1197 - 1209; PMID: 20309546; http://dx.doi.org/10.1007/s00262-010-0844-y
- Kellner C, Bruenke J, Stieglmaier J, Schwemmlein M, Schwenkert M, Singer H, et al. A novel CD19-directed recombinant bispecific antibody derivative with enhanced immune effector functions for human leukemic cells. J Immunother 2008; 31:871 - 884; PMID: 18833000; http://dx.doi.org/10.1097/CJI.0b013e318186c8b4
- Kügler M, Stein C, Kellner C, Mentz K, Saul D, Schwenkert M, et al. A recombinant trispecific single-chain Fv derivative directed against CD123 and CD33 mediates effective elimination of acute myeloid leukaemia cells by dual targeting. Br J Haematol 2010; 150:574 - 586; PMID: 10.1111/j.1365-2141.2010.08300.x
- Schubert I, Kellner C, Stein C, Kugler M, Schwenkert M, Saul D, et al. A single-chain triplebody with specificity for CD19 and CD33 mediates effective lysis of mixed lineage leukemia cells by dual targeting. MAbs 2011; 3:21 - 30; PMID: 21081841; http://dx.doi.org/10.4161/mabs.3.1.14057
- Singer H, Kellner C, Lanig H, Aigner M, Stockmeyer B, Oduncu F, et al. Effective elimination of acute myeloid leukemic cells by recombinant bispecific antibody derivatives directed against CD33 and CD16. J Immunother 2010; 33:599 - 608; PMID: 20551837; http://dx.doi.org/10.1097/CJI.0b013e3181dda225
- Bruenke J, Barbin K, Kunert S, Lang P, Pfeiffer M, Stieglmaier K, et al. Effective lysis of lymphoma cells with a stabilised bispecific single-chain Fv antibody against CD19 and FcgammaRIII (CD16). Br J Haematol 2005; 130:218 - 228; PMID: 16029450; http://dx.doi.org/10.1111/j.1365-2141.2005.05414.x
- Kügler M, Stein C, Schwenkert M, Saul D, Vockentanz L, Huber T, et al. Stabilization and humanization of a single-chain Fv antibody fragment specific for human lymphocyte antigen CD19 by designed point mutations and CDR-grafting onto a human framework. Protein Eng Des Sel 2009; 22:135 - 147; PMID: 19188138; http://dx.doi.org/10.1093/protein/gzn079
- Baum W, Steininger H, Bair HJ, Becker W, Hansen-Hagge TE, Kressel M, et al. Therapy with CD7 monoclonal antibody TH-69 is highly effective for xenografted human T-cell ALL. Br J Haematol 1996; 95:327 - 338; PMID: 8904888; http://dx.doi.org/10.1046/j.1365-2141.1996.d01-1900.x
- Beckhove P, Warta R, Lemke B, Stoycheva D, Momburg F, Schnolzer M, et al. Rapid T cell-based identification of human tumor tissue antigens by automated two-dimensional protein fractionation. J Clin Invest 2010; 120:2230 - 2242; PMID: 20458140; http://dx.doi.org/10.1172/JCI37646
- Preuss KD, Zwick C, Bormann C, Neumann F, Pfreundschuh M. Analysis of the B-cell repertoire against antigens expressed by human neoplasms. Immunol Rev 2002; 188:43 - 50; PMID: 12445280; http://dx.doi.org/10.1034/j.1600-065X.2002.18805.x
- Rosenberg SA, Yang JC, Restifo NP. Cancer immunotherapy: moving beyond current vaccines. Nat Med 2004; 10:909 - 915; PMID: 15340416; http://dx.doi.org/10.1038/nm1100
- Burkhardt C, Himmelein S, Britt W, Winkler T, Mach M. Glycoprotein N subtypes of human cyto-megalovirus induce a strain-specific antibody response during natural infection. J Gen Virol 2009; 90:1951 - 1961; PMID: 19420160; http://dx.doi.org/10.1099/vir.0.010967-0
- Baldick CJ Jr, Shenk T. Proteins associated with purified human cytomegalovirus particles. J Virol 1996; 70:6097 - 6105; PMID: 8709233
- Mantovani B, Rabinovitch M, Nussenzweig V. Phagocytosis of immune complexes by macrophages. Different roles of the macrophage receptor sites for complement (C3) and for immunoglobulin (IgG). J Exp Med 1972; 135:780 - 792; PMID: 5018051; http://dx.doi.org/10.1084/jem.135.4.780
- Olejniczak SH, Stewart CC, Donohue K, Czuczman MS. A quantitative exploration of surface antigen expression in common B-cell malignancies using flow cytometry. Immunol Invest 2006; 35:93 - 114; PMID: 16531332; http://dx.doi.org/10.1080/08820130500496878
- Lillemeier BF, Pfeiffer JR, Surviladze Z, Wilson BS, Davis MM. Plasma membrane-associated proteins are clustered into islands attached to the cytoskeleton. Proc Natl Acad Sci USA 2006; 103:18992 - 18997; PMID: 17146050; http://dx.doi.org/10.1073/pnas.0609009103
- Vallera DA, Todhunter DA, Kuroki DW, Shu Y, Sicheneder A, Chen H. A bispecific recombinant immunotoxin, DT2219, targeting human CD19 and CD22 receptors in a mouse xenograft model of B-cell leukemia/lymphoma. Clin Cancer Res 2005; 11:3879 - 3888; PMID: 15897589; http://dx.doi.org/10.1158/1078-0432.CCR-04-2290
- Robinson MK, Hodge KM, Horak E, Sundberg AL, Russeva M, Shaller CC, et al. Targeting ErbB2 and ErbB3 with a bispecific single-chain Fv enhances targeting selectivity and induces a therapeutic effect in vitro. Br J Cancer 2008; 99:1415 - 1425; PMID: 18841159; http://dx.doi.org/10.1038/sj.bjc.6604700
- Dick JE. Stem cell concepts renew cancer research. Blood 2008; 112:4793 - 4807; PMID: 19064739; http://dx.doi.org/10.1182/blood-2008-08-077941
- Hosen N, Park CY, Tatsumi N, Oji Y, Sugiyama H, Gramatzki M, et al. CD96 is a leukemic stem cell-specific marker in human acute myeloid leukemia. Proc Natl Acad Sci USA 2007; 104:11008 - 11013; PMID: 17576927; http://dx.doi.org/10.1073/pnas.0704271104
- Jaiswal S, Jamieson CH, Pang WW, Park CY, Chao MP, Majeti R, et al. CD47 is upregulated on circulating hematopoietic stem cells and leukemia cells to avoid phagocytosis. Cell 2009; 138:271 - 285; PMID: 19632178; http://dx.doi.org/10.1016/j.cell.2009.05.046
- Jin L, Lee EM, Ramshaw HS, Busfield SJ, Peoppl AG, Wilkinson L, et al. Monoclonal antibody-mediated targeting of CD123, IL-3 receptor alpha chain, eliminates human acute myeloid leukemic stem cells. Cell Stem Cell 2009; 5:31 - 42; PMID: 19570512; http://dx.doi.org/10.1016/j.stem.2009.04.018
- Jordan CT, Upchurch D, Szilvassy SJ, Guzman ML, Howard DS, Pettigrew AL, et al. The interleukin-3 receptor alpha chain is a unique marker for human acute myelogenous leukemia stem cells. Leukemia 2000; 14:1777 - 1784; PMID: 11021753; http://dx.doi.org/10.1038/sj.leu.2401903
- van Rhenen A, van Dongen GA, Kelder A, Rombouts EJ, Feller N, Moshaver B, et al. The novel AML stem cell associated antigen CLL-1 aids in discrimination between normal and leukemic stem cells. Blood 2007; 110:2659 - 2666; PMID: 17609428; http://dx.doi.org/10.1182/blood-2007-03-083048
- Taussig DC, Pearce DJ, Simpson C, Rohatiner AZ, Lister TA, Kelly G, et al. Hematopoietic stem cells express multiple myeloid markers: implications for the origin and targeted therapy of acute myeloid leukemia. Blood 2005; 106:4086 - 4092; PMID: 16131573; http://dx.doi.org/10.1182/blood-2005-03-1072
- Meeker TC, Miller RA, Link MP, Bindl J, Warnke R, Levy R. A unique human B lymphocyte antigen defined by a monoclonal antibody. Hybridoma 1984; 3:305 - 320; PMID: 6441771; http://dx.doi.org/10.1089/hyb.1984.3.305
- Fleit HB, Wright SD, Unkeless JC. Human neutrophil Fc gamma receptor distribution and structure. Proc Natl Acad Sci USA 1982; 79:3275 - 3279; PMID: 6808506; http://dx.doi.org/10.1073/pnas.79.10.3275
- Greil J, Gramatzki M, Burger R, Marschalek R, Peltner M, Trautmann U, et al. The acute lymphoblastic leukaemia cell line SEM with t(4;11) chromosomal rearrangement is biphenotypic and responsive to interleukin-7. Br J Haematol 1994; 86:275 - 283; PMID: 8199015; http://dx.doi.org/10.1111/j.1365-2141.1994.tb04726.x
- Sambrook J, Russel DW. Molecular Cloning: A Laboratory Manual 2001; NY Cold Spring Harbour
- Lang P, Barbin K, Feuchtinger T, Greil J, Peipp M, Zunino SJ, et al. Chimeric CD19 antibody mediates cytotoxic activity against leukemic blasts with effector cells from pediatric patients who received T-cell-depleted allografts. Blood 2004; 103:3982 - 3985; PMID: 14764538; http://dx.doi.org/10.1182/blood-2003-05-1735
- Peipp M, Kupers H, Saul D, Schlierf B, Greil J, Zunino SJ, et al. A recombinant CD7-specific single-chain immunotoxin is a potent inducer of apoptosis in acute leukemic T cells. Cancer Res 2002; 62:2848 - 2855; PMID: 12019163
- Stein C, Kellner C, Kugler M, Reiff N, Mentz K, Schwenkert M, et al. Novel conjugates of single-chain Fv antibody fragments specific for stem cell antigen CD123 mediate potent death of acute myeloid leukaemia cells. Br J Haematol 2010; 148:879 - 889; PMID: 20064159; http://dx.doi.org/10.1111/j.1365-2141.2009.08033.x
- Bruenke J, Fischer B, Barbin K, Schreiter K, Wachter Y, Mahr K, et al. A recombinant bispecific single-chain Fv antibody against HLA class II and FcgammaRIII (CD16) triggers effective lysis of lymphoma cells. Br J Haematol 2004; 125:167 - 179; PMID: 15059139; http://dx.doi.org/10.1111/j.1365-2141.2004.04893.x
- Benedict CA, MacKrell AJ, Anderson WF. Determination of the binding affinity of an anti-CD34 single-chain antibody using a novel, flow cytometry based assay. J Immunol Methods 1997; 201:223 - 231; PMID: 9050944; http://dx.doi.org/10.1016/S0022-1759(96)00227-X
- Elsässer D, Valerius T, Repp R, Weiner GJ, Deo Y, Kalden JR, et al. HLA class II as potential target antigen on malignant B cells for therapy with bispecific antibodies in combination with granulocyte colony-stimulating factor. Blood 1996; 87:3803 - 3812; PMID: 8611706