Abstract
Interleukin-21 (IL-21) is a type I four-helical bundle cytokine that exerts a variety of significant effects on many hematopoietic cells, including T and B lymphocytes and natural killer cells. IL-21 is produced predominantly by CD4+ T cells and natural killer T cells and, when aberrantly overexpressed, appears to play important roles in a wide variety of autoimmune disorders. To generate potential therapeutic reagents capable of inhibiting IL-21 for clinical use, we immunized human immunoglobulin transgenic mice with IL-21 and then identified and cloned a panel of human anti-human IL-21 binding monoclonal antibodies. IL-21 neutralizing and IL-21-binding, non-neutralizing antibodies were assigned to distinct epitope “bins” based on surface plasmon resonance competition studies. The most potent neutralizing antibodies had extremely high (sub pM) affinity for IL-21 and were able to block IL-21 activity in various biological assays using either an IL-21R-transfected pre-B-cell line or primary human B cells, and their neutralizing activity was, in some cases, superior to that of a soluble form of the high affinity heterodimeric IL-21 receptor. Characterization of this panel of IL-21 antibodies provided the basis for the selection of a therapeutic candidate antibody capable of inhibiting IL-21 activity for the treatment of autoimmune and inflammatory diseases.
Introduction
Interleukin 21 (IL-21) is a type I four-helix bundle cytokine and a member of a family of cytokines (including IL-2, IL-4, IL-7, IL-9 and IL-15) that utilize the common cytokine receptor γ chain (γc) as part of their receptor complex to exert a variety of significant effects on hematopoietic cells.Citation1–Citation3 IL-21 binds to the IL-21 receptor (IL-21R), which forms a complex with the γc and activates Janus-activated kinases (Jak)-1 and Jak-3, and these subsequently activate signal transducer and activator of transcription (STAT)-3 and STAT-1, and to a lesser degree STAT-5.Citation3 IL-21 is produced predominantly by CD4+ T cells and natural killer T (NKT) cells, and IL-21R is expressed widely on lymphohematopoietic cells, including NK cells, T cells, B cells, monocytes, macrophages and dendritic cells. Aberrant expression of IL-21R on fibroblasts, keratinocytes and intestinal epithelial cells in certain inflammatory disease settings has also been reported.
IL-21 exerts a broad array of biological effects, including increased CD4+ and CD8+ T-cell proliferation, maintenance and augmented function of CD8+ T cells and NK cells, promotion of IL-17-secreting Th17 cells and the enhancement of B-cell activation, plasma cell (PC) differentiation or B-cell death during humoral immune responses.Citation10–Citation15 The effects of IL-21 on B-cell responses is due at least in part to its autocrine activity on follicular helper T cells (TFH), CD4+ T cells that produce large amounts of IL-21 and are critical to the development and function of germinal centers.Citation16 IL-21 has also been shown to modulate human monocytes by inducing expression of a wide variety of cytokines (i.e., GM-CSF, IL-1, IL-2, IL-7, IL-15, IFNγ and TGFβ) and chemokines (i.e., IL-8, RANTES, MIP-1α, eotaxin, IP-10) in these cells,Citation17 and by maintaining monocyte CD16 expression by upregulating IL-10 expression by naïve human CD4+ T cells.Citation18 Additionally, under certain circumstances IL-21 can inhibit dendritic cell maturation and antigen presentation function.Citation19,Citation20 Thus, IL-21 has broad effects on both innate and adaptive immune cells.
Based on its functions on B cells and the observed overexpression of this cytokine in some patients with systemic autoimmune diseases such as systemic lupus erythematosus (SLE) and rheumatoid arthritis (RA), it has been proposed that IL-21 might play a critical role in the development of pathogenic autoantibodies and may contribute to other features of autoimmunity.Citation21–Citation25 IL-21 overexpression has also been linked to various organ-specific autoimmune disorders, such as inflammatory bowel disease (IBD), psoriasis and scleroderma, and a unique role for IL-21 in enhancing inflammation via aberrant IL-21R expression on local non-hematopoietic tissues has been proposed.Citation3–Citation5,Citation26–Citation29 Polymorphisms in the IL-2/IL-21 and IL-21R loci have also been associated with multiple autoimmune disorders including RA, Type 1 diabetes, IBD and SLE.Citation30–Citation47 The important role of IL-21 in promoting humoral immune responses suggest that neutralizing IL-21 activity might represent an effective therapeutic intervention for both systemic and organ-specific autoimmunity.Citation48 Indeed, blocking IL-21 activity has been shown to reduce disease symptoms in a variety of animal disease and xenograft models (ref. Citation49–Citation56 and our unpublished results).
Several different mechanistic strategies could be considered to interfere with IL-21 mediated cell signaling: antagonists directed against (or composed of) the IL-21R,Citation49,Citation50 antagonists directed against the common cytokine receptor γ chain (γc) (though these would impact other members of this cytokine family), or antagonists directed against IL-21 itself.Citation51,Citation52 We describe here the isolation and characterization of neutralizing monoclonal antibodies (mAbs) directly targeting IL-21 and interfering with its binding to IL-21R or the IL-21R/γc heterodimer.
Using IL-21-immunized human immunoglobulin (Ig) transgenic (TG) mice, a panel of human anti-human IL-21 specific mAbs was generated. From this panel, a subset of high affinity mAbs was identified that potently neutralize IL-21 activity in multiple in vitro biological assays. Inhibition was observed in assays utilizing transfected target cells overexpressing IL-21R, as well as in assays utilizing primary peripheral blood mononuclear cells (PBMC) isolated from healthy human donors. Additional functional characterization of the antibodies using surface plasmon resonance (BIAcore) was used to both differentiate between the mAbs on the basis of their binding affinity and kinetics, and to assign the mAbs to “epitope bins” based on their ability to bind IL-21 simultaneously or compete for binding to IL-21. The mAbs that neutralized IL-21 activity were clearly associated with two of the three assigned epitope bins. The ability to associate particular epitope bins with specific functional properties, such as neutralization, will provide the foundation for more detailed studies to identify the specific epitopes on human IL-21 bound by the neutralizing mAbs.
Results
Immunization of human immunoglobulin TG mice.
IL-21 exhibits a high degree of inter-species homology and cross-species activity and is known to have significant effects on B-cell proliferation, survival and Ig class switching, and can also inhibit antigen presentation by dendritic cells. It is likely that these properties contributed to the difficulties we encountered in eliciting a potent immunological response to human IL-21 (which weakly cross-reacts on mouse IL-21R) in mice when it was administered in a wide variety of formats and adjuvant conditions. A very limited number of mice responded to IL-21 immunization with a neutralizing titer and this response required that IL-21 be conjugated to a highly charged and effective carrier protein, and administered in a complex adjuvant formulation to the mice. Consistent with the potential involvement of human IL-21 or neutralizing anti-IL-21 antibodies on IgG production in the mice, only IL-21 highly cross-linked with formaldehyde to bovine serum albumin (BSA) or keyhole limpet hemocyanin (KLH) produced an effective titer in the mice, and in no case were we able to identify mice that could generate both a potent neutralizing anti-human IL-21 and anti-mouse IL-21 antibody response.
Male “KM” mice (Kirin human Ig TG mice cross-bred with the Medarex HuMab mouse) were initially immunized by subcutaneous (SC) injection of purified recombinant IL-21 conjugated with BSA or IL-21 conjugated with KLH-DSS in combination with CpG and GM-CSF and Emulsigen®-P adjuvant. In addition, female HuMab mice were immunized with IL-21 conjugated with KLH-DSS. Following the initial immunization, each of the mice received three additional SC injections of IL-21 in Emulsigen®-P adjuvant via the SC route in weekly intervals. Seven days after the fourth immunization, serum was collected from the mice for analysis of its ability to bind to IL-21. Serial 10-fold dilutions of sera were assessed in a direct ELISA using immobilized IL-21 and both IgG and IgM anti-IL-21 titers were measured. In parallel, sera from the immunized mice were also evaluated for neutralizing activity in a cell-based STAT3-phosphorylation assay. The level of STAT3 phosphorylation in a murine pre-B-cell line (BaF3) transfected with the human IL-21RCitation10 treated with IL-21 was measured using anti-phospho-STAT3 (pSTAT3) antibody-conjugated beads. Inhibition of IL-21 activity, determined by pre-incubating the sera from the immunized mice with IL-21 prior to adding it to the BaF3/IL-21R cells, was determined based on the decrease in pSTAT3 levels using an EC50 concentration of IL-21 and a titration of antagonist serum. We incorporated this bioassay into the primary screen of the immunized mice to improve our chances of selecting for mice that were producing neutralizing, rather than just binding, anti-IL-21 mAbs.
Fusions to generate IL-21 neutralizing mAbs.
Mice with the highest IL-21-specific, neutralizing titers were immunized a final time with 30 µg of non-conjugated IL-21 in PBS via SC injection. Four days later, the spleen and lymph nodes of each mouse were harvested, prepared as a single cell suspension and fused to a myeloma cell line at a 2:1 lymphoid cell:myeloma cell ratio with polyethylene glycol (PEG). Wells were assayed 10 d after plating of the fusion.
Hybridoma culture supernatants were initially screened for the presence of human IgG antibodies. Wells containing hybridomas secreting human IgG as determined by ELISA were selected for further evaluation. Since high affinity mAbs were desired, those hybridomas predominantly secreting human IgM were discarded. Subsequently, hybridomas secreting IL-21-specific IgG were identified based on their ability to bind to IL-21 immobilized in microtiter plates in the direct ELISA used to screen the mouse sera.
In the first of three fusions (Fusion 362), a total of 72 IgG+/Ig-kappa+ wells were identified by ELISA, of which 14 master wells had significant IL-21 neutralizing activity in the pSTAT3 assay. In Fusion 366, a total of 96 IgG+/kappa+ wells were identified (13 with neutralizing activity), and in Fusion 367, 27 IgG+/Ig kappa+ wells were identified, with five master wells selected for neutralizing activity.
Binding kinetics and epitope binning of human anti-hIL-21 mAbs.
Purified mAbs from hybridomas secreting IL-21-binding human IgG were tested using BIAcore surface plasmon resonance to determine their approximate binding kinetics and affinity. The distribution of association (ka) and dissociation (kd) rate constants for a subset of the mAbs and the heterodimeric soluble receptor is shown in . Competitive binding studies were also performed to assign antibodies to “epitope bins” based on their ability to bind simultaneously to human IL-21. The epitope bins associated with various mAbs are annotated in . Antibodies that bind to the same or overlapping epitopes on IL-21 are not able to bind simultaneously and were functionally assigned into a single family or epitope bin. The human IL-21 mAbs were assigned to three unique epitope bins. Additionally, a subset of human mAbs was identified that competed for binding with mAbs assigned to both Bin #1 and Bin #2, and thus were assigned to an overlap “Bin #1/2.” A set of mouse anti-human IL-21 mAbs generated previously (data not shown) were also characterized and these mAbs were assigned to Bin 1, Bin 2 and two new epitope bins (Bins 4&5; not shown). All of the mAbs with neutralizing activity fell into Bins #1, 2 or “1/2.” None of the mAbs in Bins #3, 4 or 5 had neutralizing activity, indicating that Bins #1 and #2 contain mAbs that disrupt a key point of interaction between IL-21 and its receptor complex.
Variable region sequencing.
The Ig heavy and light chain variable region genes from a subset of the IL-21 mAbs were cloned and sequenced, and their Ig heavy chain isotypes were also identified (). Of the three mAbs in Bin #1 whose Ig genes were sequenced (362.78, 362.564, and 362.597), all three express the VH3–33 heavy chain gene and the VkIIIA27 light chain gene, and these mAbs are identical or nearly identical to one another. Based on the sequence of their heavy chain diversity (D) segments, these three mAbs are likely to be derived from the same B-cell precursor in vivo. In fact, the mAbs 362.78 (IgG4) and 362.564 (IgG4) are identical to one another and likely derived from the same original B-cell clone. The D segment shared by these three mAbs is most closely related to (and likely derived from) D segment D6–19. All six of the Bin #1/2 mAbs express the VH4 4–59 gene and either the VkI L5, VkI L19 or VkIII A27 light chain genes ( and data not shown). All mAbs tested were either IgG1 or IgG4 isotypes, which is not uncommon for the KM mice. Of the two Bin #2 mAbs sequenced, 366.328 (IgG4) expresses VH3 3–21 and VkI L4, whereas 362.75 (IgG1) expresses VH4 4–39 and VkIII L6 (). Alignments of CDR sequences from the neutralizing antibodies across all the bins did not show any homology suggestive of a functional correlation between specific amino acid residues and neutralizing activity (data not shown).
Recombinant versions of mAbs 366.552 and 362.78 were generated by fusing the VH chain from each mAb to a modified human Ig gamma 1 (IgG1) constant region (“IgG1.1”) containing five amino acid substitutions to greatly reduce complement and FcR binding.Citation53 The VL chain from each mAb was fused to the human Ig kappa constant region and the H and L chains were co-expressed in Chinese hamster ovary (CHO) cells (see Methods). The resulting recombinant mAbs retained their original IL-21 binding and neutralizing activity while lacking Fc effector function (data not shown).
Functional activity in cell-based assays.
The human IL-21 mAbs were also characterized in a set of assays for biological activity utilizing a murine pre-B-cell line (BaF3) transfected with both the human IL-21R and a STAT-responsive luciferase reporter gene, KZ134 (“BaF3/hIL-21R”).Citation10 The human IL-21R can associate with the endogenous murine γc in the BaF3 cells and enable IL-21 signaling, and the BaF3/hIL-21R cell line is responsive to recombinant human, mouse, rat and cynomolgus monkey IL-21 (data not shown). The first screening assay using this cell line consisted of a brief (10 min) incubation of the cells with human IL-21 that had been pre-incubated or not with the hybridoma supernatants. IL-21 activity was determined by measuring the level of STAT3 phosphorylation using anti-pSTAT3 antibody-conjugated beads (binding of IL-21 to IL-21R/γc leads to phosphorylation of STAT3 and, to a lesser extent, STAT1 and STAT5).Citation54,Citation55 As a positive control, the human IL-21R/γc-Fc protein was included to demonstrate neutralization activity. Relative neutralization activity was determined based on the decrease in pSTAT3 levels using a pre-determined EC50 concentration of IL-21 and a titration of antagonist. Results for mAbs from three of the hybridomas representing Bins #1, 2 and 1/2 are shown in , and IC50 values are summarized in . The heterodimeric soluble IL-21R and the two mAbs with faster association rate constants, 362.78 and 366.552, were more efficient neutralizers than mAbs from clone 366.328 in this short-term assay. The hybridomas secreting the mAbs with the most potent IL-21 neutralizing activity were further subcloned using limiting dilution and the mAbs purified for further evaluation.
An additional neutralization screening assay utilized the same BaF3/hIL-21R cells, co-transfected with the KZ134 reporter construct, which produces luciferase in the presence of phosphorylated STAT1, 3, 4, 5 or 6. The cells were incubated with IL-21 and hybridoma supernatants or purified mAbs for 24 h and then lysed and mixed with a solution containing the luciferase substrate luciferin, and the amount of relative luminescence was measured. By using a fixed concentration of IL-21 and a titration of mAb, the relative neutralizing activity of each IL-21 mAb was determined ( and ). As expected, given the longer duration and elevated IL-21 concentration required in this assay (), a higher concentration of each mAb was required to fully inhibit IL-21 activity ( and ). Of note, the neutralizing activity of the homodimeric (consisting of two IL-21Rα chains fused to Fc) and heterodimeric (IL-21R/γc-Fc) forms of the soluble IL-21 receptor was also measured using the STAT-luciferase assay, and the IL-21R homodimer was at least 20-fold less potent than the heterodimeric IL-21R/γc-Fc (data not shown).
A subset of the IL-21 neutralizing mAbs was also tested for their ability to inhibit IL-21-mediated human B-cell proliferation. Freshly isolated human B cells were purified from healthy donor PBMC and cultured for 4 d in the presence of IL-21, anti-CD40 antibody and a titration of each IL-21 mAb. The IL-21 neutralizing activity of many of these mAbs was actually superior to that of the positive control antagonist, IL-21R/γc-Fc. IL-21 mAb 366.328 was the most potent neutralizer in this assay, followed in order by 362.78, 366.552 and IL-21R/γc-Fc ( and ).
IL-21 has been shown to drive B-cell Ig class switching and to promote differentiation of memory B cells to Ig-producing PC.Citation14,Citation56 Based on published protocols, we developed an in vitro human PC differentiation assay in order to evaluate the potency of three of the IL-21-neutralizing mAbs, 362.78 (Bin #1), 366.328 (Bin #2) and 366.552 (Bin #1/2). All three clones potently blocked IL-21 activity at least as well as the IL-21R/γc-Fc control and reduced B-cell differentiation, as assessed by the loss of IgD and gain of CD38 expression (data for clone 362.78 shown in ). Additionally, we assessed the levels of IgG in the supernatants of the cultured B cells and showed that clone 362.78 inhibited the secretion of IgG1 () in a dose-dependent manner. We were unable to accurately assess the levels of IgG4, given the interference of the added clone 362.78 (IgG4) in the human IgG ELISA. The results demonstrate that at least a few of the IL-21 mAbs effectively neutralize IL-21 activity even after 7 days in culture, suggesting that the mAbs are stable and active for a prolonged period at 37°C.
Given that all of the mAb screening assays were performed using recombinant IL-21, we next confirmed that the mAbs could also bind and neutralize native human IL-21. A representative mAb, clone 362.78, was conjugated to AlexaFluor 647 and then used to stain intracellular IL-21 in activated human CD4+ T cells. As shown in , IL-21 mAb 362.78 bound a similar fraction of activated T cells as did the control IL-21 staining mAb purchased from a vendor. In order to demonstrate that mAb 362.78 can both bind native IL-21 and also neutralize its activity, we performed the pSTAT3 assay using culture supernatants from human T cells activated under Th1-skewing conditions as the source of native IL-21. Clone 362.78 inhibited STAT3-phosphorylation induced in BaF3/IL-21R cells by activated T cell supernatants generated from all four healthy human blood donors tested ().
In order to attempt to differentiate between their activity, mAbs 366.552 and 362.78 were also tested in a modified version of the STAT3-phosphorylation assay described in . Instead of pre-incubating the mAb with IL-21 for 30 min prior to adding it to the BaF3/hIL-21R cells, the mAbs and IL-21 were added separately or were pre-mixed for 5, 15 or 30 min prior to addition. While clone 366.552 required at least a 30 min pre-incubation to effectively block IL-21 activity in this assay (), clone 362.78 was able to fully inhibit IL-21-induced STAT3 phosphorylation when added just 5 min prior to stimulation, and could partially inhibit IL-21 activity even with no pre-incubation step (). This result likely reflects mAb 362.78's 20-fold faster association rate constant (as compared with mAb 366.552) for association with IL-21 ( and ). In contrast, the soluble IL-21R/γc-Fc control could only partially inhibit IL-21 activity under each of these conditions, despite having both association rate and disassociation rate constants comparable to those of mAb 362.78 ().
Interference of IL-21 mAbs with IL-21-IL-21R binding.
To further elucidate how the neutralizing human mAbs interact with IL-21 relative to the IL-21 receptor, BIAcore was used to perform competition experiments similar to those initially used to assign the mAbs to epitope bins () to determine which of the mAbs compete for binding to IL-21 with both the homodimeric and heterodimeric forms of the soluble IL-21 receptor. Five purified mAbs, 362.78 (Bin #1), 366.552 (Bin #1/2), 362.75 (Bin #2), 366.328 (Bin #2) and 366.345 (non-neutralizing Bin #3) were tested ( and data not shown). The mAbs 362.78 and 366.552 competed with both forms of the soluble receptor, while mAbs 366.328 and 366.345 did not compete with either form of the soluble receptor, despite the observation that 366.328 can neutralize IL-21 bioactivity in cell-based assays (). The mAb 362.75 partially and weakly competed with both forms of the soluble receptor, though its relatively low affinity for IL-21 may contribute to incomplete competition in this assay format (data not shown).
Binding assessment using direct ELISA and western blotting.
The IL-21 mAbs were tested against native IL-21 and denatured proteins, an IL-21 mutein, and short synthetic peptides derived from the native IL-21 sequence, in an initial attempt to determine the scope of key regions of IL-21 involved in antibody binding. For the peptide binding assays, four peptides of 18–29 amino acids each were plated in an ELISA format to assess mAb binding (). These peptides were designed to provide significant coverage of the cytokine polypeptide while focusing on domains predicted from cytokine mutein studies and from the structure of IL-21-related cytokines to be important in receptor binding or activation. The summary of the binding results are shown in . None of the mAbs tested (clones 362.75, 362.78, 362.597, 366.328, 366.345 or 366.552) demonstrated any binding to Peptides 1–3 (which include portions of helices A, B and C), and only mAbs 362.75 and 366.552 could bind Peptide 4 (which includes helix D) ( and ). The mAbs 362.78, 362.597 and 366.328 exhibited fairly poor reactivity to the peptides and denatured proteins overall, suggesting the epitopes recognized by these potent neutralizing mAbs likely require significant conformational integrity ().
The IL-21 mutein tested contains two mutations in helix D (Q116D and I119D; ) that do not affect the mutein's ability to bind IL-21R, but do prevent signaling through the IL-21R/γc complex.Citation57,Citation58 Two of the mAbs that exhibited the best reactivity under denaturing conditions in western blot and peptide ELISA, mAbs 366.552 and 362.75, showed no reactivity to the human IL-21 mutein, and only mAb 362.75 was able to bind Peptide 4, which includes the amino acids affected in the IL-21 mutein (). These data indicate that the region of IL-21 containing these two mutated amino acids (Q116D, I119D) comprises a critical feature in the epitope recognized by mAbs 366.552 and 362.75. However, other neutralizing mAbs such as 362.78 and 362.597 were able to bind the IL-21 mutein, demonstrating only slightly reduced binding to the IL-21 mutein relative to unmutated IL-21 (), suggesting that this region of helix D is at best a minor component of their epitopes.
Discussion
IL-21 plays multiple roles within the immune system and, when dysregulated, can induce autoimmunity and inflammation, presumably by over- or improperly stimulating one or more of its target cell types. Thus, antagonizing IL-21 activity is a promising new approach for treating various autoimmune diseases such as SLE, RA and IBD.
We have described here the generation and characterization of a panel of human anti-hIL-21 mAbs generated in human Ig TG mice. Many attempts were made initially to immunize the human Ig TG mice with unconjugated rhIL-21, but the mice failed to mount strong anti-IL-21 neutralizing titers until they were immunized with IL-21 cross-linked to carrier proteins such as BSA and KLH. The weak neutralizing antibody responses to hIL-21 in the absence of carrier protein might be a result of cross-species effect of large quantities of hIL-21 on B cells in these mice, which may have adversely affected their ability to mount strong IgG responses. Another explanation may be that neutralizing antibodies generated in the mice may block the B-cell stimulatory effects of endogenous mIL-21, thus decreasing the antibody response and Ig class switch. However, in no case were we able to identify mice that had both a potent neutralizing anti-human IL-21 and anti-mouse IL-21 antibody serum titer, though this could at least in part be due to sequence dissimilarity in the key regions of mouse and human IL-21 required for neutralization. None of the anti-hIL-21 mAbs we isolated from the subsequent fusions were able to neutralize mouse IL-21, though clones 362.75 and 366.552 were able to bind denatured mIL-21 in a western blot ( and and data not shown).
The most potent hIL-21 neutralizing mAbs identified (362.78 and 362.597) proved equivalent or superior to the soluble hIL-21R/γc-Fc heterodimer, which itself is a better neutralizer than the hIL-21R homodimer (hIL-21R-Fc) in a range of biological assays (unpublished results). Notably, in the kinetic inhibition experiments shown in , mAb 362.78 proved superior to the soluble IL-21R/γc-Fc, which could only partially inhibit IL-21 activity despite having association and disassociation rate constants comparable to those of mAb 362.78. For reasons that are thus far unclear, in this assay the mAb appears to better prevent dissociation events and subsequent binding to cell surface IL-21R than does the soluble receptor. From the neutralization activity and other data collected for the panel of IL-21 mAbs, several candidates were selected for further clinical development to evaluate efficacy of an IL-21 mAb in the treatment of patients with autoimmune disease. Other groups have demonstrated anti-disease activity in mouse models using a homodimeric soluble mouse IL-21R-Fc reagent.Citation49,Citation51,Citation59–Citation64 Given that all of our neutralizing mAbs consistently neutralize IL-21 activity at least as potently as the heterodimeric human IL-21R/γc-Fc, and by inference far more potently than the human homodimeric IL-21R-Fc, it appears likely that an anti-IL-21 mAb will also be able to suppress IL-21-mediated autoimmunity and inflammation in vivo. We have also confirmed using high affinity rat anti-mIL-21 or mouse anti-mIL-21 mAbs that neutralizing mIL-21 in vivo in mouse models of colitis/psoriasis and arthritis can elicit therapeutic benefit (unpublished results).
Several of the IL-21 mAbs were also assessed for cross-reactivity to IL-21 from other species in preparation for toxicological assessments in non-human species. In particular, clones 362.78, 362.597, 366.328 and 366.552 all bind and neutralize cynomolgus monkey IL-21, but do not neutralize IL-21 derived from mouse, rat or rabbit ( and and data not shown). Future epitope mapping studies of the IL-21 mAbs will likely reveal residue dissimilarities within the key binding sites between human/cynomolgus monkey IL-21 and mouse/rat/rabbit IL-21 that account for this lack of cross-reactivity. Similarly and not surprisingly, these mAbs do not cross-react on the related, but fairly dissimilar, γc cytokines IL-2, −4, −7, −9 or −15 ( and data not shown). Notably, some of the neutralizing IL-21 mAb clones derived from the human Ig Tg mice were very similar to one another. Two of the anti-IL-21 hybridomas, 362.78 and 362.597, express Ig light and heavy chains that are results of productive rearrangements from the same variable region germline genes, VH3–33 and VkIII A27 (). The variable region sequences from the two hybridomas differ by only one amino acid located in CDR1 of the heavy chain (data not shown). The high degree of shared sequence identity suggests that in each case the heavy and light chain variable regions were derived from the same variable region germline gene. Both hybridomas express Ig kappa light chains, but 362.78 expresses an IgG4 heavy chain while 362.597 expresses an IgG1 heavy chain (). It appears that both of these hybridomas are derivatives of the same initial B-cell immunoglobulin loci rearrangement events and that 362.78 is a result of a subsequent class switch to an IgG4 heavy chain. Either prior to or after the Ig class switching event, 362.78 and 362.597 diverged by the incorporation of further somatic mutations that led to the single amino acid difference in the heavy chain. IL-21 mAbs 362.78 and 362.564 were cloned out separately from the same fusion, but were later determined to be identical in sequence and isotype. Thus, these two hybridomas were likely derived from “sister” clones from the same original B cell generated in vivo.
BIAcore competition experiments testing mAbs representative of each epitope bin against the homodimeric and heterodimeric soluble IL-21Rs revealed some important insights into the mechanism by which these mAbs neutralize IL-21 activity (). mAbs 362.78 and 366.552 competed with both forms of the soluble receptor, while mAbs 366.328 and 366.345 (a non-neutralizing mAb) did not compete with either form of the receptor, despite the observation that 366.328 can neutralize IL-21 bioactivity in cell-based assays ( and ). Theoretically, mAb 366.328 might therefore be able to bind, if only transiently, to IL-21/IL-21Rα complexes on the cell surface. In contrast, mAbs 362.78 and 366.552 directly block IL-21 from binding initially to IL-21Rα and completely prevent further ligand/receptor assembly and signaling. We could not obtain a clear result in the BIAcore competition experiments for mAb 362.75, which appears to bind to a linear epitope in the D helix of IL-21 and is only a weak neutralizer of IL-21 activity ( and ). Clone 362.75 partially and weakly competed with both forms of the soluble receptor in BIAcore, though its relatively low affinity for IL-21 may contribute to incomplete competition in this assay format (data not shown). Consistent with these data, clone 362.75 was also the most effective mAb tested in western blot and anti-peptide ELISA assays ().
While 366.552 can directly block IL-21 binding to its receptor, and can moderately bind to a linear peptide (Peptide 4) encompassing the D helix of IL-21, but not to the IL-21 D helix mutein ( and ), it requires longer pre-incubation times with IL-21 to fully inhibit IL-21 activity in the short term pSTAT3 bioassay (). This may either be due to the location of its binding site on IL-21, which seems to differ from that of 362.78 (), or due to its ∼20-fold slower association rate constant for association with IL-21 ( and ). Future epitope mapping studies may help to resolve these possible explanations. Clone 366.552 did, however, perform well as an IL-21 neutralizer in the longer term cell-based assays ( and data not shown).
To begin to address whether the IL-21 mAbs would retain their function in vivo, an assessment of IL-21 mAb activity was also performed in both short-term and long-term BaF3/hIL-21R-based bioassays in the presence of 5–10% serum from four healthy subjects or from four RA patients (data not shown). Although the serum itself from both donor sets was slightly toxic to the BaF3 cells in both STAT3-P and proliferation assays, there was no significant difference in IL-21 mAb neutralizing activity in the presence of healthy vs. RA serum. One of the IL-21 mAbs described herein (clone 362.597) was recently evaluated for neutralizing activity in vivo. Blazar et al. investigated the role of IL-21 in a human xenogeneic GVHD model, and observed that human IL-21 secreting cells were present in the colon of GVHD recipients and were associated with elevated serum IL-21 levels. When the IL-21 neutralizing mAb was administered prophylactically, it significantly reduced GVHD-associated weight loss and mortality, resulting in a concomitant increase in Tregs and a decrease in T cells secreting IFNγ or granzyme B.Citation66
We have described the generation and initial characterization of a panel of human anti-hIL-21 mAbs from human Ig Tg mice. Clinical studies using a therapeutic anti-IL-21 mAb (NNC114-0005) derived from this panel are underway to determine whether antagonizing IL-21 activity will be a promising new approach for treating various autoimmune diseases.
Materials and Methods
Proteins and peptides.
Recombinant human IL-21 (rhIL-21), cynomolgus (cyno) IL-21 and mouse IL-21 were prepared by recombinant expression and refolding from an E. coli expression system. Mature human and cynomolgus monkey IL-21 (amino acids 30–162) and murine IL-21 (amino acids 18–146) were expressed in E. coli strain W3110 (American Type Culture Collection, #27325) from 500 mL Superbroth II medium (BD Difco, #299316), supplemented with antifoam (Sigma-Aldrich, #A8311) at 100 µl/L and kanamycin (Sigma-Aldrich, #60615) at 25 µg/mL in 2L side-baffled flasks. The cultures were grown at 37°C with shaking at 250 rpm and induced with 1 mM IPTG (Calbiochem, #420322-10GM) when the cultures reached an OD600 of 1.5. Cultures were allowed to grow for an additional 4 h post-induction. The cultures were harvested by centrifugation and IL-21 protein was refolded from the resulting cell pellets. Recombinant C-terminal His6 tagged rat IL-21 was expressed from HEK 293 cells and recovered from the conditioned media by metal affinity chromatography on Ni-NTA Superflow (Qiagen, #30430). Recombinant C-terminal His6 tagged rabbit IL-21 was expressed from HEK 293 cells and evaluated from the conditioned media without further purification. The human Q116D/I119D IL-21 mutein was expressed and purified from E. coli as described above. Four peptides, predicted to be part of the exposed faces of hIL-21, were synthesized by the peptide synthesis core facility at ZymoGenetics. Peptide #1: helix A+added C-terminal Cys (pyroGlu1-Lys21-Cys), Peptide #2: Asn25-Cys42, Peptide #3: Asn68-Cys93 and Peptide #4: helix D, Cys96-Ser124 (see ). Two versions of soluble human IL-21 receptors were expressed as wild type human IgG1 Fc dimers in CHO cells: a homodimeric IL-21R extracellular domain-Fc fusion protein with C-terminal Glu-Glu tags and a heterodimeric IL-21R extracellular domain-Fc/γc extracellular domain-Fc with a C-terminal Glu-Glu tag on the IL-21R-Fc and a C-terminal HisCitation6 tag on the γc-Fc. The homodimeric IL-21R-Fc fusion protein was purified from filtered CHO cell conditioned media using Protein A Sepahrose (GE Healthcare, #17-1279-01) affinity chromatography and size exclusion chromatography (Superdex 200; GE Healthcare, #17-1071-01). The heterodimeric IL-21R-Fc/γc-Fc was purified from CHO cells co-transfected with two separate expression constructs encoding each receptor. Following Protein A Sepharose affinity chromatography and size exclusion chromatography to remove aggregated protein, the heterodimer and homodimer populations were resolved by metal affinity chromatography on Ni-NTA Superflow (Qiagen, #30430). Protein content was determined by amino acid analysis.
Production of anti-human IL-21 mAbs.
Genetically modified Ultimab™ mice from the Kirin-Medarex (KM) Mouse® colony and HuMab mice (HCo20 × BALB/c) were obtained by agreement from Medarex, Inc., (Milipitas, CA) and housed and immunized at ZymoGenetics. All experimental animals used in these studies were included in a protocol approved by the Institutional Animal Care and Use Committee (IACUC) at ZymoGenetics. For Fusions 362 and 366, KM mice were immunized and boosted repeatedly with rhIL-21 conjugated to BSA (Imject Pharmalink Immunogen Kit, ThermoScientific Pierce, #PI77158), or to keyhole limpet hemocyanin (mcKLH Thermoscientific Pierce #77608) utilizing BS3-DSS. For Fusion 367, HuMab mice were immunized with rhIL-21 conjugated to KLH-DSS. Each immunogen was composed of an emulsion of Emulsigen-P adjuvant (MVP Laboratories, Inc.,) and additional CpG (oligonucleotide murine TLR9 ligand; ZymoGenetics, Inc.,) and recombinant mouse granulocyte macrophage colony stimulatory factor (GM-CSF; R&D #415-ML/CF). Immune sera were assayed for human anti-human IL-21 mAb binding activity by enzyme-linked immunosorbent assay (ELISA) and neutralizing activity was assessed by cell-based assays. Splenocytes and lymph node cells from the mice with the highest neutralizing anti-human IL-21 titers were fused with mouse P3-X63-Ag8.653 myeloma cells (ATCC #CRL1580) to generate human antibody-producing hybridomas. Hybridoma culture supernatants were screened for the presence of human IgG/kappa isotypes using a direct ELISA to capture and detect mAbs containing human IgG and kappa constant regions. ELISA plates were first coated with polyclonal goat anti-human IgG, Fc specific (Jackson Immuno Research Laboratories, #109-005-008) antibody, incubated overnight at 4°C, and then washed. Hybridoma supernatant samples were then transferred to the assay plate and, following incubation at RT for 1 h, the wells were aspirated and washed. Horseradish peroxidase (HRP)-labeled goat anti-human kappa specific (Southern Biotech, #2061-05) antibody at a dilution of 1:5,000 was then added and the plates incubated at RT for 1 h. After washing, tetra methyl benzidine (TMB) (BioFX Laboratories, # TMBW-1000-01), was added to each well and color development was stopped by the addition of Stop Reagent (BioFX Laboratories, #STPR-1000-01). The absorbance values were measured with Molecular Devices Spectra MAX 340. Hybridomas secreting IL-21-specific IgG were identified based on their ability to bind to IL-21 immobilized in microtiter plates and on their activity in a cell-based assay for IL-21 neutralizing activity (described below). Specific mAbs were identified by the clone designation of the hybridomas that produced them. For each clone number designation (e.g., 362.78.1), the first number indicates the fusion number (362), followed by the master well from the fusion (78), and each subsequent number indicates a subcloning event (1). For simplicity, we have used here only the master well designations (e.g., 362.78) to refer to any and all subclones derived from the original hybridoma.
Hybridoma cell culture.
Hybridoma lines were cultured in IMDM (Hyclone, #SH30228.01) with 1x GlutMax (Gibco, #35050), 1x Penicillin/Streptomycin (Hyclone, #SV30010), 10% Fetalclone 1 serum, non-heat inactivated (Hyclone, #SH30080.03), 10% Hybridoma Cloning Factor (BM Condimed H1; Roche Diagnostic #11088947001). During fusion and the initial passages of fused cells, complete IMDM medium was applied with the addition of 1x HAT (hypoxanthine, aminopterin, thymidine; Mediatech #25-046-CI) for hybridoma selection. During the cloning process, cells were cultured with the addition of 1x HT (hypoxanthine, thymidine; Gibco/Invitrogen # 0521) replacing HAT.
Cloning and selection of first round clones.
Cells from anti-IL-21 mAb-producing master wells were cloned in order to isolate a single monoclonal hybridoma. Cells were cloned in hybridoma growth medium supplemented with 1x HT and distributed into 96-well microtiter cell culture plates using a standard low-density dilution (<1 cell per well) approach. Monoclonality was assessed by microscopic examination of wells for a single foci of growth prior to assay. As a back-up, 10 cell/well plates were also seeded. Selection of first round clones occurred 10 d post-plating. All wells on the plates indicating outgrowth of one colony were screened with the phosphorylated-STAT3 (pSTAT3) assay for their neutralizing activity. Positive wells were expanded into 24-well cultures and when the density were approximately 4–6 × 105 cells/mL, the supernatant (∼1.5 mL) was individually collected and stored for each well and the cells from each well cryo-preserved. When cloning of master wells did not produce single clones, cloning was repeated from the 10 cell per well plate in order to obtain a single colony outgrowth. The cloning process was repeated to obtain second round clones.
STAT3-phosphorylation assay.
A murine B cell line, BaF3, was transfected with the human IL-21R gene and a STAT-luciferase reporter construct KZ134.Citation10 Recombinant IL-21, IL-21 antibodies or soluble IL-21 heterodimeric receptor (IL-21R/γc-Fc) (ZymoGenetics, Inc.,) were serially diluted and mixed 30 min prior to initiation of reaction. Cells were plated at 5 × 104 cells per well in a 96-well U-bottom plate and incubated for 30 min at 37°C. To initiate the reaction, IL-21/antagonist solutions were mixed with the BaF3 cells and incubated for 15 min at 37°C, unless otherwise noted. Cells were lysed and the level of intracellular pSTAT3 was measured using a bead-based Phospho-STAT3 Assay (Bio-Rad Laboratories, #171-V22522).
STAT-luciferase assay.
BaF3/IL-21R/KZ134 cells were plated at 50,000 cells per well in a 96-well, flat bottom, opaque white assay plate (Corning/Costar, #3917) and incubated for 30 min at 37°C. Dilutions of IL-21, IL-21 antibodies or IL-21 soluble heterodimeric receptor were prepared in a separate 96-well plate and allowed to equilibrate for 30 min at 37°C. The treatment solutions were then transferred to the BaF3 cell plate and incubated at 37°C. After 20–24 h, the cell plate was allowed to equilibrate to RT. Steady-Glo luciferase reagent (Promega Corp., # E2510) was then added to each well and relative luminescence measured.
B-cell proliferation assay.
PBMC were isolated by Ficoll-Paque (GE Healthcare, #17-1440-03) gradient centrifugation from heparinized peripheral blood drawn from healthy human volunteers. Blood samples were obtained per the ZymoGenetics protocol, following written informed consent. B cells were negatively selected using the B Cell Isolation Kit (Miltenyi Biotec, #130-090-862) following the manufacturer's protocol. B-cell purity (CD19+), assessed by flow cytometry, was >97% from each donor. B cells were incubated with 0.1 µg/mL anti-CD40 (R&D Systems, #AF632) plus a titration of rhIL-21 (ZymoGenetics, Inc.). For neutralization assays, 0.1 µg/mL anti-CD40 and 50 ng/mL recombinant IL-21 were mixed with a titration of IL-21 antagonist (IL-21 antibodies or soluble IL-21R). Following a 3d incubation, cells were pulsed with 1 µCi/well of [3H]-thymidine (MP Biomedicals, LLC., #012404301) and incorporation measured after 16 h.
Plasma cell differentiation assay.
Primary human B cells were isolated as described and plated at 1.5 × 105 cells/well in a 96-well flat bottom plate (Becton Dickinson, #353072). Cells were then cultured with 0.1 µg/mL anti-CD40, 10 ng/mL rhIL-4 (R&D Systems, #204-IL-010) and 25 ng/mL rhIL-21 (ZymoGenetics, Inc.). Titrations of IL-21 mAb and sIL-21R were then added and cells incubated at 37°C and 5% CO2 in a humidified tissue culture incubator. After 7 d, conditioned media was collected for antibody titers and the cells pelleted for analysis by flow cytometry. Cells were incubated with antibodies to CD19, CD38, CD138 and IgD (BD Biosciences Inc., #555415, 555461, 347205, 555779, respectively) following a standard surface staining protocol. PC were defined as having the following phenotype: CD19+, IgDlow, CD38+ and CD138. IgG isotype levels in conditioned media samples were measured using the Human IgG Subclass Isotyping Kit (Millipore Corporation, #48-301).
Detection of intracellular IL-21.
Primary human CD4+ T cells were isolated using magnetic beads (Miltenyi Biotec, Inc., #130-091-155) and skewed to a Th1 phenotype by incubating for 48 h in the presence of plate-bound anti-CD3 (BD Biosciences Inc., #555336) and soluble IL-12 (R&D Systems, #219-IL-005), IFNγ (R&D Systems, #285-IF), anti-CD28 (BD Biosciences Inc., #555725) and anti-IL-4 (BD Biosciences Inc., # 54515). Conditioned media was then collected and cells stimulated for 6 h with PMA/ionomycin (Calbiochem, Inc., #524400 and 407950) and the Golgi-transport blocker GolgiPlug (BD Biosciences Inc., #555029). Cells were then collected, permeabilized and fixed in Cytofix/Cytoperm buffer (BD Biosciences Inc., #554722). Cells were stained with commercially available anti-IL-21-allophycocyanin (APC) (eBiosciences Inc., #51-7219-71), anti-IFNγ-PE (BD Biosciences Inc., #554552) or APC-conjugated IL-21 mAb clone 362.78 (ZymoGenetics, Inc.). Data was collected on a FACSCalibur (BD Biosciences Inc.).
Neutralization of native IL-21.
Conditioned media from Th1-skewed CD4+ T cells was added to BaF3/hIL-21R cells for 10 min and pSTAT3 measured to determine relative IL-21 concentration. An EC90 dilution of conditioned media was mixed with a titration of IL-21 mAb clone 362.78 and incubated for 30 min prior to mixing with BaF3/hIL-21R cells. pSTAT3 was measured following a 10 min incubation using the same protocol described above.
Bioassays to assess mAb cross reactivity to related γc cytokines.
A primary B-cell proliferation assay was used to test for IL-21 mAb cross-reactivity to IL-4. Untouched B cells were isolated as described previously and plated at 40,000 cells/well with 1 µg/mL plate-bound goat anti-human IgM (Southern Biotech, Cat #2020-01), 10 ng/mL human IL-4 (R&D Systems, Cat#204-IL-010) and a titration of IL-21 neutralizing antibodies or IL-21R/γc-Fc. Cells were incubated for 3 d and [3H]-thymidine incorporation measured during the final 18 h. A proliferation assay utilizing the murine T-cell line CTLL-2 was used to measure cross-reactivity of IL-21 mAbs to human IL-2 and IL-15. CTLL-2 cells were plated at 50,000 cells/well with either 3.0 ng/mL IL-2 (R&D Systems Inc., Cat# 202-IL-010) or 0.5 ng/mL IL-15 (R&D Systems Inc., Cat# 247-IL-005), and a titration of IL-21 mAbs. Cells were then incubated for 24 h and [3H]-thymidine incorporation measured during an additional 18 h of culture. IL-2 and IL-15 neutralizing antibodies were included as controls (R&D Systems Inc., Cat#MAB202 and MAB2471, respectively).
Antibody production and purification.
Conditioned media containing human IgG was harvested and passed through a 0.2 µm filter. Antibody protein was purified from the filtered conditioned media using a combination of Protein G Sepharose Affinity Chromatography (GE Healthcare, #17-0618-03) and Superdex 200 Size Exclusion Chromatography (GE Healthcare, #17-1071-01). Enriched protein content was estimated by absorbance at A280 and the quality evaluated by analytical size exclusion high performance liquid chromatography and SDS PAGE.
Generation of recombinant human antibodies.
Hybridoma cells corresponding to the supernatant samples that bound and neutralized human IL-21 were cloned in order to isolate monoclonal hybridomas (designated as 362.78 and 366.552) producing the neutralizing mAb of interest. RNA was isolated using the Qiagen RNeasy kit (Qiagen, #74104). Variable regions were cloned using the SMART RACE cDNA Amplification Kit (Clontech, #634914), utilizing 5′ RACE technology and gene specific 3′ primers specific to human constant region sequences. Heavy and light variable region sequences were cloned using the TOPO TA Cloning Kit for Sequencing (Invitrogen, #K4575_01). Gene sequences were verified by comparing the translated DNA sequence to the experimentally determined N-terminal amino acid sequencing performed on antibody purified from hybridomas 362.78 and 366.552. Recombinant forms of the mAbs produced by clones 362.78 and 366.552 were generated and used for some of the experiments shown. Recombinant mAbs 362.78 and 366.552 were expressed in CHO cells using separate heavy chain and light chain expression vectors. Human anti-human IL-21 mAb variable region sequences were spliced with human constant region kappa or human immunoglobulin gamma 1.1 (IgG1.1), an effector-minus variant of wild-type IgG1 that has mutations resulting in the reduction of Fc γ receptor binding and ability to fix complement.Citation53 Constructs were generated utilizing overlap PCRCitation65 and homologous recombination in yeast using a propriety mammalian expression vector with a dihydrofolate reductase (DHFR) selectable marker for the heavy chain and puromycin resistance (puroR) for the light chain. The human anti-human IL-21 heavy and light chain expression plasmids were co-transfected into CHO DXB-11 host cells. Puromycin selection was followed by methotrexate selection to obtain high, stable expression of human anti-human IL-21 mAbs. Conditioned media was harvested and protein was purified as described above.
BIAcore binding kinetics.
Binding kinetics measurements were performed either on a BIACORE T100™ system or a BIACORE-3000 system (GE Healthcare). An antibody capture surface was prepared by directly immobilizing a goat anti-human IgG-Fc-gamma specific antibody (Jackson ImmunoResearch; P/N 109-005-098) onto a CM4 chip using a mixture of 0.4 M EDC [N-ethyl-N′-(3-diethylamino-propyl) carbodiimide] and 0.1 M NHS (N-hydroxysuccinimide). After immobilization of the mAb, the active sites on the flow cell were blocked with 1 M ethanolamine. After preparation of the capture surface, the individual anti-IL-21 mAbs were captured onto separate flow cells of the chip. Serial dilutions of IL-21 from 0 to 200 nM were flowed over the surface and allowed to bind to the captured anti-IL-21 mAb. Binding kinetics were performed with a flow rate of 50 µL/min, an association time of 7 min and a dissociation time of 15 min. The experiment was run at a temperature of 25°C, and the buffer for these studies consisted of 10 mM Hepes, 0.3 M NaCl, 0.05% surfactant P20, 5 mM CaCl2, 1 mg/mL BSA, pH 8.0. Between cycles, the flow cell was washed with 20 mM hydrochloric acid to regenerate the surface. The sensorgrams were processed by double referencing (the signal from the reference flow cell was subtracted from the binding curves, followed by subtraction of the signal from the buffer injections). The binding curves were globally fit to the 1:1 binding model. Similar experiments were run to test for cross-reactivity of clone 362.78 to the related γc cytokines IL-2, IL-4, IL-7, IL-9 and IL-15.
Epitope binning and competition experiments.
Epitope binning studies were performed on a BIACORE T100™ system (GE Healthcare). Individual anti-IL-21 mAbs were covalently immobilized to separate flow cells of a CM4 sensor chip (GE Healthcare, P/N BR-1005-34) using a mixture of EDC and NHS as described above. After immobilization of the mAb, the active sites on the flow cell were blocked with 1 M ethanolamine. The IL-21 antigen was flowed over the surface and allowed to specifically bind to the mAb immobilized on the sensor chip. Following the binding of the IL-21 antigen, a secondary anti-IL-21 mAb was injected and allowed to bind. If the secondary anti-IL-21 mAb was capable of binding the antigen simultaneously with the primary mAb, an increase in mass on the surface of the chip, or binding, was detected. If the secondary anti-IL-21 mAb was not capable of binding the antigen simultaneously with the primary mAb, no additional mass or binding, was detected. Once the level of background signal (RU) associated with the negative controls was established (the same anti-IL-21 mAb used as both the primary and secondary mAb), the binning results were reported as either positive or negative binding. The differential between positive and negative response values in this experiment was significant and allowed for an unambiguous assignment of the anti-IL-21 mAbs into distinct families, or epitope bins, as described in Results. Competition studies with soluble receptors (IL-21R-Fc and IL-21R/γc-Fc) were performed in a similar manner. The experiment was set up as detailed above, except the soluble receptors were used in place of the secondary anti-IL-21 mAb. As a positive control for this assay, each anti-IL-21 mAb was tested against an anti-IL-21 mAb from a different epitope bin to determine the level of positive (binding) signal.
Epitope binning and competition experiments were performed with a flow rate of 30 µL/min and a temperature of 25°C. The buffer for these studies consisted of 10 mM Hepes, 0.3 M NaCl, 0.05% surfactant P20, 5 mM CaCl2, 1 mg/mL BSA, pH 8.0. Between cycles, the flow cell was washed with 20 mM hydrochloric acid to regenerate the surface. Data was compiled using BIACORE T100™ Evaluation software (version 1.1.1).
Binding assessment using western blotting and direct ELISA.
The ability of the mAbs to bind to hIL-21 and IL-21 orthologs was assessed using western blotting. Recombinant human, cynomolgus and mouse IL-21, human IL-21 peptides (#1–4) conjugated to maleimide activated-ovalbumin (OVA) (Thermoscientific Pierce, #PI77126), human IFN-λ1 control protein (ZymoGenetics, Inc.,) and human IL-2, IL-4, IL-7, IL-9 and IL-15 (R&D Systems) were subjected to SDS polyacrylamide gels electrophoresis (SDS-PAGE) and transferred to nitrocellulose membranes. Membranes were blocked with western Blocking Reagent [20 mM Tris pH 7.4, 0.5 mM EDTA, 0.5% v/v IGEPAL CA-630, 150 mM NaCl, 0.25% gelatin: with 1% added hydrolyzed casein (Roche Laboratories, #11921673001)] and were subsequently incubated with 10–100 ng/mL mAb in blocking reagent for 2 h at ambient temperature. Following washing, the membranes were incubated for 2 h with HRP-conjugated donkey anti-human IgG (Jackson ImmunoResearch Laboratories, #709-035-149), washed and visualized using Supersignal DuraWest peroxidase substrate solution (Pierce, #34075). The ability of the mAbs to bind to hIL-21, the hIL-21 Q116D/I119D mutein and hIL-21 sequence derived peptides (conjugated or not to OVA) was also assessed using a direct ELISA format. Recombinant antigen was immobilized onto the surface of polystyrene plates at 1 µg/mL in 0.1 M Na2CO3 pH 9.6. Plates were incubated overnight at 4°C and the plates were washed with PBS-Tween wash buffer (137 mM NaCl, 2.2 mM KCL, 6.7 mM Na2PO4, 2.0 mM KH2PO4 pH 7.2, 0.05% v/w polysorbate 20). Wells were blocked with wash buffer containing 1% w/v BSA and antibody solutions (1 µg/mL) were prepared in 5% fetal bovine serum (FBS)/Iscove's modified Dulbecco's Media (IMDM) and added to the wells for 1 h at ambient temperature. Following additional washing, HRP-conjugated goat anti-human IgG-Fc specific (Jackson Immunoresearch Laboratories, #109-035-098) was added at ambient temperature for 1 h. Plates were washed a final time and developed using TMB (BioFx Laboratories). The ability of the anti-hIL-21 binding and neutralizing mAbs to bind to hIL-21 and hIL-21 sequence derived synthetic peptides was demonstrated in the direct ELISA assay format. Binding to rhIL-21 (ZymoGenetics), or to hIL-21 sequence derived synthetic peptides (Peptides #1–4, defined above), was assessed by ELISA as described for recombinant protein.
Disclosure of Potential Conflicts of Interest
All authors are current or former employees and shareholders of ZymoGenetics, Inc.
Abbreviations
| APC | = | allophycocyanin |
| CDR | = | complementarity-determining region |
| d | = | day(s) |
| DSS | = | disuccinimidyl suberate |
| ELISA | = | enzyme-linked immunosorbent assay |
| FBS | = | fetal bovine serum |
| IL-21 | = | interleukin 21 |
| IL-21R | = | interleukin 21 receptor |
| IL-21R/γc | = | heterodimeric human interleukin 21 receptor/IL-2R common gamma chain |
| hIL-21 | = | human interleukin 21 |
| mIL-21 | = | mouse interleukin 21 |
| HRP | = | horseradish peroxidase |
| IBD | = | inflammatory bowel disease |
| Ig | = | immunoglobulin |
| Jak | = | janus activated kinase |
| KLH | = | keyhole limpet hemocyanin |
| mAb | = | monoclonal antibody |
| min | = | minute |
| NK | = | natural killer |
| NK-T | = | natural killer T cell |
| OVA | = | ovalbumin |
| PBMC | = | peripheral blood mononuclear cells |
| PC | = | plasma cell |
| pSTAT | = | phosphorylated signal transducer and activator of transcription |
| RA | = | rheumatoid arthritis |
| rhIL-21 | = | recombinant human IL-21 |
| SLE | = | systemic lupus erythematosus |
| STAT | = | signal transducer and activator of transcription |
| TFH | = | follicular helper T cells |
| TG | = | transgenic |
| TMB | = | tetra methyl benzidine |
Figures and Tables
Figure 1 Affinity and epitope bin distribution. (A) The mAbs were screened for binding affinity to rhIL-21 via surface plasmon resonance (BIAcore). Association rate constants (ka) and dissociation rate constants (kd) were plotted in an affinity isotherm plot for mAbs in Bin 1 (filled circles), Bin 2 (open squares), Bin 1/2 (asterisks), Bin 3 (filled triangles) and the soluble heterodimeric receptor (open triangle). Values for a specific subset of the mAb clones are indicated with letters. (B) Epitope bin assignment of the mAbs.
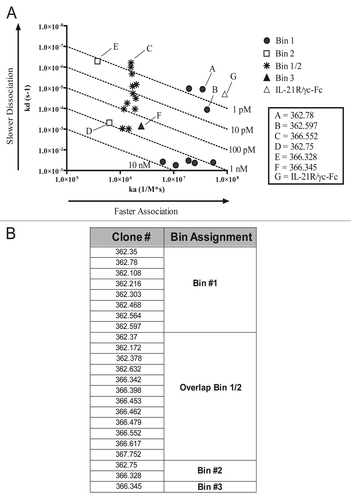
Figure 2 Neutralization of IL-21-mediated activity in vitro. (A) Neutralization of STAT3 phosphorylation by IL-21 mAbs and the soluble receptor. Thirty-two pM rhIL-21 was incubated with three CHO-expressed recombinant IL-21 neutralizing mAb clones 362.78 (filled squares), 366.328 (open triangles), 366.552 (X's), or soluble IL-21R/γc-Fc (asterisks) prior to stimulation of BaF3 cells expressing IL-21R. Phospho-STAT3 was measured following a 15 min incubation. (B) Neutralization of STAT-mediated luciferase activity. rhIL-21 (641 pM) was incubated with IL-21 mAbs or soluble IL-21R/γc for 30 min prior to culture with BaF3/IL-21R cells. After 20 h, STAT-mediated luciferase activity was measured. (C) Neutralization of IL-21-mediated B cell proliferation. Primary human B cells were incubated for 4 d with 3.21 nM (50 ng/mL) IL-21, 100 ng/mL anti-CD40 or a titration of IL-21 mAb or soluble IL-21R/γc-Fc. Incorporation of 3H-thymidine during the final 16 h was measured. Results shown are representative of three independent experiments.
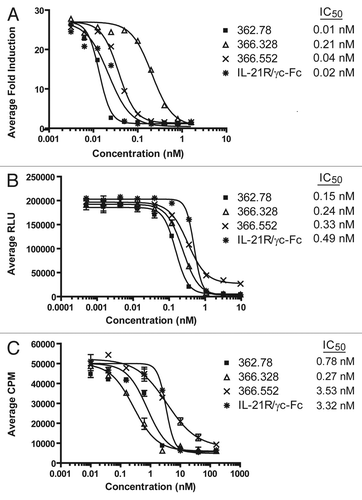
Figure 3 Neutralization of IL-21-mediated B cell differentiation and IgG1 production. (A) Inhibition of B-cell differentiation to a PC phenotype. A subset of primary human B cells, when cultured for 8 d with 25 ng/mL IL-21, 100 ng/mL anti-CD40 and 10 ng/mL IL-4, differentiate to an IgDlo, CD38+ PC phenotype. Addition of IL-21 neutralizing mAbs (produced from 1st round hybridoma clones) or soluble IL-21R blocked PC differentiation in a dose-dependent manner. Data for clone 362.78 is shown, though similar results were obtained with clones 362.328 and 366.552. Results shown are representative of two separate experiments. (B) IL-21 mAbs reduced IgG1 production. PC supernatants were collected at the end of the 8 d differentiation period and concentration (ng/mL) of total IgG1 measured. Inhibition of IL-21-mediated PC differentiation by the IL-21 mAbs correlated with a reduction in the amount of IgG1 produced.
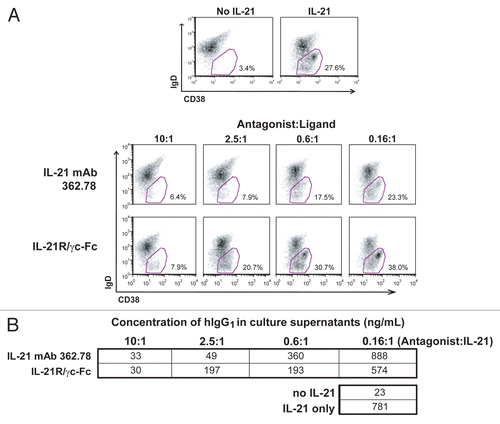
Figure 4 IL-21 mAb binds to and neutralizes native IL-21. (A) APC-labeled IL-21 mAb (362.78; purified from the 2nd round hybridoma clone) binds intracellular IL-21 in PMA+ionomycin-stimulated (upper panels), but not unstimulated (lower panels) human T cells. Total PBMC were stained with anti-CD3-FITC and counterstained with either mAb 362.78 (left panels), a commercially available APC-labeled positive control IL-21 mAb (IgG4) (right panels), or an APC-labeled IgG4 isotype control mAb (not shown). The APC staining from the isotype control mAb was used to set the quadrants shown. Results shown are representative of three independent experiments. (B) IL-21 mAb (clone 362.78) neutralized native IL-21-induced STAT3 phosphorylation. CD4+ T cells were isolated from four healthy donors and stimulated for 16 h with PMA+ionomycin to generate conditioned media containing native IL-21. Media was then incubated with a titration of IL-21 mAbs, cultured with BaF3/IL-21R cells for 15 min and pSTAT3 was measured. IL-21-mediated pSTAT3 from all four donors was blocked in a dose-dependent manner by IL-21 mAb.
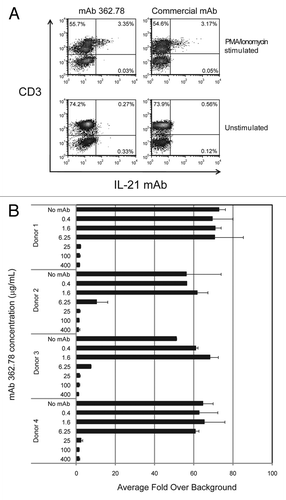
Figure 5 Binding kinetics correlate with efficacy in the IL-21-mediated pSTAT3 assay. Serial dilutions of IL-21 antagonists were pre-incubated with 96 pM IL-21 for 0 (filled squares), 5 (open triangles), 15 (filled inverted triangles) or 30 (open diamonds) min prior to initiation of the pSTAT3 assay. Recombinant CHO-expressed mAbs were used in these experiments. (A) With a relatively slow association rate, clone 366.552 requires a longer pre-incubation period than clone 362.78 or IL-21R to completely neutralize IL-21-mediated STAT3 phosphorylation. (B) IL-21 mAb clone 362.78 (very fast association rate) demonstrated the most potent inhibition of IL-21 mediated pSTAT3 with complete neutralization following 5, 15 and 30 min pre-incubation periods. (C) Soluble IL-21R/γc neutralized IL-21-mediated pSTAT3 in a time-dependent manner, but not as effectively as clone 362.78.
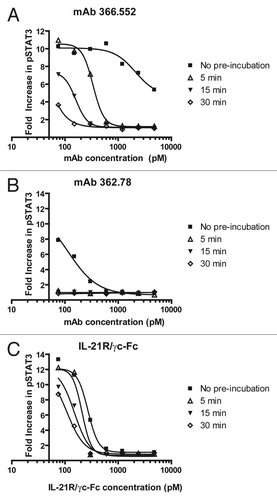
Figure 6 Representative mAbs from the neutralizing epitope bins (Bin #1, Bin #1/2 and Bin #2) were tested for competition with soluble homodimeric or heterodimeric IL-21R. Sensorgrams are shown for co-binding with (A) mAb 362.78 (Bin #1), (B) mAb 366.552 (Bin #1/2) and (C) mAb 366.328 (Bin #2). The lists of competitors tested against each mAb are shown in the boxes on the right.
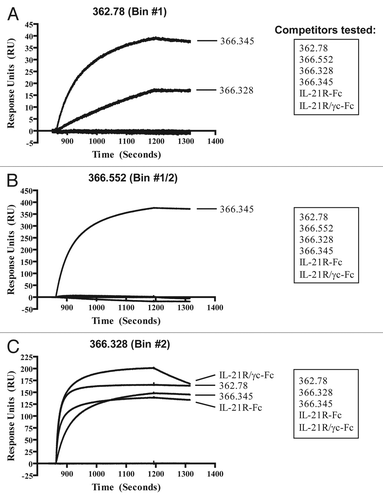
Figure 7 Amino acid sequence of mature hIL-21. Residues 1–133 of the mature hIL-21 polypeptide, lacking the signal sequence, are shown. Helices A–D are boxed, and the four synthetic peptides used to assess binding of the IL-21 mAbs by ELISA and western blot () are indicated. The two arrows denote the residues mutated in helix D (Q116D and I119D) of the IL-21 mutein; these two mutations do not affect the ability of the mutein to bind IL-21R, but prevent signaling through the IL-21R/γc complex, and are thought to be critical residues for γc binding.Citation57,Citation58
Table 1 Characteristics of key IL-21 mAbs and the soluble IL-21R/γc-Fc heterodimeric receptor
Table 2 Affinity and neutralizing activity of key IL-21 mAbs and the soluble IL-21R/γc-Fc heterodimeric receptor
Table 3 Summary of binding of key IL-21 mAbs to various forms of IL-21 and to peptides derived from IL-21
Acknowledgments
We gratefully acknowledge the helpful discussions, generation of reagents, and other contributions to this project by many of our past and present ZymoGenetics colleagues, including: Ty Brender, Jennifer Brody, Chung-Leung Chan, Mike Dodds, Frank Grant, Jane Gross, Matt Holdren, Tracey (Pownder) Jurista, Cecile Krejsa, Joe Kuijper, Megan Lantry, Katherine Lewis, Hong Liu, Pat McKernan, Kathy Mink, Julie Parrish-Novak, Scott Presnell, Fred Ramsdell, Brian Reardon, P.V. Sivakumar, Marrissa Smith, Mike Stamm, Kim Waggie, Jim West, Rob West and Eugene Yi. This work was funded by ZymoGenetics, Inc.
References
- Monteleone G, Pallone F, Macdonald TT. Interleukin-21 (IL-21)-mediated pathways in T cell-mediated disease. Cytokine Growth Factor Rev 2009; 20:185 - 191; PMID: 19261537; http://dx.doi.org/10.1016/j.cytogfr.2009.02.002
- Ettinger R, Kuchen S, Lipsky PE. Interleukin 21 as a target of intervention in autoimmune disease. Ann Rheum Dis 2008; 67:83 - 86; PMID: 19022821; http://dx.doi.org/10.1136/ard.2008.098400
- Leonard WJ, Zeng R, Spolski R. Interleukin 21: a cytokine/cytokine receptor system that has come of age. J Leukoc Biol 2008; 84:348 - 356; PMID: 18467657; http://dx.doi.org/10.1189/jlb.0308149
- Monteleone G, Caruso R, Fina D, Peluso I, Gioia V, Stolfi C, et al. Control of matrix metalloproteinase production in human intestinal fibroblasts by interleukin 21. Gut 2006; 55:1774 - 1780; PMID: 16682426; http://dx.doi.org/10.1136/gut.2006.093187
- Distler JH, Jüngel A, Kowal-Bielecka O, Michel BA, Gay RE, Sprott H, et al. Expression of interleukin-21 receptor in epidermis from patients with systemic sclerosis. Arthritis Rheum 2005; 52:856 - 864; PMID: 15751077; http://dx.doi.org/10.1002/art.20883
- Jüngel A, Distler JH, Kurowska-Stolarska M, Seemayer CA, Seibl R, Forster A, et al. Expression of interleukin-21 receptor, but not interleukin-21, in synovial fibroblasts and synovial macrophages of patients with rheumatoid arthritis. Arthritis Rheum 2004; 50:1468 - 1476; PMID: 15146416; http://dx.doi.org/10.1002/art.20218
- Li J, Shen W, Kong K, Liu Z. Interleukin-21 induces T-cell activation and proinflammatory cytokine secretion in rheumatoid arthritis. Scand J Immunol 2006; 64:515 - 522; PMID: 17032244; http://dx.doi.org/10.1111/j.1365-3083.2006.01795.x
- Caruso R, Fina D, Peluso I, Fantini MC, Tosti C, Del Vecchio Blanco G, et al. IL-21 is highly produced in Helicobacter pylori-infected gastric mucosa and promotes gelatinases synthesis. J Immunol 2007; 178:5957 - 5965; PMID: 17442980
- Caruso R, Fina D, Peluso I, Stolfi C, Fantini MC, Gioia V, et al. A functional role for interleukin-21 in promoting the synthesis of the T-cell chemoattractant, MIP-3alpha, by gut epithelial cells. Gastroenterology 2007; 132:166 - 175; PMID: 17241869; http://dx.doi.org/10.1053/j.gastro.2006.09.053
- Parrish-Novak J, Dillon SR, Nelson A, Hammond A, Sprecher C, Gross JA, et al. Interleukin 21 and its receptor are involved in NK cell expansion and regulation of lymphocyte function. Nature 2000; 408:57 - 63; PMID: 11081504; http://dx.doi.org/10.1038/35040504
- Korn T, Bettelli E, Gao W, Awasthi A, Jäger A, Strom TB, et al. IL-21 initiates an alternative pathway to induce proinflammatory T(H)17 cells. Nature 2007; 448:484 - 487; PMID: 17581588; http://dx.doi.org/10.1038/nature05970
- Nurieva R, Yang XO, Martinez G, Zhang Y, Panopoulos AD, Ma L, et al. Essential autocrine regulation by IL-21 in the generation of inflammatory T cells. Nature 2007; 448:480 - 483; PMID: 17581589; http://dx.doi.org/10.1038/nature05969
- Zhou L, Ivanov II, Spolski R, Min R, Shenderov K, Egawa T, et al. IL-6 programs T(H)-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat Immunol 2007; 8:967 - 974; PMID: 17581537; http://dx.doi.org/10.1038/ni1488
- Kuchen S, Robbins R, Sims GP, Sheng C, Phillips TM, Lipsky PE, et al. Essential role of IL-21 in B cell activation, expansion and plasma cell generation during CD4+ T cell-B cell collaboration. J Immunol 2007; 179:5886 - 5896; PMID: 17947662
- Vogelzang A, McGuire HM, Yu D, Sprent J, Mackay CR, King C. A fundamental role for interleukin-21 in the generation of T follicular helper cells. Immunity 2008; 29:127 - 137; PMID: 18602282; http://dx.doi.org/10.1016/j.immuni.2008.06.001
- Spolski R, Leonard WJ. IL-21 and T follicular helper cells. Int Immunol 2010; 22:7 - 12; PMID: 19933709; http://dx.doi.org/10.1093/intimm/dxp112
- Fuqua CF, Akomeah R, Price JO, Adunyah SE. Involvement of ERK-1/2 in IL-21-induced cytokine production in leukemia cells and human monocytes. Cytokine 2008; 44:101 - 107; PMID: 18707899; http://dx.doi.org/10.1016/j.cyto.2008.06.010
- Liu Y, Yang B, Ma J, Wang H, Huang F, Zhang J, et al. Interleukin-21 maintains the expression of CD16 on monocytes via the production of IL-10 by human naïve CD4+ T cells. Cell Immunol 2011; 267:102 - 108; PMID: 21227406; http://dx.doi.org/10.1016/j.cel-limm.2010.12.003
- Brandt K, Bulfone-Paus S, Foster DC, Rückert R. Interleukin-21 inhibits dendritic cell activation and maturation. Blood 2003; 102:4090 - 4098; PMID: 12893770; http://dx.doi.org/10.1182/blood-2003-03-0669
- Brandt K, Bulfone-Paus S, Jenckel A, Foster DC, Paus R, Rückert R. Interleukin-21 inhibits dendritic cell-mediated T cell activation and induction of contact hypersensitivity in vivo. J Invest Dermatol 2003; 121:1379 - 1382; PMID: 14675186; http://dx.doi.org/10.1046/j.1523-747.2003.12603.x
- Ettinger R, Kuchen S, Lipsky PE. The role of IL-21 in regulating B-cell function in health and disease. Immunol Rev 2008; 223:60 - 86; PMID: 18613830; http://dx.doi.org/10.1111/j.1600-065X.2008.00631.x
- Rasmussen TK, Andersen T, Hvid M, Hetland ML, Hørslev-Petersen K, Stengaard-Pedersen K, et al. Increased interleukin 21 (IL-21) and IL-23 are associated with increased disease activity and with radiographic status in patients with early rheumatoid arthritis. J Rheumatol 2010; 37:2014 - 2020; PMID: 20682664; http://dx.doi.org/10.3899/jrheum.100259
- Niu X, He D, Zhang X, Yue T, Li N, Zhang JZ, et al. IL-21 regulates Th17 cells in rheumatoid arthritis. Hum Immunol 2010; 71:334 - 341; PMID: 20079789; http://dx.doi.org/10.1016/j.humimm.2010.01.010
- Li J, Pan HF, Cen H, Tian J, Ma Y, Tao JH, et al. Interleukin-21 as a potential therapeutic target for systemic lupus erythematosus. Mol Biol Rep 2011; 38:4077 - 4081; PMID: 21107711; http://dx.doi.org/10.1007/s11033-010-0527-y
- Yuan FL, Hu W, Lu WG, Li X, Li JP, Xu RS, et al. Targeting interleukin-21 in rheumatoid arthritis. Mol Biol Rep 2011; 38:1717 - 1721; PMID: 20848219; http://dx.doi.org/10.1007/s11033-010-0285-x
- Monteleone G, Monteleone I, Fina D, Vavassori P, Del Vecchio Blanco G, Caruso R, et al. Interleukin-21 enhances T-helper cell type I signaling and interferon-gamma production in Crohn's disease. Gastroenterology 2005; 128:687 - 694; PMID: 15765404; http://dx.doi.org/10.1053/j.gastro.2004.12.042
- Fina D, Caruso R, Pallone F, Monteleone G. Interleukin-21 (IL-21) controls inflammatory pathways in the gut. Endocr Metab Immune Disord Drug Targets 2007; 7:288 - 291; PMID: 18220949; http://dx.doi.org/10.2174/187153007782794308
- Caprioli F, Sarra M, Caruso R, Stolfi C, Fina D, Sica G, et al. Autocrine regulation of IL-21 production in human T lymphocytes. J Immunol 2008; 180:1800 - 1807; PMID: 18209077
- Caruso R, Costanzo A, Monteleone G. Pathogenic role of interleukin-21 in psoriasis. Cell Cycle 2009; 8:3629 - 3630; PMID: 19884801; http://dx.doi.org/10.4161/cc.8.22.9964
- Asano K, Ikegami H, Fujisawa T, Nishino M, Nojima K, Kawabata Y, et al. Molecular scanning of interleukin-21 gene and genetic susceptibility to type 1 diabetes. Hum Immunol 2007; 68:384 - 391; PMID: 17462506; http://dx.doi.org/10.1016/j.humimm.2007.01.009
- van Heel DA, Franke L, Hunt KA, Gwilliam R, Zhernakova A, Inouye M, et al. A genome-wide association study for celiac disease identifies risk variants in the region harboring IL2 and IL21. Nat Genet 2007; 39:827 - 829; PMID: 17558408; http://dx.doi.org/10.1038/ng2058
- Sawalha AH, Kaufman KM, Kelly JA, Adler AJ, Aberle T, Kilpatrick J, et al. Genetic association of interleukin-21 polymorphisms with systemic lupus erythematosus. Ann Rheum Dis 2008; 67:458 - 461; PMID: 17720724; http://dx.doi.org/10.1136/ard.2007.075424
- Liu Y, Helms C, Liao W, Zaba LC, Duan S, Gardner J, et al. A genome-wide association study of psoriasis and psoriatic arthritis identifies new disease loci. PLoS Genet 2008; 4:1000041; PMID: 18369459; PMID: 10.1371/journal.pgen.1000041
- Chatterjee R, Batra J, Ghosh B. A common exonic variant of interleukin21 confers susceptibility to atopic asthma. Int Arch Allergy Immunol 2009; 148:137 - 146; PMID: 18802358; http://dx.doi.org/10.1159/000155744
- Festen EA, Goyette P, Scott R, Annese V, Zhernakova A, Lian J, et al. Genetic variants in the region harbouring IL2/IL21 associated with ulcerative colitis. Gut 2009; 58:799 - 804; PMID: 19201773; http://dx.doi.org/10.1136/gut.2008.166918
- Daha NA, Kurreeman FA, Marques RB, Stoeken-Rijsbergen G, Verduijn W, Huizinga TW, et al. Confirmation of STAT4, IL2/IL21 and CTLA4 polymorphisms in rheumatoid arthritis. Arthritis Rheum 2009; 60:1255 - 1260; PMID: 19404967; http://dx.doi.org/10.1002/art.24503
- Glas J, Stallhofer J, Ripke S, Wetzke M, Pfennig S, Klein W, et al. Novel genetic risk markers for ulcerative colitis in the IL2/IL21 region are in epistasis with IL23R and suggest a common genetic background for ulcerative colitis and celiac disease. Am J Gastroenterol 2009; 104:1737 - 1744; PMID: 19455118; http://dx.doi.org/10.1038/ajg.2009.163
- Márquez A, Orozco G, Martínez A, Palomino-Morales R, Fernández-Arquero M, Mendoza JL, et al. Novel association of the interleukin 2-interleukin 21 region with inflammatory bowel disease. Am J Gastroenterol 2009; 104:1968 - 1975; PMID: 19471255; http://dx.doi.org/10.1038/ajg.2009.224
- Webb R, Merrill JT, Kelly JA, Sestak A, Kaufman KM, Langefeld CD, et al. A polymorphism within IL21R confers risk for systemic lupus erythematosus. Arthritis Rheum 2009; 60:2402 - 2407; PMID: 19644854; http://dx.doi.org/10.1002/art.24658
- Coenen MJ, Trynka G, Heskamp S, Franke B, van Diemen CC, Smolonska J, et al. Common and different genetic background for rheumatoid arthritis and coeliac disease. Hum Mol Genet 2009; 18:4195 - 4203; PMID: 19648290; http://dx.doi.org/10.1093/hmg/ddp365
- Guo Y, Zhang LS, Yang TL, Tian Q, Xiong DH, Pei YF, et al. IL21R and PTH may underlie variation of femoral neck bone mineral density as revealed by a genome-wide association study. J Bone Miner Res 2010; 25:1042 - 1048; PMID: 19874204
- Hinks A, Eyre S, Ke X, Barton A, Martin P, Flynn E, et al. Childhood Arthritis Prospective Study; UKRAG Consortium; BSPAR Study Group. Association of the AFF3 gene and IL2/IL21 gene region with juvenile idiopathic arthritis. Genes Immun 2010; 11:194 - 198; PMID: 20072139; http://dx.doi.org/10.1038/gene.2009.105
- Nohra R, Beyeen AD, Guo JP, Khademi M, Sundqvist E, Hedreul MT, et al. RGMA and IL21R show association with experimental inflammation and multiple sclerosis. Genes Immun 2010; 11:279 - 293; PMID: 20072140; http://dx.doi.org/10.1038/gene.2009.111
- Maiti AK, Kim-Howard X, Viswanathan P, Guillén L, Rojas-Villarraga A, Deshmukh H, et al. Confirmation of an association between rs6822844 at the Il2-Il21 region and multiple autoimmune diseases: evidence of a general susceptibility locus. Arthritis Rheum 2010; 62:323 - 329; PMID: 20112382; http://dx.doi.org/10.1002/art.27222
- Hollis-Moffatt JE, Gearry RB, Barclay ML, Merriman TR, Roberts RL. Consolidation of evidence for association of the KIAA1109-TENR-IL2-IL21 rs6822844 variant with Crohn's disease. Am J Gastroenterol 2010; 105:1204 - 1205; PMID: 20445516; http://dx.doi.org/10.1038/ajg.2010.34
- Petukhova L, Duvic M, Hordinsky M, Norris D, Price V, Shimomura Y, et al. Genome-wide association study in alopecia areata implicates both innate and adaptive immunity. Nature 2010; 466:113 - 117; PMID: 20596022; http://dx.doi.org/10.1038/nature09114
- Warren RB, Smith RL, Flynn E, Bowes J, Eyre S, Worthington J. UKRAG Consortium. A systematic investigation of confirmed autoimmune loci in early-onset psoriasis reveals an association with IL2/IL21. Br J Dermatol 2011; 164:660 - 664; PMID: 21375519
- Sarra M, Franzè E, Pallone F, Monteleone G. Targeting interleukin-21 in inflammatory diseases. Expert Opin Ther Targets 2011; 15:695 - 702; PMID: 21391901; http://dx.doi.org/10.1517/14728222.2011.561319
- Vugmeyster Y, Guay H, Szklut P, Qian MD, Jin M, Widom A, et al. In vitro potency, pharmacokinetic profiles and pharmacological activity of optimized anti-IL-21R antibodies in a mouse model of lupus. MAbs 2010; 2:335 - 346; PMID: 20424514; http://dx.doi.org/10.4161/mabs.2.3.11850
- Vugmeyster Y, Allen S, Szklut P, Bree A, Ryan M, Ma M, et al. Correlation of pharmacodynamic activity, pharmacokinetics and anti-product antibody responses to anti-IL-21R antibody therapeutics following IV administration to cynomolgus monkeys. J Transl Med 2010; 8:41; PMID: 20420683; http://dx.doi.org/10.1186/1479-5876-8-41
- Herber D, Brown TP, Liang S, Young DA, Collins M, Dunussi-Joannopoulos K. IL-21 has a pathogenic role in a lupus-prone mouse model and its blockade with IL-21R.Fc reduces disease progression. J Immunol 2007; 178:3822 - 3830; PMID: 17339481
- Zhang JL, Foster D, Sebald W. Human IL-21 and IL-4 bind to partially overlapping epitopes of common gamma-chain. Biochem Biophys Res Commun 2003; 300:291 - 296; PMID: 12504082; http://dx.doi.org/10.1016/S0006-291X(02)02836-X
- Gross JA, Dillon SR, Mudri S, Johnston J, Littau A, Roque R, et al. TACI-Ig neutralizes molecules critical for B cell development and autoimmune disease. impaired B cell maturation in mice lacking BLyS. Immunity 2001; 15:289 - 302; PMID: 11520463; http://dx.doi.org/10.1016/S1074-7613(01)00183-2
- Asao H, Okuyama C, Kumaki S, Ishii N, Tsuchiya S, Foster D, et al. Cutting edge: the common gamma-chain is an indispensable subunit of the IL-21 receptor complex. J Immunol 2001; 167:1 - 5; PMID: 11418623
- Habib T, Senadheera S, Weinberg K, Kaushansky K. The common gamma chain (gammac) is a required signaling component of the IL-21 receptor and supports IL-21-induced cell proliferation via JAK3. Biochemistry 2002; 41:8725 - 8731; PMID: 12093291; http://dx.doi.org/10.1021/bi0202023
- Ettinger R, Sims GP, Fairhurst AM, Robbins R, da Silva YS, Spolski R, et al. IL-21 induces differentiation of human naive and memory B cells into antibody-secreting plasma cells. J Immunol 2005; 175:7867 - 7879; PMID: 16339522
- Bondensgaard K, Breinholt J, Madsen D, Omkvist DH, Kang L, Worsaae A, et al. The existence of multiple conformers of interleukin-21 directs engineering of a superpotent analogue. J Biol Chem 2007; 282:23326 - 23336; PMID: 17565991; http://dx.doi.org/10.1074/jbc.M701313200
- Kang L, Bondensgaard K, Li T, Hartmann R, Hjorth SA. Rational design of interleukin-21 antagonist through selective elimination of the gammaC binding epitope. J Biol Chem 2010; 285:12223 - 12231; PMID: 20167599; http://dx.doi.org/10.1074/jbc.M110.101444
- Young DA, Hegen M, Ma HL, Whitters MJ, Albert LM, Lowe L, et al. Blockade of the interleukin-21/interleukin-21 receptor pathway ameliorates disease in animal models of rheumatoid arthritis. Arthritis Rheum 2007; 56:1152 - 1163; PMID: 17393408; http://dx.doi.org/10.1002/art.22452
- Bubier JA, Bennett SM, Sproule TJ, Lyons BL, Olland S, Young DA, et al. Treatment of BXSB-Yaa mice with IL-21R-Fc fusion protein minimally attenuates systemic lupus erythematosus. Ann NY Acad Sci 2007; 1110:590 - 601; PMID: 17911475; http://dx.doi.org/10.1196/annals.1423.063
- Fina D, Sarra M, Fantini MC, Rizzo A, Caruso R, Caprioli F, et al. Regulation of gut inflammation and th17 cell response by interleukin-21. Gastroenterology 2008; 134:1038 - 1048; PMID: 18395085; http://dx.doi.org/10.1053/j.gastro.2008.01.041
- Caruso R, Botti E, Sarra M, Esposito M, Stolfi C, Diluvio L, et al. Involvement of interleukin-21 in the epidermal hyperplasia of psoriasis. Nat Med 2009; 15:1013 - 1015; PMID: 19684581; http://dx.doi.org/10.1038/nm.1995
- Bucher C, Koch L, Vogtenhuber C, Goren E, Munger M, Panoskaltsis-Mortari A, et al. IL-21 blockade reduces graft-versus-host disease mortality by supporting inducible T regulatory cell generation. Blood 2009; 114:5375 - 5384; PMID: 19843883; http://dx.doi.org/10.1182/blood-2009-05-221135
- McGuire HM, Walters S, Vogelzang A, Lee CM, Webster KE, Sprent J, et al. Interleukin-21 is critically required in autoimmune and allogeneic responses to islet tissue in murine models. Diabetes 2011; 60:867 - 875; PMID: 21357471; http://dx.doi.org/10.2337/db10-1157
- Horton RM, Hunt HD, Ho SN, Pullen JK, Pease LR. Engineering hybrid genes without the use of restriction enzymes: gene splicing by overlap extension. Gene 1989; 77:61 - 68; PMID: 2744488; http://dx.doi.org/10.1016/0378-1119(89)90359-4
- Hippen KL, Bucher C, Schirm DK, Bearl AM, Brender T, Mink KA, et al. Blocking IL-21 signaling ameliorates xenogeneic GVHD induced by human lymphocytes. Blood 2011; 119:619 - 628; PMID: 22077059; http://dx.doi.org/10.1182/blood-2011-07-368027