Abstract
Monoclonal antibodies are widely used for the treatment of cancer, inflammatory and infectious diseases and other disorders. Most of the marketed antibodies are monospecific and therefore capable of interacting and interfering with a single target. However, complex diseases are often multifactorial in nature, and involve redundant or synergistic action of disease mediators or upregulation of different receptors, including crosstalk between their signaling networks. Consequently, blockade of multiple, different pathological factors and pathways may result in improved therapeutic efficacy. This result can be achieved by combining different drugs, or use of the dual targeting strategies applying bispecific antibodies that have emerged as an alternative to combination therapy. This review discusses the various dual targeting strategies for which bispecific antibodies have been developed and provides an overview of the established bispecific antibody formats.
Introduction
With more than 20 monoclonal antibodies (mAbs) approved for therapy, and many more in clinical development, this class of molecules has become an established treatment modality for a variety of diseases.Citation1,Citation2 Antibody engineering is routinely applied to adapt the composition and activity for therapeutic applications in humans, e.g., to reduce immunogenicity or to increase or abrogate ADCC through the modification of Fc-mediated effector functions.Citation3,Citation4 Unmodified mAbs possess a defined specificity for a single epitope of an antigen, and thus can interact with only a singular target. However, complex diseases such as cancer or inflammatory disorders are usually multifactorial in nature, involving a redundancy of disease-mediating ligands and receptors, as well as crosstalk between signal cascades. For example, several proinflammatory cytokines such as TNF, IL-1 and IL-6 have been identified as key players in inflammatory diseases.Citation5 In cancer, tumor cells often upregulate different growth-promoting receptors that can act either independently or crosstalk intracellulary through signaling networks.Citation6,Citation7 Of note, an acquisition of resistance to therapy is often associated with upregulation of alternative receptors as well as pathway switching between two receptors.Citation8,Citation9 Consequently, therapy with mAbs that target only a singular antigen has limitations.
Blockade of multiple targets or multiple sites on one target should result in improved therapeutic efficacy. This can be achieved by combination therapy with mAbsCitation10 but also other therapeutic compounds. Improved efficacy in cancer therapy has been demonstrated with combinations of mAbs targeting different receptor tyrosine kinases on cancer cells or growth factors involved in angiogenesis, or a combination of both. Furthermore, combinations of mAbs targeting two different epitopes on a single target have shown promising results. In these studies, different mAbs, with a focus on approved antibodies such as cetuximab (Erbitux®), trastuzumab (Herceptin®) and bevacizumab (Avastin®), were combined to treat solid tumors, including metastatic pancreatic cancer and breast cancer known to be dependent on expression of tyrosine kinase receptors EGFR and HER2, as well as angiogenesis induced by VEGF. However, combination therapy requires the development and approval of the individual antibodies, which involves substantial investment of resources for manufacturing, clinical studies and regulatory review. New approaches for combination therapy, therefore, include use of oligoreactive (polyclonal) antibody mixtures for the treatment of complex diseases.Citation11,Citation12 For example, Sym004, a mixture of two anti-EGFR antibodies, has shown promising results in preclinical studies,Citation13,Citation14 and is currently undergoing evaluation in a Phase 2 study (NCT01417936) in patients with squamous cell cancer of the head and neck who responded to previous anti-EGFR mAb-based therapy and subsequently became resistant to that therapy.
During the past decade, dual targeting with bispecific antibodies has emerged as an alternative to combination therapy or use of mixtures. The concept of dual targeting with bispecific antibodies is based on the targeting of multiple disease-modifying molecules with one drug. From a technological and regulatory perspective, this makes development less complex because manufacturing, preclinical and clinical testing is reduced to a single, bispecific molecule. Therapy with a single dual-targeting drug rather than combinations should also be less complicated for patients.
Dual Targeting Strategies
Dual targeting strategies using bispecific antibodies can be divided into two types: (i) those that directly act on target structures, e.g., cell surface receptors or soluble factors () and (ii) those that use dual targeting for delivery (retargeting) of a therapeutically active moiety, e.g., effector molecules and effector cells (). Direct actions include binding and neutralization of two ligands or two receptors, neutralization of a receptor and a ligand, activation of two receptors, activation of one receptor and neutralization of another receptor or a soluble factor, but also neutralization by binding to different epitopes of one receptor or ligand (). Indirect actions include ADCC and CDC mediated by an Fc region, retargeting of immune effector cells through a further binding site, targeting of an effector molecule, e.g., a toxin, a cytokine or a prodrug-converting enzyme and targeting of drug-loaded nanoparticles (). Direct and indirect actions can be combined within one molecule to further improve efficacy.
Applications of dual targeting strategies are likewise manifold, with the main indications being cancer therapy and the treatment of inflammatory and infectious diseases ( and ). Here, the same mechanisms used for combination therapy of antibodies can be targeted with bispecific antibodies. Multiple diseases mediators and signaling pathways thus can be addressed and simultaneously inhibited by the dual targeting antibody.Citation15 This includes targets that act independently on different pathways, but also targets that are capable of cross-talking. Of further interest are bispecific antibodies targeting different epitopes on a disease mediator, which can lead to increased binding and enhanced neutralization.
Bispecific Antibody Formats
Bispecific antibodies with defined dual specificity suitable for therapeutic use must be generated through biochemical or genetic means ().Citation16,Citation17 Bispecific IgG molecules can be produced by somatic hybridization of two antibody-secreting hybridoma cells. These hybrid hybridomas (quadromas) produce within a cell two different heavy and light chains. Random assembly results in a mixture of IgG molecules, some of which are bispecific. These bispecific IgGs must be purified by two-step affinity chromatography. However, the isotypes of heavy and light chains depend on the origin of the parental antibodies, which allows little flexibility. Interestingly, however, the first bispecific antibody (catumaxomab) approved in the European Union for the treatment of malignant ascites is produced from a mouse/rat quadroma cell line,Citation18 demonstrating the feasibility of this approach to generate therapeutic bispecific antibodies. Alternatively, bispecific antibodies can be generated from existing antibodies by chemical conjugation, e.g., of two IgG molecules or two Fab' fragments, using homo- or hetero-bifunctional coupling reagents.Citation19 A different chemical coupling approach is used to produce CovX-Bodies, which comprise a catalytic IgG molecule covalently coupled to reactive, bispecific peptides.Citation20
Bispecific antibodies can be also produced by genetic engineering and more than 45 different formats have been established in the past two decades ().Citation16 A clear advantage of this approach is the greater flexibility regarding the origin of the binding site (e.g., implementing humanized or human antibodies), the composition (e.g., size, valency, isotype, presence or absence of an Fc region) and production (e.g., applying prokaryotic or eukaryotic expression systems).
A large group of recombinant bispecific antibodies are IgG-like molecules. In most of these formats, binding sites of a second specificity are fused to the N- or C-terminus of the heavy or light chain, e.g., in the form of an scFv fragment or a variable single domain, resulting in bispecific, tetravalent molecules. Bispecific molecules generated through fusion of an scFv fragment to a mAb offer great flexibility (). ScFv molecules have been fused to the N-terminus but also the C-terminus of the of the heavy or light chain of a mAb,Citation21–Citation23 generally without compromising productivity or antigen-binding activity, although issues regarding stability have recently been addressed.Citation24 This group of IgG-like bispecific molecules also includes DVD-Igs, where a second VH and VL domain is fused to the heavy and light chain, respectively, of a mAb,Citation25 two-in-one antibodies, where a second specificity is introduced into the natural binding site of an IgG molecule,Citation26 and mAb2 molecules, where a second specificity is build into the CH3 domain of the Fc region.Citation27 A characteristic feature of all these molecules is a symmetry caused by dimeric assembly of two identical heavy chains, an intrinsic property of these chains. A different approach is the generation of asymmetric IgG molecules. This can be achieved with the knobs-into-holes strategy.Citation28 Here, amino acids at the contact site between the CH3 domains are substituted by larger or smaller residues forcing a heterodimeric assembly of heavy chains. One drawback is, however, that there is still random association with the light chains. This has been addressed by generating bispecific molecules with common light chains,Citation29 or, more recently, by domain swapping between one heavy and light chain resulting in CrossMabs.Citation30 Heavy chain heterodimerization was also achieved by engineering a charged CH3 interface to introduce an electrostatic steering effect or using the strand-exchange engineered domain technology (SEEDbody) with CH3 sequences composed of alternating segments from human IgA and IgG.Citation31,Citation32 In contrast to the bispecific IgG-like molecules, these bispecific antibodies are bivalent with a size basically identical to that of IgG. Fc heterodimerization was recently applied to generate a trivalent, bispecific molecule fusing a VH and a VL domain to the C-termini of the engineered heavy chains (HA-TF Fc variant).Citation33
Bispecific antibodies with a molecular mass in the range of 50–100 kDa can be generated by combining the variable domains of two antibodies.Citation16,Citation34 For example, two scFv have been connected by a more or less flexible peptide linker in a tandem orientation (tandem scFv, taFv, tascFv), which can be extended further by additional scFv, e.g., generating bispecific or trispecific triple bodies (sctb).Citation35 Diabodies are heterodimeric molecules composed of the variable domains of two antibodies arranged either in the order VHA-VLB and VHB-VLA (VH-VL orientation) or in the order VLA-VHB and VLB-VHA (VL-VH orientation). The linker connecting the two domains within one chain is approximately 5 residues leading, after co-expression of the two chains within one cell, to a head-to-tail assembly and hence formation of a compact molecule with two functional binding sites.Citation36 The diabody (Db) format was further stabilized by introducing interchain disulfide bonds (dsDb, DART molecules) or by generating a single-chain derivative (scDb).Citation37–Citation39 ScDbs can be converted into tetravalent molecules by reducing the middle linker, resulting in homodimerzation of two chains.Citation40 Small bispecific molecules have also been produced by fusing a scFv to the heavy or light chain of a Fab fragment.Citation41 Furthermore, tandem scFv, diabodies and scDb have been fused to the Fc or a CH3 domain to generate tetravalent derivatives. Also, scFv can be combined with Fc or CH3 domains to generate tetravalent molecules, e.g., fusing scFvs to the N- and C-terminus of an Fc fragment, or using the knobs-into-holes approach to generate bivalent scFv-Fc or scFv-CH3 molecules. A different approach for the generation of bispecific antibodies is realized by the dock-and-lock method (DNL). Here, antibody fragments are fused to a homodimerizing docking domain (DDD) from human cAMP-dependent protein kinase A (PKA) and the anchoring domain (AD) from A-kinase anchor protein (AKAP) leading to the formation of bispecific, trivalent molecules.Citation42
Many of the established bispecific antibody formats can also be combined with additional proteins and components, e.g., drugs, toxins, enzymes and cytokines, enabling dual targeting and delivery of a fusion partner. In addition, fusion to plasma proteins such as serum albumin or albumin-binding moieties can be applied to extend the plasma half-life of bispecific antibodies.Citation43,Citation44
Dual Targeting of Two Receptors in Cancer Therapy
Tumor development and progression often depend on growth signals mediated by receptors, which are consequently upregulated or amplified in many tumor cells. Examples include members of the EGF receptor family, i.e., EGFR, HER2, HER3 and HER4,Citation45 and the IGF-1 receptor (IGF-1R),Citation46 which play essential roles in regulating cell proliferation, survival, differentiation and migration. These receptors, with some exceptions, e.g., HER3 which is by its own signaling incompetent, act through multiple downstream pathways including the Ras/Raf/ERK/MAPK and the PI3K/AKT signal pathways. An acquisition of resistance to antibody therapy against a singular receptor is often associated with pathway switching between two receptors, i.e., through a compensatory upregulation and activation of the reciprocal receptor as shown for EGFR and IGF-1R, thus maintaining the malignant phenotype and leading to a relapse of the disease.Citation7–Citation9 Furthermore, co-expression and crosstalk of different growth-promoting receptors such as EGFR and IGF-1R have been found for many tumors.Citation47,Citation48 Consequently, it was postulated that targeting two different receptors on a tumor cell should increase the anti-proliferative effect and help to avoid the development of resistance.
Several bispecific molecules targeting EGFR and IGF-1R have been developed, including bispecific diabodies, IgG-like tetravalent Di-diabodies, IgG-scFv fusion proteins and bispecific Adnectins™ ().Citation9,Citation49–Citation52 Bispecific diabodies targeting EGFR and IGF-1R were generated from the anti-EGFR antibody 11F8 and the anti-IGF-1R antibody A12 and retained binding activity for the respective receptors. However, it was found that the affinity was influenced by the domain orientation and arrangement, with the VL-VH orientation being superior over the VH-VL orientation.Citation50 This diabody was further converted into an IgG-like bispecific and tetravalent molecule by fusion of one of the chains to a human IgG Fc region, including the hinge region.Citation51 This Di-diabody was produced in NS0 cells and the purified protein was shown to be capable of binding both antigens simultaneously. Inhibition of tumor cell proliferation was demonstrated in vitro, although the Di-diabody exhibited an approximately 25-fold lower inhibitory activity than the parental antibodies alone or in combination. Furthermore, the Di-diabody blocked signaling pathways stimulated by EGF and IGF-1, while the parental mAb showed inhibitory activity only for the respective pathway (Akt pathway for IGF-1R and MAPK p44/p42 for EGF). Importantly, the Di-diabody also mediated ADCC toward cells expressing either EGFR or IGF-1R, or both receptors, while the parental antibodies were only active toward tumor cells expressing their target antigen. Finally, antitumor activity was demonstrated in two xenograft mouse tumor models. In the HT29 tumor model, the bispecific tetravalent antibody was superior to treatment with the individual parental mAbs, with an activity similar to that of the combination of the two parental mAbs. The same group further developed tetravalent bispecific IgG-like molecules (scFv4-Ig) by fusing an anti-IGF-1R scFv to the constant VL domain and an anti-EGFR scFv to the first CH1 domain of the IgG heavy chain, or vice versa.Citation49 With these constructs, similar results as for the Di-diabody were observed in vitro. The results also lead to the assumption that therapeutic response of targeting two different receptors may depend on the expression levels of the receptors and activation status of each receptor and its downstream signaling molecules.
Another IgG-like anti-EGFR x anti-IGF-1R bispecific antibody was generated by genetic fusion of a stability-engineered anti-IGF-1R scFv to the C-terminus of a chimeric aglycosylated IgG4.P/IgG1 antibody derived from an affinity-matured variant of an EGFRvIII-specific antibody isolated from a semi-synthetic phage library.Citation9 This antibody (EI-04) demonstrated simultaneous binding of both antigens with similar affinity as the parental antibodies and concurrent blockade of ligand binding and receptor activation with IC50 values in the low nanomolar range. Interestingly, in a head and neck squamous cell carcinoma cell line, the bispecific antibody efficiently reduced EGFR phosphorylation, while the parental anti-EGFR antibody showed little effect. This finding indicates that the bispecific antibody is capable of inhibiting receptor pathway crosstalk in this cell line. The bispecific antibody also inhibited proliferation to a similar extent as the combination of the two monospecific antibodies. This was confirmed with a panel of tumor cell lines establishing expanded growth inhibition compared with the parental antibodies. Potent antitumor activities of EI-04 were demonstrated in tumor xenograft models. Here, the bispecific antibody administered at all dose levels was statistically more efficacious than the two parental antibodies alone using equimolar dosages. For one tumor cell line (BxPC3), EI-04 was also statistically more potent than the combination of the two parental antibodies, while with a second cell line (GEO) a similar efficacy was observed, further highlighting the complexity of target receptor biology.Citation9
Dual targeting of EGFR and IGF-1R was further investigated for bispecific Adnectins.Citation52 Adnectins™ represent an antibody-mimetic alternative scaffold derived from a human fibronectin domain. The bispecific Adnectins™ were generated by connecting two Adnectins™ with a flexible linker composed of ten glycine-serine repeats. Affinities of these tandem molecules were similar to those of the monospecific Adnectins™. To increase plasma half-life, the tandem Adnectins™ were coupled to a 40 kDa branched PEG chain, which resulted in a 10- to 20-fold reduction in binding, although inhibition of EGFR and IGF-1R phosphorylation in vitro was only slightly affected. Importantly, compared with the monospecific Adnectins™, the bispecific Adnectins™ were more potent in inhibiting proliferation of lung cancer cell line H292 expressing high levels of EGFR and IGF-1R. A functional benefit of having both domains within one molecule was deduced from in vivo experiments with BxPC3 xenografts. Here, the tandem Adnectin™ showed significantly better tumor growth inhibition compared with the individual monospecific Adnectins™ or a mixture of both molecules.
Dual targeting of HER2- and HER3-expressing tumor cells was described for a bispecific molecule generated by fusing scFvs directed against HER2 and HER3 to the N- and C-terminus of human serum albumin (scFv-HSA-scFv).Citation53 This molecule (MM-111) combines targeting of HER2-overexpressing tumor cells with potent inhibition of ligand-induced phosphorylation of HER3 with IC50 values in the sub-nanomolar range. Computational physicochemical modeling was applied to optimize the monovalent binding affinities to increase potency and specificity for tumor cells. MM-111 is currently undergoing evaluation in three Phase 1 studies in patients with advanced HER2-amplified cancers (NCT00911898, NCT01097460 and NCT01304784).
Simultaneous receptor blockage with bispecific antibodies was also applied for platelet-derived growth factor receptor α (PDGFRα) and β (PDGFRβ).Citation54 These receptors are activated by members of the PDGF family and are capable of forming homo- as well as heterodimeric receptor complexes. PDGFRs have been identified on a number of tumor types and are involved in stimulation of tumor cells, but also angiogenesis.Citation55,Citation56 Bispecific IgG-like antibodies against the two PDGF receptors were generated by fusion of an anti-mouse PDGFRα single variable domain (sVD) either to the N-terminus of the light chain (sVD-IgG) or the C-terminus of the heavy chain (IgG-sVD) of an anti-mouse PDGFRβ IgG (see also ). Simultaneous binding of antigens was demonstrated for the bispecific antibodies and both antibodies were capable of blocking binding of the ligands PDFG-AA and PDFG-BB to its receptors, which resulted in inhibition of ligand-mediated receptor phosphorylation. In these assays, the IgG-sVD fusion protein showed better effects than the sVD-IgG fusion protein indicating that the position of adding a second binding site has a direct influence on bioactivity.
Another sVD-IgG construct was generated by fusing the anti-mouse PDGFRα sVD to the N-terminus of the light chain of an anti-mouse VEGFR2 IgG.Citation57 Thus, this bispecific antibody targets another receptor involved in tumor angiogenesis.Citation58 The bispecific antibody was able to recognize both receptors simultaneously and to inhibit PDGF- and VEGF-induced stimulation of murine endothelial cells. Neutralization of stimulating receptors of tumor endothelial cells was also studied with a bispecific diabody directed against VEGFR2 (KDR) and VEGFR3 (Flt-4) or a Di-diabody (diabody-CH3 fusion protein) directed against VEGFR1 (Flt-1) and VEGFR2.Citation59,Citation60 For these constructs, inhibition of ligand binding and VEGF-induced cell migration was described.
Based on results from a Phase 2 study of combination therapy with mAbs for the treatment of B cell lymphoma combining an anti-CD20 antibody (rituximab) with an anti-CD22 antibody (epratuzumab),Citation61 it was postulated that bispecific antibodies should also be applicable for the treatment of hematologic malignancies. A tetravalent IgG-like bispecific antibody generated by fusing an anti-CD22 scFv from epratuzumab to the C-terminus of the heavy chain of an anti-CD20 IgG veltuzumab showed improved inhibition of cell proliferation as well as ADCC against Daudi cells in vitro.Citation62 In vivo, beneficial therapeutic effects of the bispecific antibody were, however, seen only at the highest dose. Of interest was the finding that the bispecific antibody induces translocation and accumulation of B cell receptors (BCR) in lipid rafts, presumably due to a stronger interaction of CD22 with the BCR, which was thought to lead to increased growth inhibition and apoptosis. This approach was extended by the same group using a hexavalent IgG-Fab molecule produced by the dock-and-lock method.Citation63 Potent inhibition of cell proliferation was also observed for this bispecific antibody. Again, antitumor activity was similar to that observed for a combination of the parental antibody.Citation63,Citation64 In another study, a CD20 × CD22-specific bispecific F(ab')2 molecule generated by chemical conjugation showed significantly improved antitumor effects compared with treatment of the parental antibodies rituximab (CD20) and HB22.7 (CD22) alone or in combination.Citation65 All the studies established that the bispecific antibodies induce a stronger p38 phosphorylation, which might be responsible for the increased cytotoxic effects observed in vitro. Furthermore, it was discussed that the bispecific antibody prevents rapid internalization of CD22 through binding to non-internalizing CD20, thus affecting downstream signaling.
The studies with anti-CD20 and anti-CD22 bispecific antibodies demonstrated that targeting of relevant cell surface receptors can lead to an efficient activation of signaling pathways and increased anti-proliferative and cytotoxic effects without utilizing ADCC or CDC. Induction of cell death through apoptosis is also known for members of the death receptor family, e.g., TRAIL receptors, which has led to the development of agonistic antibodies for cancer therapy.Citation66 The applicability of bispecific antibodies as agonists of death receptors was investigated with IgG-scFv fusion proteins targeting TRAIL receptor 2 (TRAIL-R2, DR5) and lymphotoxin-β receptor (LTβR), which are co-expressed on the surface of a variety of cell lines from tumors of epithelial origin.Citation67 Bispecific IgG-scFvs were produced by fusing a stability-improved disulfide-linked anti-LTβR scFv either to the N- or C-terminus of the anti-TRAIL-R2 IgG heavy chain. An enhanced antitumor activity was observed for a subset of tumor cell lines in vitro relative to the combination of parental antibodies. In vivo, superior tumor growth inhibition was observed for MDA-MB-231 xenografts and a bispecific antibody with the scFv fused to the heavy chain C-terminus, while for another cell line (WiDr) effects were similar to that of the combination therapy, in accordance with the observed in vitro activity.
Dual Targeting of Two Receptors for the Treatment of Inflammatory Diseases
Self-perpetuating B lymphocytes have been identified as contributors to the development and progression of inflammatory and autoimmune rheumatic diseases.Citation68,Citation69 Therefore, B cell depletion or inactivation represents a viable therapeutic approach, as shown by the use of rituximab in the treatment of rheumatoid arthritis.Citation70 B cell activation is induced by antigen binding to the B cell receptor (BCR) and regulated by a negative feedback loop mediated by the inhibitory Fcγ receptor IIb (FcγRIIb; CD32B) induced by a close contact between the two receptors upon binding of antigen to the BCR and soluble immunoglobulins.Citation71 To recapitulate this antigen-driven proximity of the activating and inhibitory receptors, a bispecific DART molecule directed against CD79b, which is a signaling-competent molecule of the BCR and CD32B was generated.Citation72 Simultaneous binding to both receptors resulted in inhibition of B cell proliferation and secretion of immunoglobulins and reduced disease severity of collagen-induced arthritis in mice, indicating that bispecific antibodies as activation-dependent inhibitors are useful for the treatment of autoimmunity. A similar approach was applied for the treatment of allergic diseases. Crosslinking of the inhibitory CD32B receptor to the FcεRI delivers a dominant-negative signal that efficiently suppresses all activating signals of the FcεRI receptor expressed by mast cells and basophils. This was shown previously with a dimeric dual targeting Fcγ-Fcε bifunctional fusion protein.Citation73 A bispecific IgG recognizing FcεRI and CD32B was generated using a knobs-into-holes approach. This antibody inhibited the activation of mast cells in vitro and in a passive cutaneous anaphylaxis (PCA) model.Citation74 Furthermore, coaggregation of FcεRI and CD32B and inhibition of histamine release was also achieved using a bispecific F(ab')2 molecule directed against IgE and FcγRII.Citation75
Dual Targeting of Two Receptors for the Treatment of Infectious Diseases
Many pathogens interact through specific adhesion molecules with receptors on host cell or use surface-exposed enzymes to facilitate infection. Interference with these molecules can, therefore, be employed to inhibit infectivity and pathogen spreading, e.g., by passive immunization with pathogen-reactive antibodies or serum preparations.Citation76,Citation77 Treatment with these reagents is especially appropriate in patients with an impaired immune system or who have become refractory to treatment with anti-microbial drugs. Here, dual targeting can improve an anti-microbial response.
Candidiasis, a fungal infection caused by the opportunistic, mucosal pathogen Candida albicans, can develop into a life-threading disease in severely immuno-compromised individuals.Citation78 Mannoprotein MP65 and the secreted protease Sap2 represent important virulent factors. A bispecific antibody against these two targets was generated by fusing two human domain antibodies (dAb) in tandem, and it was shown to inhibit adherence to vaginal epithelium and to protect against experimental vaginal candidiasis.Citation79 Interestingly, an accelerated fungal clearance from vagina was observed for the bispecific dAb molecule compared with the monospecific molecules.
Dual Targeting of Different Epitopes of One Receptor or Ligand
MAbs against cell surface receptors can exert their inhibitory potential through different mechanisms, including competitive and allosteric blockage of ligand binding. For IGF-1R, which can be activated by two ligands, IGF-1 and IGF-2, it was shown that antibodies recognizing different epitopes of the receptor exhibit an increased neutralizing potential in vitro and in vivo.Citation80 Two of these antibodies, one being a competitive and the other being an allosteric inhibitor of ligand binding, were combined into tetravalent, bispecific IgG-like molecules by fusing scFv fragments either to the N- or C-terminus of the IgG heavy chain.Citation23 A comparison of various molecules revealed that only one of these antibodies, BIIB4-5scFv, was capable of binding with all of its arms to IGF-1R and showed in vitro an inhibitory activity comparable to that of the combination of the two mAbs. Using established xenograft models of SJSA-1 tumors, an increased antitumor activity was observed for BIIB4-5scFv compared with the single mAbs or the bispecific antibody 5scFv-BIIB4 that had the scFv fused to the N-terminus of the heavy chain. However, using other xenograft models, the superior activity of BIIB4-5scFv could not be repeated or was not statistically significant. One reason for this finding might be that these tumor cells depend on multiple growth and survival signals and that inhibition of IGF-1R signaling is not sufficient to suppress tumor growth.
Dual targeting of different epitopes of one receptor was also applied for the treatment of HIV infections using stability-improved IgG-scFv fusion proteins fusing scFv either to the N- or C-terminus of the heavy or light chain, respectively.Citation24 These bispecific antibodies block two alternative docking sites of the CCR5 co-receptor of HIV. To improve the stability of the fusion proteins, the scFv fragment was further stabilized by introducing a disulfide-bond between the VH and VL domain or by increasing the linker length connecting the VH and VL domain from 15 residues to 30 residues, which drastically reduced the aggregation tendency. While binding to CCR5-expressing CHO cells was increased only 1.3- to 1.7-fold, an 18- to 57-fold increased antiviral activity was observed for the different variants compared with the parental antibodies. Importantly, the bispecific antibodies were also capable of inhibiting virus strains resistant to the treatment with the single parental antibodies. These findings demonstrate that dual targeting with bispecific antibodies can improve antiviral activity and can even overcome resistance to treatment with monospecific mAbs.
Application of the emerging class of scaffold proteins for dual targeting was shown for a bispecific designed ankyrin repeat protein (DARPin) targeting two epitopes of the high-affinity Fcε receptor (FcεRI).Citation81 Aggregation of this receptor by allergen-induced cross-linking of bound IgE induces degranulation of basophils and mast cells and can induce allergy symptoms by the release of proinflammatory molecules. Treatment options include anti-IgE antibodies, which prevent binding of IgE to FcεRI.Citation82 Omalizumab, a humanized antibody directed against the Fc part of IgE is approved for the therapy of severe asthma.Citation83 Therapy should also be possible with antibodies directed against the FcεRI; however, it is essential that binding does not induce cross-linking and activation of the receptor. Eggel and coworkersCitation81 addressed this problem by generating a bispecific DARPin targeting the two IgE-binding sites of FcεRI located in the extracellular FcεRIα chain. Bispecific DARPins were generated connecting two DARPins by a flexible peptide linker. One of these molecules, DARPin 30/85, showed a strongly increased affinity compared with the parental DARPins and simultaneously blocked both epitopes of the receptor without forming receptor aggregates. However, it was shown that anti-DARPin antibodies can induce cross-linking and degranulation, which might limit therapeutic applications.
Dual targeting can also be applied to efficiently neutralize toxins, e.g., from bacteria, fungi, plants or animals. Feasibility was demonstrated, for example, with a bispecific heavy chain antibody generated by fusing a VHH domain to the C-terminus of a llama heavy chain antibody.Citation84 This tetravalent, bispecific HCAb targets two subunits (LucS-PV and LucF-PV) of Staphylococcus leukotoxin Panton-Valentine leukocidin (PVL), which is associated with human pyogenic necrotizing skin infections and more severe septic infections. The antibody bound both antigens simultaneously and inhibited formation of new pores by preventing binding of the toxin components to membranes of peripheral mononuclear cells, monocytes and lymphocytes. However, the antibody had no effect on already formed pores. Effectiveness was further demonstrated in vivo in a non-infectious PVL-induced rabbit endophthalmitis model. Compared with equimolar amounts of the bivalent, monospecific HCAbs, inhibition of inflammatory reactions and tissue destruction was more efficient with the tetravalent, bispecific molecule.
In another study, a bispecific Nanobody was developed for the treatment of Androctonus australis hector (Aah) scorpion envenoming.Citation85 The venom contains three small toxins with a molecular mass of 7 kDa that rapidly distribute in the blood and tissues. Currently, intoxicated people are treated with a polyclonal equine F(ab')2-based antivenom. However, these fragments reach the tissue much slower than the toxin, which necessitates high doses applied intravenously.Citation86 The bispecific Nanobody NbF12–10 directed against AahI' and AaHII, possessing a size of only 29 kDa, was highly potent in protecting mice from lethal doses of the scorpion venom when administered subcutaneously, in contrast to treatment with the plasma antivenom serum-derived F(ab')2 which was ineffective under these conditions.
Dual Targeting of Two Ligands in Cancer Therapy
The growth of solid tumors depends on neovascularization promoted by vascular growth factors.Citation87 These angiogenic factors induce endothelial cell proliferation and migration, extracellular matrix remodeling, increased vascular permeability and survival of the newly formed blood vessels.Citation88 Besides VEGF-A, several other proteins with angiogenic activity have been identified, including angiopoietin-2 (Ang-2) and osteopontin. Neutralization of these factors with mAbs interferes with the formation of novel blood vessels, as shown for bevacizumab, an anti-VEGF antibody approved for the treatment of metastatic colorectal cancer and various other solid tumors. Simultaneous neutralization of different angiogenic molecules should further improve the anti-angiogenic activity. This was demonstrated for bispecific DVD-Igs generated by fusing either the variable domains of an anti-osteopontin antibody (hu1A12) to the N-terminus of the heavy and light chains of bevacizumab (VEGF/OPN-BsAb) or the other way round (OPN/VEGF-BsAb).Citation89 Both antibodies showed similar binding behavior as the parental antibodies and VEGF/OPN-BsAb was chosen for further analysis. The bispecific antibody efficiently inhibited growth of endothelial cells in vitro, reduced strongly the micro-vessel density (MVD) in a hepatocellular carcinoma model (HCCLM3) and potently suppressed the growth of primary tumors and the formation of spontaneous lung metastases, suggesting that this approach has potential in treating metastatic cancers. In all these experiments, the activity was increased compared with treatment with the bevacizumab and hu1A12 alone, but similar to treatment with a combination of both parental antibodies.
In another study, the CrossMab format was applied to generate bivalent, bispecific IgG molecules directed against VEGF-A and Ang-2.Citation30 One of these antibodies, CrossMabCH1-CL, showed favorable stability properties and was capable of simultaneously binding to both antigens with comparable affinities as the parental antibodies bevacizumab and LC06. Inhibition of Colo205 tumors by the CrossMab was similar to treatment with a combination of bevacizumab and LC06 and more effective that single antibody treatment. Furthermore, similar results were observed for inhibition of VEGF-induced corneal angiogenesis, emphasizing the versatility of dual targeting strategies.
VEGF and Ang-2 were also targeted with a bispecific CovX-Body.Citation20 These molecules are produced by chemical coupling of a peptide to a heavy chain lysine of an aldolase catalytic IgG.Citation90 Bispecific CovX-Bodies are generated using branched peptides directed against two different targets. The VEGF- and Ang-2-specific bispecific CovX-Body CVX-241 was able to bind simultaneously to both ligands and inhibit binding of the ligands to their respective receptors with subnanomolar IC50 values. In xenograft tumor models, a significant reduction of tumor growth was observed with CVX-241, which was superior to the monospecific CovX-Bodies and comparable with the combination of both parental CovX-Bodies. These findings established that peptides coupled to IgG exhibit antibody-like properties such as a long half-life and are therapeutically effective.
Dual Targeting of Two Ligands in the Treatment of Inflammatory and Autoimmune Diseases
Multiple disease modulators play an essential role in the pathogenesis of inflammatory and autoimmune diseases having either a redundant activity, i.e., acting on the same signaling cascade, or acting on two or more independent pathways. Simultaneous inhibition of different disease modulators should therefore be beneficial for therapy, although studies from combination therapies, e.g., with etanercept (Enbrel®) and abatacept (Orencia®), did not reveal improved efficacy but an increase in infectious complications,Citation91 underlining the adage that targets have to be carefully selected.
Dual targeting of disease-modulating cytokines was evaluated with various bispecific antibodies. A tetravalent, bispecific DVD-Ig that simultaneously bound and neutralized IL-12 and IL-18 was generated.Citation25 This antibody bound to the two cytokines with similar affinities as the parental antibodies and efficiently inhibited IL-12 and IL-18-induced IFNγ release in vitro. Therapeutic efficacy was demonstrated for Staphylococcus aureus dried cell (SAC)-induced IFNγ production in SCID mice. Here, the bispecific antibody almost complete abrogated IFNγ production and was as efficient as a combination of the two parental antibody and more potent than the mAbs alone. In the same study, a bispecific DVD-Ig directed against mouse IL-1α and IL-1β was generated. This bispecific antibody inhibited both pro-inflammatory cytokines with IC50 in the low nanomolar range. However, compared with the parental antibodies, the neutralizing activity was 10-fold reduced for IL-1α and 2-fold reduced for IL-1β. Nevertheless, in a collagen-induced arthritis (CIA) model, the bispecific antibody strongly inhibited disease progression, similar to a combination of the parental antibodies, as well as potency-matching antibodies. It was further found that the orientation and the linker lengths influenced stability, aggregation tendency, as well as binding and affinity. Thus, a DVD-Ig directed against human IL-1α and IL-1β was generated and further optimized by varying the domain orientation and the length of the linkers connecting the variable domains.Citation92
Dual targeting and neutralization was further demonstrated for bispecific antibodies directed against IL-17A and IL-23.Citation93 IL-23 is a cytokine that stimulates the differentiation and regulation of Th17 helper T cells, which in turn produce several pro-inflammatory cytokines such as IL-17.Citation94 Th17 cells are thus dominating cell types associated with autoimmune disorders, e.g., rheumatoid arthritis, with IL-23 and IL-17 being important disease-promoting factors.Citation95 Stable scFv molecules directed against IL-17A and IL-23 were selected from a phage library and employed for the generation of various tetravalent, bispecific antibodies: a tandem scFv-Fc fusion protein (tascFv-Fc), a scFv-Fc-scFv fusion protein and an IgG-scFv with the scFv fused to the C-terminus of the heavy chain.Citation91 An increased thermal stability was determined for the IgG-scFv fusion protein compared with the two other formats. All bispecific variants exhibited 6- to 8-fold decreased affinity for the two cytokines, but were capable of binding both cytokines simultaneously. Compared with soluble IL-23 receptor, an up to 10-fold increased neutralization activity was observed in a murine splenocyte assay. Pharmacokinetic studies demonstrated that the tascFv-Fc and IgG-scFv fusion proteins had superior serum half-lives.
Dual Targeting of a Receptor and a Ligand in Cancer Therapy
Signal transduction is induced by binding of a ligand to a receptor. Inhibition of growth and differentiation-promoting signals can be achieved by inhibiting either the receptor or the ligand, as illustrated by the various examples discussed. This offers also an approach for dual targeting by simultaneously inhibiting a receptor and a ligand for the same or another receptor. Feasibility was shown for dual inhibition of HER2 and VEGF with a combination of trastuzumab and VEGF-trap, thus, combining targeting of tumor cells with an anti-angiogenic approach.Citation96 This combination is of special interest because HER2-overexpressing tumor cells have been shown to secret elevated levels of VEGF.Citation97 A bispecific antibody against HER2 and VEGF was recently generated applying the “two-in-one” antibody strategy.Citation26 In this study, a second specificity for VEGF was grafted into the binding site of the anti-HER2 trastuzumab. This reduced the affinities for both antigens. Affinity improved variants were therefore selected from phage libraries. One of these variants, bH1–44, inhibited the growth of HUVECs and BT474 tumor cells in vitro to a similar extend as bevacizumab or trastuzumab, respectively, and also showed potent anti-tumor activity in two xenograft tumor models, which was similar or even better than a combination of bevacizumab and trastuzumab.
Dual targeting of a receptor and a ligand can also be applied for inhibition of different signals involved in angiogenesis. For example, beneficial effects of co-inhibition of PDFG- and VEGF-mediated signaling were shown for a combination therapy with receptor-specific small molecule tyrosine kinase inhibitors or with mAbs directed against PDFGRβ and VEGFR.Citation98,Citation99 In another study, a bispecific antibody was applied to simultaneously inhibit PDGFRβ and VEGF-A.Citation100 The antibody was generated by fusing different scFvs to the N-terminus and C-terminus of an Fc fragment (scFv-Fc-scFv), resulting in a tetravalent, bispecific molecule with the two chains covalently linked by disulfide bonds of the hinge region. This bispecific antibody inhibited proliferation of HUVECs, as well as human brain vascular pericytes (HBVPs), with IC50 values in the picomolar range. In a co-culture sprouting assay mimicking the in vivo generation of blood vessels from endothelial cells and mesenchymal stem cells (MSC), the bispecific antibody inhibited endothelial sprouting and pericyte dissociation from endothelial cells. Although not cross-reactive with mouse VEGF and PDGFRβ, inhibition of tumor growth and reduction of the micro-vessel density (MVD) were observed in a xenograft mouse model, similar to treatment with bevacizumab.
Dual Retargeting of Effector Cells
Bispecific antibodies have been widely used for the retargeting of immune effector cells to tumor cells.Citation101 In this case, one binding site is directed against a tumor-associated antigen and the second antigen against a trigger molecule on the effector cells. Thus, bispecific antibodies have been employed for the retargeting of T cells by binding to CD3, which is part of the T cell receptor complex, or of natural killer (NK) cells by binding to the FcγRIII (CD16).Citation16,Citation102 Blinatumomab, a recombinant bispecific tandem scFv molecule (bispecific T cell engager, BiTE) directed against CD3 and CD19 is in clinical trials and has shown promising results in Phase 1 and 2 studies in non-Hodgkin lymphoma and ALL patients.Citation103,Citation104 The tandem scFv format was further improved by adding a third binding site, thereby generating a trispecific triple body (sctb).Citation105 Applicability was shown for a triple body directed against CD16 and the two tumor-associated antigens CD33 and CD123 for dual retargeting of NK cells to myeloid leukemia cells. In this construct, the anti-CD16 scFv was placed in the middle and further stabilized by a disulfide bond. Simultaneous binding to both antigens was demonstrated by flow cytometry with CD33-positive cells incubated with triple body and a CD123-red fluorescent protein (RFP) fusion protein. Improved ADCC was observed with an AML-derived cell line and primary leukemia cells. Using a double-positive cell line, the contribution of both antigens to cell lysis was shown by blocking experiments with scFvs. In another study, a triple body directed against CD16 and the tumor-associated antigens CD33 and CD19 was investigated.Citation106 Strong lysis of double-positive tumor cells was observed in vitro. The beneficial effect of increased avidity was shown in comparison with a bispecific tandem scFv directed against CD16 and either CD33 or CD19. These experiments demonstrate that effector cell retargeting that utilizes dual targeting can result in improvements in efficacy and selectivity.
Dual Retargeting of Toxins
Extensive work has been performed for dual retargeting of toxins. Targeted toxins are generated by coupling or fusion of a toxin, e.g., ricin or others derived from bacteria, fungi or plants, to antibodies or other ligands.Citation107,Citation108 In immunotoxins, the cell-binding moiety of the toxin is replaced by the antibody, thus redirecting the toxin to a tumor cell and leading to target-mediated internalization of the fusion protein. The toxin fragment contains a translocation domain necessary for release of the catalytic domain into the cytosol. Feasibility of this approach is demonstrated with denileukin diftitox (Ontak®), which is approved for the treatment of cutaneous T cell lymphoma.Citation109 Denileukin diftitox is a fusion protein comprising a Diphtheria toxin fragment (DAB389) fused to IL-2 as ligand for IL-2R expressed on activated T cells. Various antibody-toxin fusion proteins have been generated, e.g., by fusion of an scFv or disulfide-stabilized Fv fragment to a toxin, for the treatment of hematologic and solid tumors and several have already entered clinical trials.Citation108 Despite the clinical responses observed, these studies also revealed various challenges of this approach, including nonspecific toxicities, stability and production issues and immunogenicity. The latter has been addressed by use of humanized or human antibody fragments and by generating deimmunized versions of the toxin, e.g., as shown for Pseudomonas exotoxin A.Citation110
Dual targeting strategies have been developed to improve efficacy of immunotoxins ().Citation111 Initial studies applied bispecific IgG molecules conjugated with a toxin. Thus, an immunotoxin was generated using a quadroma-derived bispecific IgG directed against CD4 and CD26 coupled to a ricin holotoxin for the elimination of activated T cells, e.g., to prevent graft-vs.-host disease and autoimmunity.Citation112 The bispecific antibody caused a drastically increased internalization and 2- to 3-fold increased cytotoxicity compared with the parental antibodies. Similar results were described for an IgG-immunotoxin directed against CD4 and CD29 developed for targeting and depletion of restricted T-cell subsets.Citation113
More recently, the concept was evaluated with recombinant bispecific immunotoxins generated either by fusing a tandem scFv to the N-terminus of an exotoxin A fragment or to the C-terminus of a Diphtheria toxin fragment, respectively.Citation114–Citation116 These bacterially produced bispecific immunotoxins were directed against CD19 and CD22 and demonstrated an improved efficacy against mouse xenograft models of B cell malignancies and metastases. Because of their dual specificity, it was discussed that these types of immunotoxins broaden the reactivity against most lymphomas and B cell leukemia, as shown for the Diphtheria immunotoxin.Citation114 Problems arising from an aggregation tendency were addressed by domain swapping within the tandem scFv and use of an aggregation-reducing linker, which also improved therapeutic efficacy in animal models.Citation115 Furthermore, a deimmunized version of exotoxin A was used to generate a bispecific immunotoxin directed against CD19 and CD22, which strongly reduced the production of a neutralizing antitoxin immune response while maintaining its cytotoxic activity. This should allow multiple drug treatments and result in improved therapeutic activity.Citation116
In another study, a bispecific immunotoxin comprising an anti-HER2 scFv fused to a Diphtheria toxin-anti-EpCAM immunotoxin was generated for the treatment of solid tumors overexpressing HER2 and EpCAM.Citation117 Compared with monospecific immunotoxins, increased cytotoxicity toward tumor cells expressing both antigens was observed in vitro and in xenograft tumor models.
As an alternative to the use of antibody fragments, various bispecific ligand-directed toxins (BLT) were generated using natural ligands such as growth factors and cytokines for targeting. For example, dual targeted toxins were generated fusing IL-13 and EGF with Diphtheria toxin.Citation118–Citation122 In other studies, IL-13 was combined with uPA,Citation123–Citation125 IL-4 with EGF,Citation126,Citation127 or EGF with uPA,Citation128 fused either to Diphtheria toxin or Pseudomonas exotoxin A (). Superior activities were seen with this BLTs, e.g., after intratumor injections into subcutaneous xenograft tumors. The interaction of the ligands with receptors on normal cells was reduced by applying a ToxBloc method. This involves a bolus IP injection of recombinant bispecific fusion protein without toxin prior to application of the immunotoxin, which increased the MTD by 15-fold, as shown for an Diphtheria toxin-EGF-IL-13 fusion protein.Citation122 These studies illustrate the great flexibility of this system.
Dual Retargeting of Cytokines
A growing number of immunostimulatory and apoptosis-inducing cytokines are being developed for tumor therapy, including interleukins, interferons, growth factors and TNF family members. Interleukin-2 (IL-2) was approved for the treatment of RCC and metastatic melanoma and TNF is used for the treatment of sarcoma and melanoma by isolated limb perfusion in combination with chemotherapy.Citation129 Furthermore, GM-CSF and its PEGylated derivative are used for the treatment of neutropenia, e.g., during myelosuppressive chemotherapy. Targeted delivery of these cytokines, e.g., by fusion to an antibody fragment, can result in enhanced therapeutic efficacy by increased tumor localization.Citation130 Examples include antibodies and antibody fragments fused to IL-2, IL-10, IL-12, IL-15, IFNα, IFNβ, IFNγ, TNF, TRAIL and FasL. Several of these fusion proteins have already entered clinical Phase 1 studies.Citation131 Dual targeting was applied to an IFNα2b immunocytokine (20-C2-2b) by combining two copies of IFNα2b with a humanized and stabilized F(ab)2 directed against HLA-DR and a humanized anti-CD20 IgG using the dock-and-lock method.Citation132 An increased cytotoxicity against various lymphoma and myeloma cell lines was observed in vitro compared with a monospecific antibody-IFNα fusion protein targeting only CD20 or a mixture of the parental antibodies. Furthermore, the bispecific immunocytokine was more potent in killing tumor cells expressing CD20 and HLA-DR than the monospecific immunocytokine, indicating that binding to both antigens enhances the cytotoxic effect of the cytokine. This study also showed that sensitivity to the cytokine, as well as expression and density of the target antigens, determine responsiveness toward the immunocytokine.
Dual Targeting of Liposomes and Nanoparticles
Nanoparticles are versatile carrier systems for the delivery of drugs.Citation133,Citation134 Use of liposomes attracted early interest, which resulted in the approval of various liposomal drugs, e.g., for cancer therapy and treatment of fungal infections.Citation135 Carrier systems are able to protect the drug from rapid elimination and degradation. Furthermore, long-circulating particles such as PEGylated liposomes, utilize the enhanced permeability and retention (EPR) effect to passively accumulate in the tumor tissue.Citation136 PEGylated liposomal doxorubicin (Doxil®, Caelyx®) is approved for the treatment of ovarian cancer, multiple myeloma and AIDS-related Kaposi's sarcoma.Citation137 Delivery of nanocarriers to tumor cells or other structures can be improved by insertion of ligands into the particle surface, thereby enabling active targeting. For example, sterically-stabilized immunoliposomes are generated by coupling antibody or antibody fragments to PEG chains inserted into the lipid bilayer.Citation138,Citation139 Internalization of drug-loaded nanocarriers has been identified as important prerequisite for efficient drug delivery.Citation140,Citation141 This can be achieved using ligands or antibodies binding to rapidly internalizing receptors. Furthermore, intracellular release of the encapsulated drug from the endosomal compartment has been found to be equally important. Strategies to facilitate endosomal release include the implementation of pH-sensitive mechanisms.Citation142
Preferably, immunoliposomes are generated using antibody fragments devoid of an Fc region to avoid uptake by cells of the reticuloendothelial system.Citation143 A first immunoliposomal formulation of doxorubicin (MM-302) comprising an anti-HER2 scFv for targeting of HER2-positive cancers is currently in a Phase 1 trial to evaluate safety and pharmacokinetic properties in patients with advanced breast cancer (NCT01304797),Citation144 illustrating that even complex therapeutics composed of lipids, antibodies and drugs can be formulated for clinical use.
Nanoparticles should also benefit from targeting different antigens on a cancer cell, thus improving binding, selectivity and drug delivery. Dual targeting of nanocarriers with antibodies was demonstrated for PEGylated liposomes conjugated with monoclonal IgG directed against CD19 and CD20.Citation145 These dual targeting immunoliposomes were generated by inserting equal amounts of anti-CD19 and anti-CD20 IgG coupled to maleimide-PEG-DSPE. In this study, additive effects were observed for binding and internalization of a combination of anti-CD19 and anti-C20 immunoliposomes, similar to effects seen with dual targeting immunoliposomes at equal antibody and liposome concentrations, which translated into a tendency to somewhat lower IC50 values in in vitro cytotoxicity assays with doxorubicin-loaded liposomes.
Dual targeting of nanocarriers can also be achieved using natural ligands and synthetic peptides. This was shown, for example, with nanoparticles conjugated with folate and an anti-EGFR antibody,Citation146 or combining an αv integrin-binding RGD peptide with ligands such as transferrin,Citation147 neurophilin,Citation148 galectin-1,Citation149 or an NGR peptide recognizing CD13.Citation150 Results from one study with paclitaxel-loaded liposomes targeted to tumor cells and the tumor vasculature support the concept that dual targeting of different cell types improves the antitumor activity of drug-loaded nanocarriers.Citation148
Conclusions
During the past decade a plethora of novel bispecific antibody formats have been developed and used for dual targeting strategies. Although a strong focus is on development of treatments of cancer and inflammatory diseases, additional applications include treatment of other disorders such as infectious and allergic diseases. Results from these studies demonstrate that, in general, bispecific antibodies outperform treatment with monospecific antibodies, but are similar to the combination of parental antibodies, although for some indications increased potency was described. The examples clearly show that bispecific antibodies targeting different disease modulators are capable of improving standard therapy. There are still issues to be addressed, e.g., manufacturing, stability and pharmacokinetic properties of the bispecific antibodies. Nevertheless, the growing interest in this field and the potential of bispecific antibodies for dual targeting strategies suggest that these molecules will enter clinical study in increasing numbers in the near future.
Abbreviations
| ADCC | = | antibody-dependent cell- mediated cytotoxicity |
| dAb | = | domain antibody |
| Db | = | diabody |
| DT | = | diphtheria toxin |
| DVD-Ig | = | dual-variable-domain immunoglobulin |
| EGF | = | epidermal growth factor |
| EGFR | = | epidermal growth factor receptor |
| EpCAM | = | epithelial cell adhesion molecule |
| ETA | = | pseudomonas exotoxin A |
| GvHD | = | graft-versus-host disease |
| HER2 | = | human epidermal growth factor receptor 2 |
| HER3 | = | human epidermal growth factor receptor 3 |
| HSA | = | human serum albumin |
| IGF-1R | = | insulin-like growth factor 1 receptor |
| IL | = | interleukin |
| PDGF | = | platelet-derived growth factor |
| PDGFR | = | platelet-derived growth factor receptor |
| scDb | = | single-chain diabody |
| scFv | = | single-chain fragment variable |
| sctb | = | single-chain triple body |
| sVD | = | single variable domain |
| taFv | = | tandem scFv |
| TRAIL | = | TNF-related apoptosis-inducing ligand |
| TRAIL-R2 | = | TRAIL receptor 2 |
| uPA | = | urokinase-type plasminogen activator |
| uPAR | = | urokinase-type plasminogen activator receptor |
| VEGF | = | vascular endothelial growth factor |
| VEGFR2 | = | VEGF receptor 2 |
Figures and Tables
Figure 1 (A–H) Dual targeting strategies utilizing bispecific antibodies: (A) neutralization of two receptor-activating ligands, (B) neutralization of two receptors, (C) neutralization of a receptor and a ligand, (D) activation of two receptors, (E) activation of a receptor and inactivation of another receptor, (F) activation of a receptor and inactivation of a ligand, (G) blockage of two epitopes of one receptor, (H) blockage of two epitopes of one ligand. (I–O) Dual retargeting strategies utilizing bispecific antibodies: (I) binding to two receptors and Fc-mediated ADCC or CDC, (K) retargeting of cytotoxic effector cells with a trispecific antibody, (L) targeting of a bispecific toxin (immunotoxin) or a bispecific antibody-drug conjugate (ADC) to two receptors, (M) targeting of a bispecific cytokine (immunocytokine) to two receptors, (N) targeting of an enzyme to two receptors, (O) targeting of a drug-loaded nanoparticle/liposome to two receptors. Strategies are exemplified with bispecific IgG and Fab molecules, respectively.
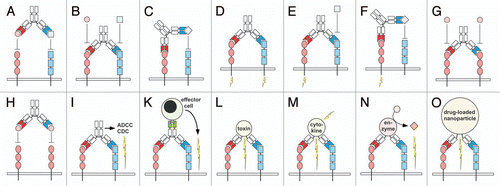
Figure 2 Bispecific antibody formats. Variable heavy chain domains (VH) are shown in dark blue and dark red, variable light chain domains (VL) are shown in light blue and light red, red and blue indicating different specificities. Antibody constant domains are shown in white boxes and fusion proteins in white circles.
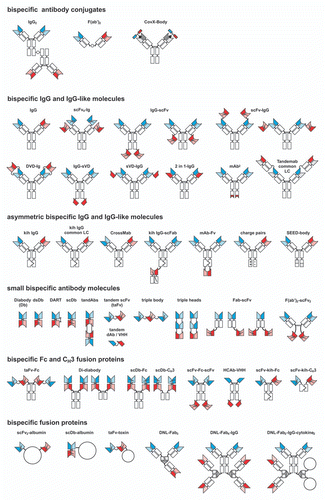
Table 1 Dual targeting approaches
Table 2 Dual retargeting approaches
References
- Reichert JM. Antibody-based therapeutics to watch in 2011. MAbs 2011; 3:76 - 99; PMID: 21051951; http://dx.doi.org/10.4161/mabs.3.1.13895
- Nelson AL, Dhimolea E, Reichert JM. Development trends for human monoclonal antibody therapeutics. Nat Rev Drug Discov 2010; 9:767 - 774; PMID: 20811384; http://dx.doi.org/10.1038/nrd3229
- Presta LG. Engineering of therapeutic antibodies to minimize immunogenicity and optimize function. Adv Drug Deliv Rev 2006; 58:640 - 656; PMID: 16904789; http://dx.doi.org/10.1016/j.addr.2006.01.026
- Presta LG. Molecular engineering and design of therapeutic antibodies. Curr Opin Immunol 2008; 20:460 - 470; PMID: 18656541; http://dx.doi.org/10.1016/j.coi.2008.06.012
- Rothe A, Rubbert A. Recombinant proteins in rheumatology—recent advances. N Biotechnol 2011; 28:502 - 510; PMID: 21473939; http://dx.doi.org/10.1016/j.nbt.2011.03.019
- Jones HE, Gee JMW, Hutcheson IR, Knowlden JM, Barrow D, Nicholson RI. Growth factor receptor interplay and resistance in cancer. Endocr Relat Cancer 2006; 13:45 - 51; PMID: 17259558; http://dx.doi.org/10.1677/erc.1.01275
- van der Veeken J, Oliveira S, Schiffelers RM, Storm G, van Bergen En Henegouwen PM, Roovers RC. Crosstalk between epidermal growth factor receptor- and insulin-like growth factor-1 receptor signaling: implications for cancer therapy. Curr Cancer Drug Targets 2009; 9:748 - 760; PMID: 19754359; http://dx.doi.org/10.2174/156800909789271495
- Morgillo F, Lee HY. Resistance to epidermal growth factor receptor-targeted therapy. Drug Resist Updat 2005; 8:298 - 310; PMID: 16172017; http://dx.doi.org/10.1016/j.drup.2005.08.004
- Dong J, Sereno A, Aivazian D, Langley E, Miller BR, Snyder WB, et al. A stable IgG-like bispecific antibody targeting the epidermal growth factor receptor and the type I insulin-like growth factor receptor demonstrates superior anti-tumor activity. MAbs 2011; 3:273 - 288; PMID: 21393993; http://dx.doi.org/10.4161/mabs.3.3.15188
- Demarest SJ, Hariharan K, Dong J. Emerging antibody combinations in oncology. MAbs 2011; 3:338 - 351; PMID: 21697653; http://dx.doi.org/10.4161/mabs.3.4.16615
- Haurum JS. Recombinant polyclonal antibodies: the next generation of antibody therapeutics?. Drug Discov Today 2006; 11:655 - 660; PMID: 16793535; http://dx.doi.org/10.1016/j.drudis.2006.05.009
- Koefoed K, Steinaa L, Søderberg JN, Kjær I, Jacobsen HJ, Meijer PJ, et al. Rational identification of an optimal antibody mixture for targeting the epidermal growth factor receptor. MAbs 2011; 3; In press PMID: 22123060
- Pedersen MW, Jacobsen HJ, Koefoed K, Hey A, Pyke C, Haurum JS, et al. Sym004: a novel synergistic anti-epidermal growth factor receptor antibody mixture with superior anticancer efficacy. Cancer Res 2010; 70:588 - 597; PMID: 20068188; http://dx.doi.org/10.1158/0008-5472.CAN-09-1417
- Skartved NJ, Jacobsen HJ, Pedersen MW, Jensen PF, Sen JW, Jørgensen TK, et al. Preclinical pharmacokinetics and safety of Sym004: a synergistic antibody mixture directed against epidermal growth factor receptor. Clin Cancer Res 2011; 17:5962 - 5972; PMID: 21825041; http://dx.doi.org/10.1158/1078-0432.CCR-11-1209
- Chan AC, Carter PJ. Therapeutic antibodies for autoimmunity and inflammation. Nat Rev Immunol 2010; 10:301 - 316; PMID: 20414204; http://dx.doi.org/10.1038/nri2761
- Müller D, Kontermann RE. Bispecific antibodies for cancer immunotherapy: Current perspectives. BioDrugs 2010; 24:89 - 98; PMID: 20199124; http://dx.doi.org/10.2165/11530960-000000000-00000
- Kontermann RE. Bispecific antibodies 2011; Springer ISBN 978-3-642-20909-3
- Seimetz D, Lindhofer H, Bokemeyer C. Development and approval of the trifunctional antibody catumaxomab (anti-EpCAM x anti-CD3) as a targeted cancer immunotherapy. Cancer Treat Rev 2010; 36:458 - 467; PMID: 20347527; http://dx.doi.org/10.1016/j.ctrv.2010.03.001
- Graziano RF, Guptill P. Chemical production of bispecific antibodies. Methods Mol Biol 2004; 283:71 - 85; PMID: 15197303
- Doppalapudi VR, Huang J, Liu D, Jin P, Liu B, Li L, et al. Chemical generation of bispecific antibodies. Proc Natl Acad Sci USA 2010; 107:22611 - 22616; PMID: 21149738
- Coloma MJ, Morrison SL. Design and production of novel tetravalent bispecific antibodies. Nat Biotechnol 1997; 15:159 - 163; PMID: 9035142; http://dx.doi.org/10.1038/nbt0297-159
- Orcutt KD, Ackerman ME, Cieslewicz M, Quiroz E, Slusarczyk AL, Frangioni JV, et al. A modular IgG-scFv bispecific antibody topology. Protein Eng Des Sel 2010; 23:221 - 228; PMID: 20019028; http://dx.doi.org/10.1093/protein/gzp077
- Dong J, Sereno A, Snyder WB, Miller BR, Tamraz S, Doern A, et al. Stable IgG-like bispecific antibodies directed toward the type I insulin-like growth factor receptor demonstrate enhanced ligand blockade and anti-tumor activity. J Biol Chem 2011; 286:4703 - 4717; PMID: 21123183; http://dx.doi.org/10.1074/jbc.M110.184317
- Schanzer J, Jekle A, Nezu J, Lochner A, Croasdale R, Dioszegi M, et al. Development of tetravalent, bispecific CCR5 antibodies with antiviral activity against CCR5 monoclonal antibody-resistant HIV-1 strains. Antimicrob Agents Chemother 2011; 55:2369 - 2378; PMID: 21300827; http://dx.doi.org/10.1128/AAC.00215-10
- Wu C, Ying H, Grinnell C, Bryant S, Miller R, Clabbers A, et al. Simultaneous targeting of multiple disease mediators by a dual-variable-domain immunoglobulin. Nat Biotechnol 2007; 25:1290 - 1297; PMID: 17934452; http://dx.doi.org/10.1038/nbt1345
- Bostrom J, Yu SF, Kan D, Appleton BA, Lee CV, Billeci K, et al. Variants of the antibody herceptin that interact with HER2 and VEGF at the antigen binding site. Science 2009; 323:1610 - 1614; PMID: 19299620; http://dx.doi.org/10.1126/science.1165480
- Wozniak-Knopp G, Bartl S, Bauer A, Mostageer M, Woisetschläger M, Antes B, et al. Introducing antigen-binding sites in structural loops of immunoglobulin constant domains: Fc fragments with engineered HER2/neu-binding sites and antibody properties. Protein Eng Des Sel 2010; 23:289 - 297; PMID: 20150180; http://dx.doi.org/10.1093/protein/gzq005
- Ridgway JB, Presta LG, Carter P. ‘Knobs-into-holes’ engineering of antibody CH3 domains for heavy chain heterodimerization. Protein Eng 1996; 9:617 - 621; PMID: 8844834; http://dx.doi.org/10.1093/protein/9.7.617
- Merchant AM, Zhu Z, Yuan JQ, Goddard A, Adams CW, Presta LG, et al. An efficient route to human bispecific IgG. Nat Biotechnol 1998; 16:677 - 681; PMID: 9661204; http://dx.doi.org/10.1038/nbt0798677
- Schaefer W, Regula JT, Bähner M, Schanzer J, Croasdale R, Dürr H, et al. Immunoglobulin domain crossover as a generic approach for the production of bispecific IgG antibodies. Proc Natl Acad Sci USA 2011; 108:11187 - 11192; PMID: 21690412; http://dx.doi.org/10.1073/pnas.1019002108
- Gunasekaran K, Pentony M, Shen M, Garrett L, Forte C, Woodward A, et al. Enhancing antibody Fc heterodimer formation through electrostatic steering effects: applications to bispecific molecules and monovalent IgG. J Biol Chem 2010; 285:19637 - 19646; PMID: 20400508; http://dx.doi.org/10.1074/jbc.M110.117382
- Davis JH, Aperlo C, Li Y, Kurosawa E, Lan Y, Lo KM, et al. SEEDbodies: fusion proteins based on strand-exchange engineered domain (SEED) CH3 heterodimers in an Fc analogue platform for asymmetric binders or immunofusions and bispecific antibodies. Protein Eng Des Sel 2010; 23:195 - 202; PMID: 20299542; http://dx.doi.org/10.1093/protein/gzp094
- Moore GL, Bautista C, Pong E, Nguyen DHT, Jacinto J, Eivazi A, et al. A novel bispecific antibody format enables simultaneous bivalent and monovalent co-engagement of distinct target antigens. MAbs 2011; 3; In press PMID: 22123055
- Kontermann RE. Alternative antibody formats. Curr Opin Mol Ther 2010; 12:176 - 183; PMID: 20373261
- Kellner C, Bruenke J, Stieglmaier J, Schwemmlein M, Schwenkert M, Singer H, et al. A novel CD19-directed recombinant bispecific antibody derivative with enhanced immune effector functions for human leukemic cells. J Immunother 2008; 31:871 - 884; PMID: 18833000; http://dx.doi.org/10.1097/CJI.0b013e318186c8b4
- Holliger P, Prospero T, Winter G. “Diabodies”: small bivalent and bispecific antibody fragments. Proc Natl Acad Sci USA 1993; 90:6444 - 6448; PMID: 8341653; http://dx.doi.org/10.1073/pnas.90.14.6444
- FitzGerald K, Holliger P, Winter G. Improved tumour targeting by disulphide stabilized diabodies expressed in Pichia pastoris. Protein Eng 1997; 10:1221 - 1225; PMID: 9488147; http://dx.doi.org/10.1093/protein/10.10.1221
- Johnson S, Burke S, Huang L, Gorlatov S, Li H, Wang W, et al. Effector cell recruitment with novel Fv-based dual-affinity re-targeting protein leads to potent tumor cytolysis and in vivo B-cell depletion. J Mol Biol 2010; 399:436 - 449; PMID: 20382161; http://dx.doi.org/10.1016/j.jmb.2010.04.001
- Brüsselbach S, Korn T, Völkel T, Müller R, Kontermann RE. Enzyme recruitment and tumor cell killing in vitro by a secreted bispecific single-chain diabody. Tumor Targeting 1999; 4:115 - 123
- Kipriyanov SM, Moldenhauer G, Schuhmacher J, Cochlovius B, Von der Lieth CW, Matys ER, et al. Bispecific tandem diabody for tumor therapy with improved antigen binding and pharmacokinetics. J Mol Biol 1999; 293:41 - 56; PMID: 10512714; http://dx.doi.org/10.1006/jmbi.1999.3156
- Schoonjans R, Willems A, Schoonooghe S, Leoen J, Grooten J, Mertens N. A new model for intermediate molecular weight recombinant bispecific and trispecific antibodies by efficient heterodimerization of single chain variable domains through fusion to a Fab-chain. Biomol Eng 2001; 17:193 - 202; PMID: 11337278; http://dx.doi.org/10.1016/S1389-0344(01)00066-1
- Goldenberg DM, Rossi EA, Sharkey RM, McBride WJ, Chang CH. Multifunctional antibodies by the Dock-and-Lock method for improved cancer imaging and therapy by pretargeting. J Nucl Med 2008; 49:158 - 163; PMID: 18077530; http://dx.doi.org/10.2967/jnumed.107.046185
- Kontermann RE. Strategies to extend plasma half-lives of recombinant antibodies. BioDrugs 2009; 23:93 - 109; PMID: 19489651; http://dx.doi.org/10.2165/00063030-200923020-00003
- Kontermann RE. Strategies for extended serum half-life of protein therapeutics. Curr Opin Biotechnol 2011; Epub ahead of print PMID: 21862310
- Wieduwilt MJ, Moasser MM. The epidermal growth factor receptor family: biology driving targeted therapeutics. Cell Mol Life Sci 2008; 65:1566 - 1584; PMID: 18259690; http://dx.doi.org/10.1007/s00018-008-7440-8
- Tao Y, Pinzi V, Bourhis J, Deutsch E. Mechanisms of disease: signaling of the insulin-like growth factor 1 receptor pathway—therapeutic perspectives in cancer. Nat Clin Pract Oncol 2007; 4:591 - 602; PMID: 17898809; http://dx.doi.org/10.1038/ncponc0934
- Ludovini V, Bellezza G, Pistola L, Bianconi F, Di Carlo L, Sidoni A, et al. High coexpression of both insulin-like growth factor receptor-1 (IGFR-1) and epidermal growth factor receptor (EGFR) is associated with shorter disease-free survival in resected non-small-cell lung cancer patients. Ann Oncol 2009; 20:842 - 849; PMID: 19153117; http://dx.doi.org/10.1093/annonc/mdn727
- Ueda S, Hatsuse K, Tsuda H, Ogata S, Kawarabayashi N, Takigawa T, et al. Potential crosstalk between insulin-like growth factor receptor type 1 and epidermal growth factor receptor in progression and metastasis of pancreatic cancer. Mod Pathol 2006; 19:788 - 796; PMID: 16575403
- Lu D, Zhang H, Ludwig D, Persaud A, Jimenez X, Burtrum D, et al. Simultaneous blockade of both the epidermal growth factor receptor and the insulin-like growth factor receptor signaling pathways in cancer cells with a fully human recombinant bispecific antibody. J Biol Chem 2004; 279:2856 - 2865; PMID: 14576153; http://dx.doi.org/10.1074/jbc.M310132200
- Lu D, Jimenez X, Witte L, Zhu Z. The effect of variable domain orientation and arrangement on the antigen-binding activity of a recombinant human bispecific diabody. Biochem Biophys Res Commun 2004; 318:507 - 513; PMID: 15120630; http://dx.doi.org/10.1016/j.bbrc.2004.04.060
- Lu D, Zhang H, Koo H, Tonra J, Balderes P, Prewett M, et al. A fully human recombinant IgG-like bispecific antibody to both the epidermal growth factor receptor and the insulin-like growth factor receptor for enhanced antitumor activity. J Biol Chem 2005; 280:19665 - 19672; PMID: 15757893; http://dx.doi.org/10.1074/jbc.M500815200
- Emanuel SL, Engle LJ, Chao G, Zhu RR, Cao C, Lin Z, et al. A fibronectin scaffold approach to bispecific inhibitors of epidermal growth factor receptor and insulin-like growth factor-I receptor. MAbs 2011; 3:38 - 48; PMID: 21099371; http://dx.doi.org/10.4161/mabs.3.1.14168
- Nielsen U, Huhalov A, Harms B, Paragas V, Adams S, Gu J, et al. MM-111: a novel bispecific antibody targeting ErbB3 with potent anti-tumor activity in ErbB2 overexpressing malignancies. Cancer Res 2009; 69:4166
- Shen J, Vil MD, Jimenez X, Zhang H, Iacolina M, Mangalampalli V, et al. Single variable domain antibody as a versatile building block for the construction of IgG-like bispecific antibodies. J Immunol Methods 2007; 318:65 - 74; PMID: 17126853; http://dx.doi.org/10.1016/j.jim.2006.09.020
- Yu J, Ustach C, Kim HRC. Platelet-derived growth factor signaling and human cancer. J Biochem Mol Biol 2003; 36:49 - 59; PMID: 12542975; http://dx.doi.org/10.5483/BMBRep.2003.36.1.049
- Board R, Jayson GC. Platelet-derived growth factor receptor (PDGFR): a target for anticancer therapeutics. Drug Resist Updat 2005; 8:75 - 83; PMID: 15939344; http://dx.doi.org/10.1016/j.drup.2005.03.004
- Shen J, Vil MD, Jimenez X, Iacolina M, Zhang H, Zhu Z. Single variable domain-IgG fusion. A novel recombinant approach to Fc domain-containing bispecific antibodies. J Biol Chem 2006; 281:10706 - 10714; PMID: 16481314; http://dx.doi.org/10.1074/jbc.M513415200
- Tugues S, Koch S, Gualandi L, Li X, Claesson-Welsh L. Vascular endothelial growth factors and receptors: anti-angiogenic therapy in the treatment of cancer. Mol Aspects Med 2011; 32:88 - 111; PMID: 21565214; http://dx.doi.org/10.1016/j.mam.2011.04.004
- Jimenez X, Lu D, Brennan L, Persaud K, Liu M, Miao H, et al. A recombinant, fully human, bispecific antibody neutralizes the biological activities mediated by both vascular endothelial growth factor receptors 2 and 3. Mol Cancer Ther 2005; 4:427 - 434; PMID: 15767551
- Lu D, Jimenez X, Zhang H, Atkins A, Brennan L, Balderes P, et al. Di-diabody: a novel tetravalent bispecific antibody molecule by design. J Immunol Methods 2003; 279:219 - 232; PMID: 12969563; http://dx.doi.org/10.1016/S0022-1759(03)00251-5
- Leonard JP, Schuster SJ, Emmanouilides C, Couture F, Teoh N, Wegener WA, et al. Durable complete responses from therapy with combined epratuzumab and rituximab: final results from an international multicenter, phase 2 study in recurrent, indolent, non-Hodgkin lymphoma. Cancer 2008; 113:2714 - 2723; PMID: 18853418; http://dx.doi.org/10.1002/cncr.23890
- Qu Z, Goldenberg DM, Cardillo TM, Shi V, Hansen HJ, Chang CH. Bispecific anti-CD20/22 antibodies inhibit B-cell lymphoma proliferation by a unique mechanism of action. Blood 2008; 111:2211 - 2219; PMID: 18025153; http://dx.doi.org/10.1182/blood-2007-08-110072
- Rossi EA, Goldenberg DM, Cardillo TM, Stein R, Chang CH. Hexavalent bispecific antibodies represent a new class of anticancer therapeutics: 1. Properties of anti-CD20/CD22 antibodies in lymphoma. Blood 2009; 113:6161 - 6171; PMID: 19372261; http://dx.doi.org/10.1182/blood-2008-10-187138
- Gupta P, Goldenberg DM, Rossi EA, Chang CH. Multiple signaling pathways induced by hexavalent, monospecific, anti-CD20 and hexavalent, bispecific, anti-CD20/CD22 humanized antibodies correlate with enhanced toxicity to B-cell lymphomas and leukemias. Blood 2010; 116:3258 - 3267; PMID: 20628151; http://dx.doi.org/10.1182/blood-2010-03-276857
- Tuscano JM, Ma Y, Martin SM, Kato J, O'Donnell RT. The Bs20 × 22 anti-CD20-CD22 bispecific antibody has more lymphomacidal activity than do the parent antibodies alone. Cancer Immunol Immunother 2011; 60:771 - 780; PMID: 21347809; http://dx.doi.org/10.1007/s00262-011-0978-6
- Ashkenazi A, Holland P, Eckhardt SG. Ligand-based targeting of apoptosis in cancer: the potential of recombinant human apoptosis ligand 2/Tumor necrosis factor-related apoptosis-inducing ligand (rhApo2L/TRAIL). J Clin Oncol 2008; 26:3621 - 3630; PMID: 18640940; http://dx.doi.org/10.1200/JCO.2007.15.7198
- Michaelson JS, Demarest SJ, Miller B, Amatucci A, Snyder WB, Wu X, et al. Anti-tumor activity of stability-engineered IgG-like bispecific antibodies targeting TRAIL-R2 and LTbetaR. MAbs 2009; 1:128 - 141; PMID: 20061822; http://dx.doi.org/10.4161/mabs.1.2.7631
- Looney RJ. B cells as a therapeutic target in autoimmune diseases other than rheumatoid arthritis. Rheumatology (Oxford) 2005; 44:13 - 17; PMID: 15851522; http://dx.doi.org/10.1093/rheumatology/keh618
- Edwards JCW, Cambridge G, Leandro MJ. B cell depletion therapy in rheumatic disease. Best Pract Res Clin Rheumatol 2006; 20:915 - 928; PMID: 16980214; http://dx.doi.org/10.1016/j.berh.2006.05.010
- Cohen SB. Targeting the B cell in rheumatoid arthritis. Best Pract Res Clin Rheumatol 2010; 24:553 - 563; PMID: 20732652; http://dx.doi.org/10.1016/j.berh.2009.11.006
- Smith KG, Clatworthy MR. FcgammaRIIB in autoimmunity and infection: evolutionary and therapeutic implications. Nat Rev Immunol 2010; 10:328 - 343; PMID: 20414206; http://dx.doi.org/10.1038/nri2762
- Veri MC, Burke S, Huang L, Li H, Gorlatov S, Tuaillon N, et al. Therapeutic control of B cell activation via recruitment of Fcγ receptor IIb (CD32B) inhibitory function with a novel bispecific antibody scaffold. Arthritis Rheum (Munch) 2010; 62:1933 - 1943
- Zhang K, Kepley CL, Terada T, Zhu D, Perez H, Saxon A. Inhibition of allergen-specific IgE reactivity by a human Ig Fcgamma-Fcepsilon bifunctional fusion protein. J Allergy Clin Immunol 2004; 114:321 - 327; PMID: 15316510; http://dx.doi.org/10.1016/j.jaci.2004.03.058
- Jackman J, Chen Y, Huang A, Moffat B, Scheer JM, Leong SR, et al. Development of a two-part strategy to identify a therapeutic human bispecific antibody that inhibits IgE receptor signaling. J Biol Chem 2010; 285:20850 - 20859; PMID: 20444694; http://dx.doi.org/10.1074/jbc.M110.113910
- Tam SW, Demissie S, Thomas D, Daëron M. A bispecific antibody against human IgE and human FcgammaRII that inhibits antigen-induced histamine release by human mast cells and basophils. Allergy 2004; 59:772 - 780; PMID: 15180766; http://dx.doi.org/10.1111/j.1398-9995.2004.00332.x
- Saylor C, Dadachova E, Casadevall A. Monoclonal antibody-based therapies for microbial diseases. Vaccine 2009; 27:38 - 46; PMID: 20006139
- Casadevall A, Dadachova E, Pirofski LA. Passive antibody therapy for infectious diseases. Nat Rev Microbiol 2004; 2:695 - 703; PMID: 15372080; http://dx.doi.org/10.1038/nrmicro974
- Lim CS, Rosli R, Seow HF, Chong PP. Candida and invasive candidiasis: back to basics. Eur J Clin Microbiol Infect Dis 2911; Epub ahead of print PMID: 21544694
- De Bernardis F, Liu H, O'Mahony R, La Valle R, Bartollino S, Sandini S, et al. Human domain antibodies against virulence traits of Candida albicans inhibit fungus adherence to vaginal epithelium and protect against experimental vaginal candidiasis. J Infect Dis 2007; 195:149 - 157; PMID: 17152019; http://dx.doi.org/10.1086/509891
- Dong J, Demarest SJ, Sereno A, Tamraz S, Langley E, Doern A, et al. Combination of two insulin-like growth factor-I receptor inhibitory antibodies targeting distinct epitopes leads to an enhanced antitumor response. Mol Cancer Ther 2010; 9:2593 - 2604; PMID: 20716637; http://dx.doi.org/10.1158/1535-7163.MCT-09-1018
- Eggel A, Baumann MJ, Amstutz P, Stadler BM, Vogel M. DARPins as bispecific receptor antagonists analyzed for immunoglobulin E receptor blockage. J Mol Biol 2009; 393:598 - 607; PMID: 19683003; http://dx.doi.org/10.1016/j.jmb.2009.08.014
- Thomson NC, Chaudhuri R, Spears M. Emerging therapies for severe asthma. BMC Med 2011; 9:102; PMID: 21896202; http://dx.doi.org/10.1186/1741-7015-9-102
- Nowak D. Management of asthma with anti-immunoglobulin E: a review of clinical trials of omalizumab. Respir Med 2006; 100:1907 - 1917; PMID: 16949266; http://dx.doi.org/10.1016/j.rmed.2005.10.004
- Laventie BJ, Rademaker HJ, Saleh M, de Boer E, Janssens R, Bourcier T, et al. Heavy chain-only antibodies and tetravalent bispecific antibody neutralizing Staphylococcus aureus leukotoxins. Proc Natl Acad Sci USA 2011; 108:16404 - 16409; PMID: 21930905; http://dx.doi.org/10.1073/pnas.1102265108
- Hmila I, Saerens D, Ben Abderrazek R, Vincke C, Abidi N, Benlasfar Z, et al. A bispecific nanobody to provide full protection against lethal scorpion envenoming. FASEB J 2010; 24:3479 - 3489; PMID: 20410443; http://dx.doi.org/10.1096/fj.09-148213
- Hammoudi-Triki D, Lefort J, Rougeot C, Robbe-Vincent A, Bon C, Laraba-Djebari F, et al. Toxicokinetic and toxicodynamic analyses of Androctonus australis hector venom in rats: optimization of antivenom therapy. Toxicol Appl Pharmacol 2007; 218:205 - 214; PMID: 17198719; http://dx.doi.org/10.1016/j.taap.2006.11.003
- Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature 2000; 407:249 - 257; PMID: 11001068; http://dx.doi.org/10.1038/35025220
- Ferrara N. Vascular endothelial growth factor: basic science and clinical progress. Endocr Rev 2004; 25:581 - 611; PMID: 15294883; http://dx.doi.org/10.1210/er.2003-0027
- Kou G, Shi J, Chen L, Zhang D, Hou S, Zhao L, et al. A bispecific antibody effectively inhibits tumor growth and metastasis by simultaneous blocking vascular endothelial growth factor A and osteopontin. Cancer Lett 2010; 299:130 - 136; PMID: 20826049; http://dx.doi.org/10.1016/j.canlet.2010.08.011
- Rader C, Sinha SC, Popkov M, Lerner RA, Barbas CF 3rd. Chemically programmed monoclonal antibodies for cancer therapy: adaptor immunotherapy based on a covalent antibody catalyst. Proc Natl Acad Sci USA 2003; 100:5396 - 5400; PMID: 12702756; http://dx.doi.org/10.1073/pnas.0931308100
- Weinblatt M, Schiff M, Goldman A, Kremer J, Luggen M, Li T, et al. Selective costimulation modulation using abatacept in patients with active rheumatoid arthritis while receiving etanercept: a randomised clinical trial. Ann Rheum Dis 2007; 66:228 - 234; PMID: 16935912; http://dx.doi.org/10.1136/ard.2006.055111
- Wu C, Ying H, Bose S, Miller R, Medina L, Santora L, et al. Molecular construction and optimization of anti-human IL-1α/β dual variable domain immunoglobulin (DVD-Ig) molecules. MAbs 2009; 1:339 - 347; PMID: 20068402; http://dx.doi.org/10.4161/mabs.1.4.8755
- Mabry R, Lewis KE, Moore M, McKernan PA, Bukowski TR, Bontadelli K, et al. Engineering of stable bispecific antibodies targeting IL-17A and IL-23. Protein Eng Des Sel 2010; 23:115 - 127; PMID: 20022918; http://dx.doi.org/10.1093/protein/gzp073
- McGeachy MJ, Cua DJ. Th17 cell differentiation: the long and winding road. Immunity 2008; 28:445 - 453; PMID: 18400187; http://dx.doi.org/10.1016/j.immuni.2008.03.001
- Cornelissen F, van Hamburg JP, Lubberts E. The IL-12/IL-23 axis and its role in Th17 cell development, pathology and plasticity in arthritis. Curr Opin Investig Drugs 2009; 10:452 - 462; PMID: 19431078
- Le XF, Mao W, Lu C, Thornton A, Heymach JV, Sood AK, et al. Specific blockade of VEGF and HER2 pathways results in greater growth inhibition of breast cancer xenografts that overexpress HER2. Cell Cycle 2008; 7:3747 - 3758; PMID: 19029832; http://dx.doi.org/10.4161/cc.7.23.7212
- Yang W, Klos K, Yang Y, Smith TL, Shi D, Yu D. ErbB2 overexpression correlates with increased expression of vascular endothelial growth factors A, C and D in human breast carcinoma. Cancer 2002; 94:2855 - 2861; PMID: 12115372; http://dx.doi.org/10.1002/cncr.10553
- Erber R, Thurnher A, Katsen AD, Groth G, Kerger H, Hammes HP, et al. Combined inhibition of VEGF and PDGF signaling enforces tumor vessel regression by interfering with pericyte-mediated endothelial cell survival mechanisms. FASEB J 2004; 18:338 - 340; PMID: 14657001
- Shen J, Vil MD, Zhang H, Tonra JR, Rong LL, Damoci C, et al. An antibody directed against PDGF receptor beta enhances the antitumor and the anti-angiogenic activities of an anti-VEGF receptor 2 antibody. Biochem Biophys Res Commun 2007; 357:1142 - 1147; PMID: 17462601; http://dx.doi.org/10.1016/j.bbrc.2007.04.075
- Mabry R, Gilbertson DG, Frank A, Vu T, Ardourel D, Ostrander C, et al. A dual-targeting PDGFRbeta/VEGF-A molecule assembled from stable antibody fragments demonstrates anti-angiogenic activity in vitro and in vivo. MAbs 2010; 2:20 - 34; PMID: 20065654; http://dx.doi.org/10.4161/mabs.2.1.10498
- Lum LG, Davol PA. Retargeting T cells and immune effector cells with bispecific antibodies. Cancer Chemother Biol Response Modif 2005; 22:273 - 291; PMID: 16110617
- Müller D, Kontermann RE. Recombinant bispecific antibodies for cellular cancer immunotherapy. Curr Opin Mol Ther 2007; 9:319 - 326; PMID: 17694444
- Bargou R, Leo E, Zugmaier G, Klinger M, Goebeler M, Knop S, et al. Tumor regression in cancer patients by very low doses of a T cell-engaging antibody. Science 2008; 321:974 - 977; PMID: 18703743; http://dx.doi.org/10.1126/science.1158545
- Topp MS, Kufer P, Gökbuget N, Goebeler M, Klinger M, Neumann S, et al. Targeted therapy with the T-cell-engaging antibody blinatumomab of chemotherapy-refractory minimal residual disease in B-lineage acute lymphoblastic leukemia patients results in high response rate and prolonged leukemia-free survival. J Clin Oncol 2011; 29:2493 - 2498; PMID: 21576633; http://dx.doi.org/10.1200/JCO.2010.32.7270
- Kügler M, Stein C, Kellner C, Mentz K, Saul D, Schwenkert M, et al. A recombinant trispecific single-chain Fv derivative directed against CD123 and CD33 mediates effective elimination of acute myeloid leukaemia cells by dual targeting. Br J Haematol 2010; 150:574 - 586; PMID: 20636437; http://dx.doi.org/10.1111/j.1365-2141.2010.08300.x
- Schubert I, Kellner C, Stein C, Kügler M, Schwenkert M, Saul D, et al. A single-chain triplebody with specificity for CD19 and CD33 mediates effective lysis of mixed lineage leukemia cells by dual targeting. MAbs 2011; 3:21 - 30; PMID: 21081841; http://dx.doi.org/10.4161/mabs.3.1.14057
- Pastan I, Hassan R, FitzGerald DJ, Kreitman RJ. Immunotoxin treatment of cancer. Annu Rev Med 2007; 58:221 - 237; PMID: 17059365; http://dx.doi.org/10.1146/annurev.med.58.070605.115320
- Choudhary S, Mathew M, Verma RS. Therapeutic potential of anticancer immunotoxins. Drug Discov Today 2011; 16:495 - 503; PMID: 21511052; http://dx.doi.org/10.1016/j.drudis.2011.04.003
- Manoukian G, Hagemeister F. Denileukin diftitox: a novel immunotoxin. Expert Opin Biol Ther 2009; 9:1445 - 1451; PMID: 19817678; http://dx.doi.org/10.1517/14712590903348135
- Onda M, Beers R, Xiang L, Lee B, Weldon JE, Kreitman RJ, et al. Recombinant immunotoxin against B-cell malignancies with no immunogenicity in mice by removal of B-cell epitopes. Proc Natl Acad Sci USA 2011; 108:5742 - 5747; PMID: 21436054; http://dx.doi.org/10.1073/pnas.1102746108
- Frankel AE, Woo JH. Bispecific immunotoxins. Leuk Res 2009; 33:1173 - 1174; PMID: 19406472; http://dx.doi.org/10.1016/j.leukres.2009.03.037
- Duke-Cohan JS, Morimoto C, Schlossman SF. Targeting of an activated T-cell subset using a bispecific antibody-toxin conjugate directed against CD4 and CD26. Blood 1993; 82:2224 - 2234; PMID: 8104537
- Duke-Cohan JS, Morimoto C, Schlossman SF. Depletion of the helper/inducer (memory) T cell subset using a bispecific antibody-toxin conjugate directed against CD4 and CD29. Transplantation 1993; 56:1188 - 1196; PMID: 7504344; http://dx.doi.org/10.1097/00007890-199311000-00027
- Vallera DA, Todhunter DA, Kuroki DW, Shu Y, Sicheneder A, Chen H. A bispecific recombinant immunotoxin, DT2219, targeting human CD19 and CD22 receptors in a mouse xenograft model of B-cell leukemia/lymphoma. Clin Cancer Res 2005; 11:3879 - 3888; PMID: 15897589; http://dx.doi.org/10.1158/1078-0432.CCR-04-2290
- Vallera DA, Chen H, Sicheneder AR, Panoskaltsis-Mortari A, Taras EP. Genetic alteration of a bispecific ligand-directed toxin targeting human CD19 and CD22 receptors resulting in improved efficacy against systemic B cell malignancy. Leuk Res 2009; 33:1233 - 1242; PMID: 19327829; http://dx.doi.org/10.1016/j.leukres.2009.02.006
- Vallera DA, Oh S, Chen H, Shu Y, Frankel AE. Bioengineering a unique deimmunized bispecific targeted toxin that simultaneously recognizes human CD22 and CD19 receptors in a mouse model of B-cell metastases. Mol Cancer Ther 2010; 9:1872 - 1883; PMID: 20530709; http://dx.doi.org/10.1158/1535-7163.MCT-10-0203
- Stish BJ, Chen H, Shu Y, Panoskaltsis-Mortari A, Vallera DA. Increasing anticarcinoma activity of an anti-erbB2 recombinant immunotoxin by the addition of an anti-EpCAM sFv. Clin Cancer Res 2007; 13:3058 - 3067; PMID: 17505009; http://dx.doi.org/10.1158/1078-0432.CCR-06-2454
- Stish BJ, Chen H, Shu Y, Panoskaltsis-Mortari A, Vallera DA. A bispecific recombinant cytotoxin (DTEGF13) targeting human interleukin-13 and epidermal growth factor receptors in a mouse xenograft model of prostate cancer. Clin Cancer Res 2007; 13:6486 - 6493; PMID: 17975161; http://dx.doi.org/10.1158/1078-0432.CCR-07-0938
- Stish BJ, Oh S, Vallera DA. Anti-glioblastoma effect of a recombinant bispecific cytotoxin cotargeting human IL-13 and EGF receptors in a mouse xenograft model. J Neurooncol 2008; 87:51 - 61; PMID: 18084721; http://dx.doi.org/10.1007/s11060-007-9499-8
- Vallera DA, Stish BJ, Shu Y, Chen H, Saluja A, Buchsbaum DJ, et al. Genetically designing a more potent antipancreatic cancer agent by simultaneously co-targeting human IL13 and EGF receptors in a mouse xenograft model. Gut 2008; 57:634 - 641; PMID: 18222985; http://dx.doi.org/10.1136/gut.2007.137802
- Oh S, Ohlfest JR, Todhunter DA, Vallera VD, Hall WA, Chen H, et al. Intracranial elimination of human glioblastoma brain tumors in nude rats using the bispecific ligand-directed toxin, DTEGF13 and convection enhanced delivery. J Neurooncol 2009; 95:331 - 342; PMID: 19517064; http://dx.doi.org/10.1007/s11060-009-9932-2
- Oh S, Stish BJ, Vickers SM, Buchsbaum DJ, Saluja AK, Vallera DA. A new drug delivery method of bispecific ligand-directed toxins, which reduces toxicity and promotes efficacy in a model of orthotopic pancreatic cancer. Pancreas 2010; 39:913 - 922; PMID: 20182395; http://dx.doi.org/10.1097/MPA.0b013e3181cbd908
- Todhunter DA, Hall WA, Rustamzadeh E, Shu Y, Doumbia SO, Vallera DA. A bispecific immunotoxin (DTAT13) targeting human IL-13 receptor (IL-13R) and urokinase-type plasminogen activator receptor (uPAR) in a mouse xenograft model. Protein Eng Des Sel 2004; 17:157 - 164; PMID: 15047912; http://dx.doi.org/10.1093/protein/gzh023
- Hall WA, Vallera DA. Efficacy of antiangiogenic targeted toxins against glioblastoma multiforme. Neurosurg Focus 2006; 20:23; PMID: 16709029; http://dx.doi.org/10.3171/foc.2006.20.4.15
- Rustamzadeh E, Vallera DA, Todhunter DA, Low WC, Panoskaltsis-Mortari A, Hall WA. Immunotoxin pharmacokinetics: a comparison of the anti-glioblastoma bi-specific fusion protein (DTAT13) to DTAT and DTIL13. J Neurooncol 2006; 77:257 - 266; PMID: 16314943; http://dx.doi.org/10.1007/s11060-005-9051-7
- Oh S, Stish BH, Sachdev D, Chen H, Dudek AZ, Vallera DA. A novel “reduced immunogenicity” bispecific targeted toxin simultaneously recognizing human EGF and IL-4 receptors in a mouse model of metastatic breast carcinoma. Clin Cancer Res 2009; 15:6137 - 6147; PMID: 19789305; http://dx.doi.org/10.1158/1078-0432.CCR-09-0696
- Stish BJ, Oh S, Chen H, Dudek AZ, Kratzke RA, Vallera DA. Design and modification of EGF4KDEL 7Mut, a novel bispecific ligand-directed toxin, with decreased immunogenicity and potent anti-mesothelioma activity. Br J Cancer 2009; 101:1114 - 1123; PMID: 19755995; http://dx.doi.org/10.1038/sj.bjc.6605297
- Tsai AK, Oh S, Chen H, Shu Y, Ohlfest JR, Vallera DA. A novel bispecific ligand-directed toxin designed to simultaneously target EGFR on human glioblastoma cells and uPAR on tumor neovasculature. J Neurooncol 2011; 103:255 - 266; PMID: 20830604; http://dx.doi.org/10.1007/s11060-010-0392-5
- Dougan M, Dranoff G. Immune therapy for cancer. Annu Rev Immunol 2009; 27:83 - 117; PMID: 19007331; http://dx.doi.org/10.1146/annurev.immunol.021908.132544
- Ortiz-Sánchez E, Helguera G, Daniels TR, Penichet ML. Antibody-cytokine fusion proteins: applications in cancer therapy. Expert Opin Biol Ther 2008; 8:609 - 632; PMID: 18407765; http://dx.doi.org/10.1517/14712598.8.5.609
- Rudman SM, Jameson MB, McKeage MJ, Savage P, Jodrell DI, Harries M, et al. A phase 1 study of AS1409, a novel antibody-cytokine fusion protein, in patients with malignant melanoma or renal cell carcinoma. Clin Cancer Res 2011; 17:1998 - 2005; PMID: 21447719; http://dx.doi.org/10.1158/1078-0432.CCR-10-2490
- Rossi EA, Rossi DL, Stein R, Goldenberg DM, Chang CH. A bispecific antibody-IFNalpha2b immunocytokine targeting CD20 and HLA-DR is highly toxic to human lymphoma and multiple myeloma cells. Cancer Res 2010; 70:7600 - 7609; PMID: 20876805; http://dx.doi.org/10.1158/0008-5472.CAN-10-2126
- Cho K, Wang X, Nie S, Chen ZG, Shin DM. Therapeutic nanoparticles for drug delivery in cancer. Clin Cancer Res 2008; 14:1310 - 1316; PMID: 18316549; http://dx.doi.org/10.1158/1078-0432.CCR-07-1441
- Malam Y, Loizidou M, Seifalian AM. Liposomes and nanoparticles: nanosized vehicles for drug delivery in cancer. Trends Pharmacol Sci 2009; 30:592 - 599; PMID: 19837467; http://dx.doi.org/10.1016/j.tips.2009.08.004
- Fenske DB, Cullis PR. Liposomal nanomedicines. Expert Opin Drug Deliv 2008; 5:25 - 44; PMID: 18095927; http://dx.doi.org/10.1517/17425247.5.1.25
- Peer D, Karp JM, Hong S, Farokhzad OC, Margalit R, Langer R. Nanocarriers as an emerging platform for cancer therapy. Nat Nanotechnol 2007; 2:751 - 760; PMID: 18654426; http://dx.doi.org/10.1038/nnano.2007.387
- Markman M. Pegylated liposomal doxorubicin: appraisal of its current role in the management of epithelial ovarian cancer. Cancer Manag Res 2011; 3:219 - 225; PMID: 21792330; http://dx.doi.org/10.2147/CMAR.S15558
- Kontermann RE. Immunoliposomes for cancer therapy. Curr Opin Mol Ther 2006; 8:39 - 45; PMID: 16506524
- Torchilin V. Antibody-modified liposomes for cancer chemotherapy. Expert Opin Drug Deliv 2008; 5:1003 - 1025; PMID: 18754750; http://dx.doi.org/10.1517/17425247.5.9.1003
- Kirpotin DB, Drummond DC, Shao Y, Shalaby MR, Hong K, Nielsen UB, et al. Antibody targeting of long-circulating lipidic nanoparticles does not increase tumor localization but does increase internalization in animal models. Cancer Res 2006; 66:6732 - 6740; PMID: 16818648; http://dx.doi.org/10.1158/0008-5472.CAN-05-4199
- Park JW, Benz CC, Martin FJ. Future directions of liposome- and immunoliposome-based cancer therapeutics. Semin Oncol 2004; 31:196 - 205; PMID: 15717745; http://dx.doi.org/10.1053/j.seminoncol.2004.08.009
- Karanth H, Murthy RS. pH-sensitive liposomes—principle and application in cancer therapy. J Pharm Pharmacol 2007; 59:469 - 483; PMID: 17430630; http://dx.doi.org/10.1211/jpp.59.4.0001
- Koning GA, Morselt HW, Gorter A, Allen TM, Zalipsky S, Scherphof GL, et al. Interaction of differently designed immunoliposomes with colon cancer cells and Kupffer cells. An in vitro comparison. Pharm Res 2003; 20:1249 - 1257; PMID: 12948023; http://dx.doi.org/10.1023/A:1025009300562
- Nellis DF, Ekstrom DL, Kirpotin DB, Zhu J, Andersson R, Broadt TL, et al. Preclinical manufacture of an anti-HER2 scFv-PEG-DSPE, liposome-inserting conjugate. 1. Gram-scale production and purification. Biotechnol Prog 2005; 21:205 - 220; PMID: 15903260; http://dx.doi.org/10.1021/bp049840y
- Mumbengegwi DL, Allen TM. Liposomes targeted via two different antibodies: assay, B-cell binding and cytotoxicity. Biochim Biophys Acta 2005; 1711:25 - 32; PMID: 15904660
- Saul JM, Annapragada AV, Bellamkonda RV. A dual-ligand approach for enhancing targeting selectivity of therapeutic nanocarriers. J Control Release 2006; 114:277 - 287; PMID: 16904220; http://dx.doi.org/10.1016/j.jconrel.2006.05.028
- Quan CY, Chang C, Wei H, Chen CS, Xu XD, Cheng SX, et al. Dual targeting of a thermosensitive nanogel conjugated with transferrin and RGD-containing peptide for effective cell uptake and drug release. Nanotechnology 2009; 20:335101; PMID: 19636104; http://dx.doi.org/10.1088/0957-4484/20/33/335101
- Meng S, Su B, Li W, Ding Y, Tang L, Zhou W, et al. Enhanced antitumor effect of novel dual-targeted paclitaxel liposomes. Nanotechnology 2010; 21:415103; PMID: 20852356; http://dx.doi.org/10.1088/0957-4484/21/41/415103
- Kluza E, van der Schaft DWJ, Hautvast PAI, Mulder WJM, Mayo KH, Griffioen AW, et al. Synergistic targeting of alphavbeta3 integrin and galectin-1 with heteromultivalent paramagnetic liposomes for combined MR imaging and treatment of angiogenesis. Nano Lett 2010; 10:52 - 58; PMID: 19968235; http://dx.doi.org/10.1021/nl902659g
- Murase Y, Asai T, Katanasaka Y, Sugiyama T, Shimizu K, Maeda N, et al. A novel DDS strategy, “dual-targeting”, and its application for antineovascular therapy. Cancer Lett 2010; 287:165 - 171; PMID: 19616372; http://dx.doi.org/10.1016/j.canlet.2009.06.008
- Friedman M, Lindström S, Ekerljung L, Andersson-Svahn H, Carlsson J, Brismar H, et al. Engineering and characterization of a bispecific HER2 x EGFR-binding affibody molecule. Biotechnol Appl Biochem 2009; 54:121 - 131; PMID: 19492986; http://dx.doi.org/10.1042/BA20090096