 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Chromatin remodelers translocate nucleosomes along the DNA chain in an ATP-dependent manner. This catalytic activity is particularly important for DNA replication and repair since both processes require a significant amount of nucleosome translocations and assembly during DNA synthesis. Recently, we have studied the mobility and interactions of the human ISWI family chromatin remodelers Snf2H and Snf2L as well as Acf1, one of the non-catalytic subunits present in the ACF and CHRAC complexes of Snf2H. We proposed that these protein complexes identify their nucleosomal substrates via a continuous sampling mechanism. It rationalizes the relatively high nuclear mobility and abundance observed for all ISWI proteins in terms of fast target location. According to our model a certain type of ISWI complex visits a given nucleosome in the human genome on the timescale of several seconds to a few minutes. Here, we show that the ISWI proteins Snf2H, Snf2L as well as Acf1 accumulate at UV-induced DNA damage sites within seconds and reach a plateau after a few minutes. These findings corroborate the predictions of the continuous sampling mechanism as an efficient way for targeting chromatin remodelers to sites in the genome that require their activity. In comparison to the mobility of PCNA (proliferating cell nuclear antigen) that also accumulates at DNA repair sites the specifics of substrate location by chromatin remodelers are further characterized.
Introduction
ATP-driven chromatin remodelers are instrumental for regulating the access of other protein factors to the information encoded in the DNA sequence. Their ATP-coupled activity repositions or evicts nucleosomes from the DNA so that nonhistone proteins can gain access to the DNA. Although chromatin remodelers have been extensively studied in vitro, much less is known about how they operate in their native environment, where a dedicated network for establishing and maintaining specific nucleosome positioning patterns exists. By using a combination of advanced fluorescence microscopy and spectroscopy techniques we recently investigated the mobility and chromatin interactions of ISWI-type chromatin remodelers in living cells.Citation1 The study yielded short interaction times of Snf2H and Snf2L with their chromatin substrate with average residence times in the range of 10–150 ms. The concentrations of the endogenous proteins amounted to roughly 1 µM. Based on these data we proposed a “continuous sampling model”: under normal conditions during G1 phase a given class of chromatin remodeling complexes continuously probes all nucleosomes of the genome within seconds to minutes in transient binding reactions to read out signals that mark them for translocation. Most of these binding events do not lead to repositioning, which means that nucleosomes stay at their positions most of the time. These findings point to a tight regulation of chromatin accessibility, realized by nucleosomes that are continuously sampled by chromatin remodelers and are only translocated if specific recruitment signals are present. In contrast, extensive remodeling activity is required at replication foci in S phase or at DNA repair sites. Accordingly, the average residence time of ISWI remodelers increased to seconds and minutes at these sites.Citation1 This change in binding activity is in line with a ‘release mechanism’ proposed previously based on in vitro experiments:Citation2 Nucleosomes that are to be translocated by a given chromatin remodeling complex display a higher binding affinity than those at positions reached at the end points of the remodeling reaction.
Here, we extend the approach used in our previous work to investigate the kinetics of ISWI-type remodelers accumulating at DNA repair sites induced by UV irradiation. In this manner we can test the prediction of fast target location on the second to minute time scale. Mammalian cells have specialized pathways to respond to DNA damage.Citation3 Since assembly of DNA repair proteins and subsequent DNA synthesis requires the translocation and (dis)assembly of nucleosomes, the activity of chromatin remodelers is needed. Remodelers of different types like SWI/SNF complexes,Citation4,Citation5 RSC,Citation6 the ISWI complexes WICHCitation7 and ACF/CHRAC,Citation8 the CHD remodeler Chd4,Citation9,Citation10 or the INO80/Swr1 complexCitation11–Citation15 have been reported to be enriched at sites of DNA repair. Here, we show that Snf2H, Snf2L and Acf1 are recruited to DNA repair sites with similar time kinetics. Accumulation started some seconds after induction of the damage and reached a plateau after 2–3 minutes. In contrast, proliferating cell nuclear antigen (PCNA) accumulated slightly earlier than the ISWI remodelers and no plateau was reached after 3 minutes. Furthermore, the concentration of soluble Snf2H-PCNA complexes was insignificant. This suggests that recruitment of PCNA to DNA damage sites does not occur simultaneously with the ISWI-type remodelers studied here.
Snf2H-GFP Rescues the Snf2H-Knockout Phenotype in U2OS Cells
For mobility measurements in living cells via fluorescence fluctuation microscopy of fluorescently tagged proteins the effect of the fusion on the protein's activity has to be assessed. In our recent work we found that the Snf2H-GFP construct behaved like endogenous Snf2H regarding intracellular localization, interaction with its Acf1 subunit and its in vitro nucleosome translocation activity.Citation1 To further test if the tagged protein is fully functional in living cells, a complementation approach was used here to rescue the S phase arrest reported previously for knockdown of endogenous Snf2HCitation16 (). Stable U2OS cell lines were constructed that carried a plasmid containing the coding sequence for Snf2H-GFP with three neutral mutations so that it was unaffected by the siRNA knockdown of the wild type protein. As shown in , siRNA transfection led to an almost complete depletion of endogenous Snf2H but did not decrease Snf2H-GFP protein levels, while a control siRNA had no effect. To assess the phenotype caused by Snf2H depletion, cells were synchronized at the G1/S border 48 hours after siRNA transfection and were analyzed by FACS 8 hours after having been released from their cell cycle block. As reported previously in reference Citation16, Snf2H-depleted cells progressed slower through S phase compared to untreated cells (). This wild type phenotype could be rescued with Snf2H-GFP as shown for two U2OS cell line clones (X1 and X3) expressing the protein at different levels ( and E), demonstrating that Snf2H-GFP is functional in its cellular context. To quantify relative activities, the Snf2H-GFP expression levels in the two U2OS clones were estimated using quantitative western blots. Average ratios between GFP-tagged and endogenous Snf2H of approximately 1.1 in the clone with low expression level (X1) and of 3.4 in the clone with moderate expression level (X3, ) were obtained. Both clones were further characterized by fluorescence correlation spectroscopy (FCS) () and flow cytometry (FCM) () analyses. In these experiments, the ratio between the Snf2H-GFP expression levels of clone X1 and X3 amounted to roughly 2.1 ± 0.5 (FCS) and 1.5 (FCM). Thus, clone X3 had a 2 to 3-fold higher expression level than clone X1. When comparing their cell cycle profiles it appeared that full rescue of the Snf2H knockdown phenotype required somewhat higher average concentrations of the GFP-tagged Snf2H construct as compared to the endogenous one ( and E). This points to a slightly reduced interaction affinity of Snf2H-GFP with cellular binding partners due to the GFP-tag. It is noted, however, that the heterogeneity with respect to the Snf2H-GFP expression level is considerable in the two clones (), and larger than that of the endogenous proteins as judged from comparison with immunostained wild type cells (data not shown). Thus, both the X1 and the X3 clones contain a significant cell fraction that expresses Snf2H-GFP at levels well below the endogenous Snf2H protein concentration of 0.83 ± 0.13 µM measured previously in reference Citation1. This could result in an incomplete rescue in this fraction of cells even at an average expression level of Snf2H-GFP above that of the endogenous protein.
Snf2H, Snf2L and Acf1 are Rapidly Recruited to DNA Damage Sites
One way to test the efficiency of chromatin remodeler recruitment via the proposed continuous sampling mechanism is the kinetic analysis of remodeler binding to de novo generated interaction sites. Here, we used DNA damage sites induced via UV laser microirradiation as a prototypic system: By illuminating a small spot in the cell nucleus with high laser power at 405 nm for a few seconds, a large number of DNA lesions are produced, to which repair factors including chromatin remodelers are subsequently recruited.Citation1,Citation8,Citation17 Corresponding time series for GFP-tagged Snf2H, Snf2L, the inactive splice variant Snf2L+13 and Acf1 are shown in . As a marker for the DNA damage site PCNA-RFP was used. Remarkably, all proteins including the Acf1 subunit of the ACF/CHRAC complex accumulated at the repair sites. This supports the conclusion that these sites require the activity of a large number of different chromatin remodeling complexes to allow for DNA repair and subsequent re-establishment of the original nucleosome organization at the damage site.
Our previous calculations of the sampling rates predicted that remodeler recruitment should occur on the time scale of several seconds to a few minutes. This behavior was indeed observed in the experiments shown in as evident from the accumulation curves that followed a single species rate process with sampling rates of 2–3 min−1 (see Materials & Methods section). PCNA was recruited to the damage site with similar kinetics but accumulated 5–20 seconds earlier. In contrast to the chromatin remodelers, no plateau phase was reached within the observation period of 3 minutes. The kinetics of both PCNA and remodeler recruitment were similar in G1/2 phase and S phase of the cell cycle (data not shown), although different repair pathways are active in different stages of the cell cycle. One level of regulation are the cell cyclespecific posttranslational modifications of PCNA like sumoylation during S phase.Citation18 The independence of recruitment kinetics and cell cycle suggests that preexisting PCNA modifications do not play a major role in the initial recruitment phase of PCNA or chromatin remodelers to DNA damage sites. To compare the recruitment kinetics of the different remodelers to each other, cells cotransfected with GFP-Snf2L/RFP-Snf2H or GFP-Snf2L/RFP-Snf2L+13 were analyzed as described above. The ratios between the different remodelers and with PCNA were plotted versus time (): Between remodelers this parameter remained constant, indicating simultaneous recruitment of Snf2H, Snf2L and Snf2L+13 to the damage site (due to more efficient bleaching of GFP than of RFP during UV microirradiation, the ratio before and after damage induction is different). In contrast, the ratio between the accumulation curves for the GFP-tagged remodelers and PCNA-RFP increased linearly during the measurement time of 3 minutes (). Thus, both the 5–20 sec delay between PCNA and remodeler binding as well as the different recruitment kinetics indicate that both factors bind as separate protein complexes to the damage site.
PCNA and Snf2H are not Detected in Soluble Complexes
ISWI remodeling complexes colocalize with PCNA at DNA damage sites and at DNA replication foci during S phase. In addition, the WSTF subunit of the Snf2H-containing WICH complex has previously been reported to mediate interaction between Snf2H and PCNA.Citation19 To directly test if soluble PCNA-Snf2H complexes are present in the nucleoplasm, a fluorescence cross-correlation spectroscopy (FCCS) interaction analysis was conducted. U2OS cells stably expressing Snf2H-GFP were transiently transfected with PCNA-RFP. As a control, wild type U2OS cells were transfected with PCNA-RFP and PCNA-GFP. Representative correlation curves are shown in . A robust interaction between PCNA-GFP and PCNA-RFP was detected in the nucleus and the cytoplasm at PCNA-GFP/RFP concentrations of approximately 3 µM or 300 nM, respectively. This is consistent with previous reports that PCNA forms trimers at nanomolar concentrations.Citation20 In contrast, the FCCS signal for the interaction between PCNA-RFP and Snf2H-GFP was only slightly above background. This argues against the presence of a significant fraction of a stable soluble complex of the two proteins in the nucleus, e.g., via WSTF-mediated interaction.
PCNA and ISWIs Adopt Different Strategies for Target Location
The diffusive mobility in the absence of chromatin interactions of both Snf2H and PCNA can be derived from the FCS measurements in the cytoplasm where almost identical values of Deff ≈ 13 µm2s−1 were determined for both proteins (). In the nucleus Snf2H and PCNA displayed different dynamic properties (): As described previously, the FCS analysis of Snf2H displayed two components in the autocorrelation function (Deff,1 ≈ 3 µm2s−1, Deff,2 ≈ 0.02 µm2s−1).Citation1 The very slow component specific for Snf2H can be explained by chromatin fiber movements that are associated with transiently bound Snf2H molecules.Citation21 Although Snf2H residence times at chromatin are short, a certain number of Snf2H molecules are always associated with a given part of the chromatin fiber in steady state, generating an additional component in the correlation function. The autocorrelation function of PCNA also displayed two components (Deff,1 ≈ 10 µm2s−1, Deff,2 ≈ 0.8 µm2s−1); however, in contrast to Snf2H the components were not well separable. The slow PCNA component is unlikely to represent chromatin dynamics since the diffusion coefficient was too large and the anomaly parameter was α ≈ 0.7 (for confined diffusion of chromatin fibers, an anomaly parameter larger than 1 is expected in the FCS fit of the data as observed for Snf2H with α = 2.1 ± 0.2,Citation1,Citation21). We conclude that the equilibrium density of bound PCNA molecules along the chromatin fiber is insufficient to be detected in the correlation function. This suggests that, in the absence of DNA repair/replication, binding sites for PCNA on chromatin are sparse. The two components from the FCS analysis are consistent with PCNA diffusing freely through the nucleus most of the time and occasional interactions with chromatin, which might include one-dimensional sliding along the DNA.Citation22
The mobility differences between Snf2H and PCNA were readily apparent in the FRAP experiments (). PCNA recovered faster than Snf2H (Deff ≈ 11 µm2s−1 vs. Deff ≈ 1 µm2s−1) and displayed no immobile fraction during G1/2 phase in the absence of DNA damage. This is in line with the results obtained previously in reference Citation23 and Citation24. The Deff value approaches the value that was observed for PCNA or ISWIs in the cytoplasm (Deff ≈ 13 µm2s−1) in the absence of chromatin binding sites. This indicates that nuclear mobility of PCNA is predominantly diffusive without chromatin binding unless specific recruitment or loading factors are present. The diffusion coefficient from the FRAP experiments is consistent with the FCS values if particles move freely with Deff ≈ 13 µm2s−1 about 80% of the time and with a reduced value of Deff ≈ 0.5 µm2s−1 the rest of the time. If particles change their mode of motion or binding behavior on length scales that are between those of FCS (∼0.3 µm) and FRAP (∼3 µm), two components appear in the FCS curves. However, only one pool translocating with the average diffusion coefficient (equal to the weighted average of the two FCS components) is retrieved from the FRAP analysis. Thus, the above mobility analysis suggests that ISWI proteins can potentially bind every nucleosome, albeit the vast majority with relatively low affinity. In contrast, PCNA binds to chromatin only very rarely in the absence of DNA repair/replication activity, since the density of its binding sites is low under these conditions.
A Model for ISWI Remodeler Recruitment to Nascent DNA Repair Sites
Our results identify ISWI chromatin remodeling complexes as a part of the early DNA damage response. They accumulate within tens of seconds, but 5–20 sec later than PCNA, which is loaded onto DNA as a clamp around the DNA double helix by replication factor C (RFC).Citation25 The latter protein has also been found to be present at DNA damage sites at very early time points.Citation26 However, it is noted that chromatin binding of PCNA to DNA repair sites can be mediated also by other factors like XPG and RPA independently of RFC and prior to the subsequent clamp loading process.Citation27 Thus, the accumulation of PCNA could include two or more binding steps with different kinetics that are not resolved in our experiments. The mechanistic conclusions from our work are depicted in the scheme shown in . The initial DNA damage signaling events lead to the binding of PCNA by a recruitment and/or loading factor like RFC, XPG or RPA. The resulting complex could target ISWI chromatin remodelers to nascent DNA damage foci in conjunction with other proteins. A previous study reported that the WSTF subunit of the Snf2H-containing WICH complex interacts directly with PCNA.Citation19 Our finding that only a very minor fraction of complexes containing both Snf2H and PCNA was present in U2OS cells suggests that the initial Snf2H recruitment occurs to a large extent independently of preformed PCNA-WICH complexes. This argues for the PCNA-chromatin complex acting as a binding platform for several factors with high turnover rates that are likely to involve also WSTF-mediated interactions between PCNA and Snf2H.Citation24,Citation28,Citation29 Other previously reported chromatin remodeler binding partners at DNA repair sites are the phosphorylated form of histone H2A.X (γH2A.X),Citation5,Citation13–Citation15 as well as the Ku70/80 proteinsCitation6,Citation8 that mark DNA double strand breaks for the DNA repair machinery. Interestingly, mammalian SWI/SNF complexes appear to promote γH2A.X induction at DSBs.Citation4,Citation5 This points to the existence of a feedback loop, in which γH2A.X phosphorylation is both enhanced by chromatin remodeling complexes and at the same time promotes their accumulation at repair sites. In support of this view, H2A.X phosphorylation has been reported to occur within some seconds to minutes after damage induction,Citation30 which is the same timescale observed here for ISWI recruitment. Both PCNA and the ISWI remodelers are very mobile and are present at relatively high micromolar nuclear concentrations. It is also note-worthy that the concentration of PCNA increases only about 2-fold from 0.8 µM in G1 to 1.2 µM in S phase.Citation31 Thus, under normal conditions during G1 phase the protein is surprisingly abundant. This can be explained in terms of the requirement for fast binding to DNA damage sites also during G1 and G2 phase. As discussed previously for the ISWI remodelers the combination of high protein concentrations and short chromatin interaction times leads to a fast location of nuclear targets within tens of seconds response time.Citation1 This could be a characteristic feature of the search mechanism employed by a number of chromatin interacting proteins.Citation32–Citation34 However, while both PCNA and ISWI remodelers fit into this scheme some specific features of chromatin remodelers can be identified from the comparative mobility study conducted here. In contrast to PCNA, ISWIs thoroughly probe a large number of nucleosomes by a continuous sampling mechanism, thus ensuring that potential targets are reliably identified. Interestingly, ISWI-type remodelers have been proposed to translocate nucleosomes according to a kinetic proof reading scheme,Citation35,Citation36 which involves the ATP-dependent generation of a high-energy intermediate. In such a model a certain time is required to decide if productive translocation should take place or if the remodeler should dissociate. Although the average time for ATP hydrolysis was estimated to equal about one second in in vitro experiments,Citation37,Citation38 the first ATP hydrolysis step could occur on the 100 ms scale in the native cellular environment. Together with a residence time on a similar time scale this would allow for a more efficient discrimination between correct and incorrect substrates as compared to the estimates reported previously in reference Citation37. Furthermore, the 100 ms timescale would be comparable to our estimates for the average ISWI remodeler residence time at chromatin in G1/2 phase of the cell cycle, suggesting the possibility that at least some binding events could be connected to the generation of a high-energy intermediate. In the absence of ATP, the lack of the proof reading step could lead to the slight mobility increase (larger Deff values) observed previously in reference Citation1. Thus, the continuous sampling model in conjunction with a potential proof reading step might be a characteristic feature of ISWI-type remodeling complexes. It could explain a reduced nuclear mobility as compared to PCNA, in which the chromatin target reaction would involve only binding to an appropriate recruitment/loading factor.
Materials and Methods
U2OS cells and GFP constructs of ISWI proteins were used as described previously in reference Citation1. To generate the plasmid coding for RFP-tagged Snf2L + 13, the coding sequence of Snf2L + 13 was cut out from GFP-Snf2L + 13 and ligated into pTagRFP-C (Evrogen). The plasmid coding for Snf2H-GFP was subjected to site-directed mutagenesis to introduce three neutral mutations in the region targeted by the siRNA against Snf2H used by Varga-Weisz and colleagues.Citation16 To this end, the plasmid sequence 5′-AAG AGG AGG ATG AAG AGC TAT-3′ was replaced by 5′-AAG AGG AGG ACG AGG AGC TCT-3′. Since both codons GAT/GAC code for aspartic acid (D), GAA/GAG code for glutamic acid (E) and CTA/CTC code for leucine (L), the Snf2H protein sequence remains unchanged. For Snf2H knockdown experiments siRNA (Ambion) was transfected at a concentration of 50 nM using Lipofectamine 2000 (Invitrogen) according to the manufacturer's protocols. The siRNA against Snf2H had the target sequence given above (the same as used by Collins et al.Citation16). A scrambled siRNA was used as negative control. For cell cycle analysis cells were synchronized at the G1/S border by supplementing the growth medium with 3 µg/ml aphidicolin for 24 hours. Progression through the cell cycle was followed by flow cytometry using a FACSCalibur instrument (BD Biosciences) after fixing the cells with ethanol and staining with 0.25 µM TO-PRO3 and an antibody against GFP. Data were analyzed with the software Weasel (WEHI).
FRAP, FCS and FCCS experiments and data analysis were conducted as described previously in reference Citation1. For UV microirradiation, the region of interest was illuminated with a 405 nm laser at full power for 4 frames (4-fold line average), resulting in a net illumination time of roughly 0.1 ms per pixel. The recruitment curves were fitted to an irreversible pseudo-first-order rate process:
Here, I0 is the maximum accumulation, ksample is the effective sampling rate constant and t0 is a time offset corresponding to the start of the recruitment.
Figures and Tables
Figure 1 Snf2H-GFP rescues the Snf2H-KO phenotype. (A) To verify that the siRNA against Snf2H targets only endogenous Snf2H, cell lysates from untreated cells and cells transfected with siRNA against Snf2H (KO) or control siRNA (ctrl) were analyzed in a Western blot. (B) Initial cell cycle profiles of siRNA-transfected U2OS cells were synchronized at the G1/S border. (C) Profiles 8 h after release from the cell cycle block. (D) U2OS clone X1 with neutrally mutated Snf2H-GFP 8 h after release from the cell cycle block. (E) Same as panel D but for clone X3 that has a 2–3 fold higher Snf2H-GFP expression level. The Snf2H-KO phenotype evident in panel C is partially complemented in the X1 clone and almost fully complemented in the X3 clone. (F) Determination of Snf2H-GFP concentrations from the amplitude of the FCS curves. (G) Histograms of GFP intensity signals determined by flow cytometry for the X1 and X3 clones. Arrows indicate the weighted averages of the two distributions.
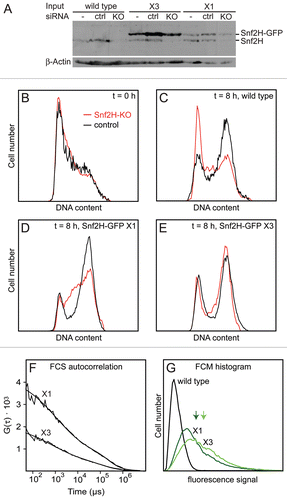
Figure 2 Snf2H, Snf2L, Snf2L+13 and Acf1 are recruited to DNA damage sites. (A) The kinetics of recruiting Snf2H, Snf2L, inactive Snf2L+13, Acf1 and PCNA to UV-induced DNA damage sites were monitored over time. Averaged curves were fitted to a single species rate process. Scale bar: 5 µm. (B) The ratio between different ISWI remodelers or the ratio between PCNA and the ISWI remodelers was plotted over time for cells co-transfected with the given protein combinations. Whereas the ratio between the different remodelers was constant, the ratio between PCNA and the remodelers increased linearly during the observation time of 3 minutes.
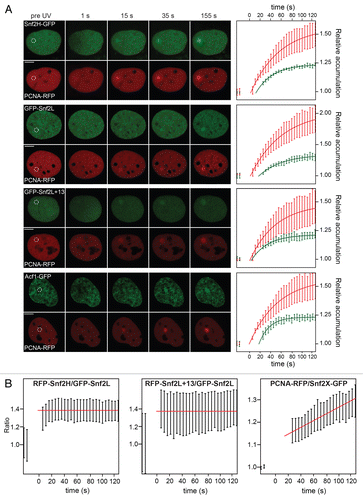
Figure 3 Snf2H and PCNA are not detected in soluble complexes. U2OS cells were co-transfected either with Snf2H-GFP/PCNA-RFP or with PCNA-GFP/PCNA-RFP and the presence of two-color labeled complexes was evaluated by FCCS. While no Snf2H-PCNA complexes were detected (left part), PCNA-PCNA interactions were present in the cytosol as well as in the nucleus (right part).
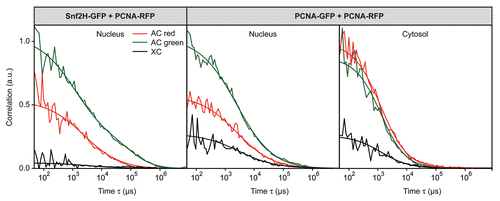
Figure 4 Mobility of Snf2H and PCNA. The mobility of Snf2H-GFP and PCNA-RFP in U2OS cells was compared. (A) The FCS analysis revealed a reduced PCNA and Snf2H mobility in the nucleus with respect to the cytosol. (B) In FRAP experiments PCNA-RFP recovered faster than Snf2H-GFP and no immobile PCNA fraction was present in G1/2 phase cells.
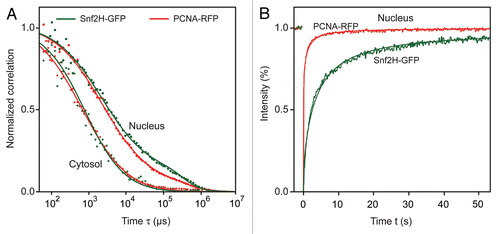
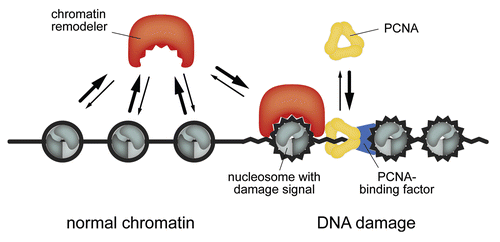
Figure 5 A model for ISWI recruitment to nascent DNA damage sites. ISWI remodelers bind transiently to chromatin and search their targets via continuous sampling. The vast majority of the proteins (.96%) is in the unbound state. Efficient target location is ensured by the relatively large concentrations in the µM range and the rather short residence times at the chromatin substrate of below 150 ms. This leads to the detection of DNA damage sites within tens of seconds after their appearance. PCNA diffuses even faster than ISWI remodelers, binds less frequently to chromatin and accumulates 5–20 seconds earlier than the ISWIs at DNA damage sites. The PCNA-chromatin complex could stabilize the interaction between ISWIs and nucleosomes carrying additional damage signals via direct protein-protein interactions.
Acknowledgements
We are grateful to Carolin Bohm, Philipp Mallm and Steffen Schmitt for their help and thank Patrick Varga-Weisz, Cristina Cardoso and Gernot Längst for discussions. Part of the fluorescence microscopy work was conducted at the DKFZ Microscopy Core Facility. This work was supported by DFG grant Ri 1283/8-1.
Extra View to: Erdel F, Schubert T, Marth C, Längst G, Rippe K. Human ISWI chromatin-remodeling complexes sample nucleosomes via transient binding reactions and become immobilized at active sites. Proc Natl Acad Sci USA 2010; 107:19873 - 19878; PMID: 20974961; http://dx.doi.org/10.1073/pnas.1003438107
References
- Erdel F, Schubert T, Marth C, Langst G, Rippe K. Human ISWI chromatin-remodeling complexes sample nucleosomes via transient binding reactions and become immobilized at active sites. Proc Natl Acad Sci USA 2010; 107:19873 - 19878
- Rippe K, Schrader A, Riede P, Strohner R, Lehmann E, Langst G. DNA sequence- and conformation-directed positioning of nucleosomes by chromatin-remodeling complexes. Proc Natl Acad Sci USA 2007; 104:15635 - 15640
- Jackson SP, Bartek J. The DNA-damage response in human biology and disease. Nature 2009; 461:1071 - 1078
- Park JH, Park EJ, Lee HS, Kim SJ, Hur SK, Imbalzano AN, et al. Mammalian SWI/SNF complexes facilitate DNA double-strand break repair by promoting gamma-H2AX induction. EMBO J 2006; 25:3986 - 3997
- Lee HS, Park JH, Kim SJ, Kwon SJ, Kwon J. A cooperative activation loop among SWI/SNF, gamma-H2AX and H3 acetylation for DNA double-strand break repair. EMBO J 2010; 29:1434 - 1445
- Shim EY, Ma JL, Oum JH, Yanez Y, Lee SE. The yeast chromatin remodeler RSC complex facilitates end joining repair of DNA double-strand breaks. Mol Cell Biol 2005; 25:3934 - 3944
- Xiao A, Li H, Shechter D, Ahn SH, Fabrizio LA, Erdjument-Bromage H, et al. WSTF regulates the H2A.X DNA damage response via a novel tyrosine kinase activity. Nature 2009; 457:57 - 62
- Lan L, Ui A, Nakajima S, Hatakeyama K, Hoshi M, Watanabe R, et al. The ACF1 complex is required for DNA double-strand break repair in human cells. Mol Cell 2010; 40:976 - 987
- Smeenk G, Wiegant WW, Vrolijk H, Solari AP, Pastink A, van Attikum H. The NuRD chromatin-remodeling complex regulates signaling and repair of DNA damage. J Cell Biol 2010; 190:741 - 749
- Larsen DH, Poinsignon C, Gudjonsson T, Dinant C, Payne MR, Hari FJ, et al. The chromatin-remodeling factor CHD4 coordinates signaling and repair after DNA damage. J Cell Biol 2010; 190:731 - 740
- Kashiwaba SI, Kitahashi K, Watanabe T, Onoda F, Ohtsu M, Murakami Y. The mammalian INO80 complex is recruited to DNA damage sites in an ARP8 dependent manner. Biochem Biophys Res Commun 2010; 402:619 - 625
- Jiang Y, Wang X, Bao S, Guo R, Johnson DG, Shen X, et al. INO80 chromatin remodeling complex promotes the removal of UV lesions by the nucleotide excision repair pathway. Proc Natl Acad Sci USA 2010; 17274 - 17279
- Morrison AJ, Highland J, Krogan NJ, Arbel-Eden A, Greenblatt JF, Haber JE, et al. INO80 and gamma-H2AX interaction links ATP-dependent chromatin remodeling to DNA damage repair. Cell 2004; 119:767 - 775
- van Attikum H, Fritsch O, Gasser SM. Distinct roles for SWR1 and INO80 chromatin remodeling complexes at chromosomal double-strand breaks. EMBO J 2007; 26:4113 - 4125
- van Attikum H, Fritsch O, Hohn B, Gasser SM. Recruitment of the INO80 complex by H2A phosphorylation links ATP-dependent chromatin remodeling with DNA double-strand break repair. Cell 2004; 119:777 - 788
- Collins N, Poot RA, Kukimoto I, Garcia-Jimenez C, Dellaire G, Varga-Weisz PD. An ACF1-ISWI chromatin-remodeling complex is required for DNA replication through heterochromatin. Nat Genet 2002; 32:627 - 632
- Mortusewicz O, Schermelleh L, Walter J, Cardoso MC, Leonhardt H. Recruitment of DNA methyl-transferase I to DNA repair sites. Proc Natl Acad Sci USA 2005; 102:8905 - 8909
- Moldovan GL, Pfander B, Jentsch S. PCNA, the maestro of the replication fork. Cell 2007; 129:665 - 679
- Poot R, Bozhenok L, van den Berg DL, Steffensen S, Ferreira F, Grimaldi M, et al. The Williams syndrome transcription factor interacts with PCNA to target chromatin remodelling by ISWI to replication foci. Nat Cell Biol 2004; 1236 - 1244
- Yao N, Turner J, Kelman Z, Stukenberg PT, Dean F, Shechter D, et al. Clamp loading, unloading and intrinsic stability of the PCNA, beta and gp45 sliding clamps of human, E. coli and T4 replicases. Genes Cells 1996; 1:101 - 113
- Erdel F, Müller-Ott K, Baum M, Wachsmuth M, Rippe K. Dissecting chromatin interactions in living cells from protein mobility maps. Chromosome Res 2011; 19:99 - 115
- Kochaniak AB, Habuchi S, Loparo JJ, Chang DJ, Cimprich KA, Walter JC, et al. Proliferating cell nuclear antigen uses two distinct modes to move along DNA. J Biol Chem 2009; 284:17700 - 17710
- Essers J, Theil AF, Baldeyron C, van Cappellen WA, Houtsmuller AB, Kanaar R, et al. Nuclear dynamics of PCNA in DNA replication and repair. Mol Cell Biol 2005; 25:9350 - 9359
- Solomon DA, Cardoso MC, Knudsen ES. Dynamic targeting of the replication machinery to sites of DNA damage. J Cell Biol 2004; 166:455 - 463
- Bowman GD, O'donnell M, Kuriyan J. Structural analysis of a eukaryotic sliding DNA clamp-clamp loader complex. Nature 2004; 429:724 - 730
- Hashiguchi K, Matsumoto Y, Yasui A. Recruitment of DNA repair synthesis machinery to sites of DNA damage/repair in living human cells. Nucleic Acids Res 2007; 35:2913 - 2923
- Overmeer RM, Gourdin AM, Giglia-Mari A, Kool H, Houtsmuller AB, Siegal G, et al. Replication factor C recruits DNA polymerase delta to sites of nucleotide excision repair but is not required for PCNA recruitment. Mol Cell Biol 2010; 30:4828 - 4839
- Hemmerich P, Schmiedeberg L, Diekmann S. Dynamic as well as stable protein interactions contribute to genome function and maintenance. Chromosome Res 2011; 19:131 - 151
- Sporbert A, Domaing P, Leonhardt H, Cardoso MC. PCNA acts as a stationary loading platform for transiently interacting Okazaki fragment maturation proteins. Nucleic Acids Res 2005; 33:3521 - 3528
- Rogakou EP, Pilch DR, Orr AH, Ivanova VS, Bonner WM. DNA double-stranded breaks induce histone H2AX phosphorylation on serine 139. J Biol Chem 1998; 273:5858 - 5868
- Morris GF, Mathews MB. Regulation of proliferating cell nuclear antigen during the cell cycle. J Biol Chem 1989; 264:13856 - 13864
- Wachsmuth M, Caudron-Herger M, Rippe K. Genome organization: Balancing stability and plasticity. Biochim Biophys Acta 2008; 1783:2061 - 2079
- Hager GL, McNally JG, Misteli T. Transcription dynamics. Mol Cell 2009; 35:741 - 753
- van Royen ME, Zotter A, Ibrahim SM, Geverts B, Houtsmuller AB. Nuclear proteins: finding and binding target sites in chromatin. Chromosome Res 2010;
- Blossey R, Schiessel H. Kinetic proofreading of gene activation by chromatin remodeling. HFSP J 2008; 2:167 - 170
- Narlikar GJ. A proposal for kinetic proof reading by ISWI family chromatin remodeling motors. Curr Opin Chem Biol 2010; 14:660 - 665
- Corona DF, Langst G, Clapier CR, Bonte EJ, Ferrari S, Tamkun JW, et al. ISWI is an ATP-dependent nucleosome remodeling factor. Mol Cell 1999; 3:239 - 245
- He X, Fan HY, Narlikar GJ, Kingston RE. Human ACF1 alters the remodeling strategy of SNF2 h. J Biol Chem 2006; 281:28636 - 28647