Abstract
TRAIL selectively kills cancer cells while bispecific antibody EpCAMxCD3 guides effector lymphocytes to cancer cells. Arming of ex vivo constructed TRAIL-lymphocytes with EpCAMxCD3 enhances contact time and affinity between lymphocytes and tumor cells and enforces tumor elimination. This boosts endogenous immune responses and augments the effect of cytotoxic tumor therapy.
Epithelial cell adhesion molecule (EpCAM; ESA; CD326) was defined as a cancer stem cell marker (CSC) and is frequently overexpressed and functionally altered by the majority of epithelial tumors and derived metastases. Although several normal tissues bear considerable levels ofl EpCAM the binding epitopes are suggested to be largely covered in normal but available in malignant tissue.Citation1 Therefore, EpCAM is suggested as an attractive target for cancer immunotherapy e.g., by bispecific antibodies (bsAb). A prominent example for an EpCAM-directed bsAb represents the trifunctional antibody catumaxomab (Removab®, Fresenius Biotech), which binds T cells via CD3 and Fcγ receptor I/III-positive accessory immune cells to EpCAM-positive tumor cells. Catumaxomab is approved in Europe for reduction of malignant ascites in palliative settings.Citation2
We constructed the bsAb EpCAMxCD3, which is directed against the T-cell receptor-CD3 complex on lymphocytes and EpCAM accessible on tumor cells (). We demonstrated the effectiveness of EpCAMxCD3 in an experimental system and found redirection of T cells to EpCAM-positive tumor cells of pancreas and prostate. This was associated with increased duration of contacts between T cells and tumor cells and followed by the release of Granzyme B, Perforin, IFNγ, TNFα, IL-10 and TGFβ in xenograft tumors.Citation3 Importantly, intraperitoneal application of our bsAb reduced malignant ascites in patients with advanced ovarian cancer,Citation4 suggesting that even late stages of cancer are susceptible. This finding is further supported by EpCAMxCD3-mediated elimination of drug-resistant established pancreatic cancer cells with CSC characteristics.Citation5 Just now, these data were confirmed by Cioffi and colleagues who demonstrated elimination of primary pancreatic CSC using a recombinant EpCAMxCD3 antibody.Citation6
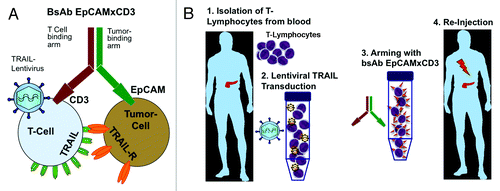
Figure 1. Cartoon of gene-immunotherapy with bispecific antibody EpCAMxCD3 and TRAIL-overexpressing lymphocytes. (A) BsAb EpCAMxCD3 binds cytotoxic T cells via CD3 and cancer cells via EpCAM. This leads to activation of cytotoxic T cells, targeting to EpCAM-overexpressing cancer cells and to an increase of contact time and binding affinity between T cells and cancer cells resulting in tumor cell lysis. This anti-tumor effect was further enhanced by lentivirus-mediated overexpression of membrane TRAIL in T-lymphocytes. TRAIL interacts with TRAIL receptors expressed on cancer cells and induces selective apoptosis in malignant, but not in normal cells. Thus, cancer cells are eliminated by a combination of T cell-specific killing via the perforin granzyme system and TRAIL-induced apoptosis, which is further enhanced by EpCAMxCD3-mediated prolonged contact time and synapse formation between TRAIL-lymphocytes and tumor cells. (B) The scenario for future therapeutic application in patients may look like follows: (1) T-lymphocytes are isolated from an individual patient followed by (2) ex vivo transduction with TRAIL lentiviral vector for overexpression of membrane-bound TRAIL (3) systemic re-injection of manipulated T cells together with bsAb EpCAMxCD3 for tumor-specific targeting.
In order to further increase the anti-tumor-activity of EpCAMxCD3-guided T cells we combined it with tumor necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL) transduction. While resting lymphocytes carry only minimal amounts of TRAIL, expression is enhanced to an intermediate level upon lymphocyte activation. By lentiviral transduction we were able to superinduce expression of membrane (m)TRAIL on the surface of freshly isolated peripheral blood lymphocytes. The main advantage of TRAIL for tumor therapy is its specific toxicity toward malignant cells together with proven low side effects found in Phase I/II clinical studies, in which recombinant soluble (s)TRAIL protein or agonistic antibodies against TRAIL-receptor 1 (mapatumumab) have been evaluated.Citation7 However, only limited TRAIL-induced antitumor activity in patients could be demonstrated, most probably due to short exposure of tumor cells to low TRAIL concentrations and to sTRAIL’s short in vivo half-life. To overcome these limitations we used for the first time lymphocytes as vehicles for membrane TRAIL and combined this approach with EpCAMxCD3-mediated targeted transport of TRAIL-lymphocytes to tumor cells.
Our study revealed that overexpression of mTRAIL in primary human lymphocytes increased T cell cytotoxicity toward cancer cells of pancreas and prostate. Importantly, combination with EpCAMxCD3 potentiated this effect as analyzed in vitro and in vivo. Toxic side effects, such as a decrease of body weight, liver toxicity or metastasis in mice were never observed. Amplification of therapeutic effects may be due to the combination of TRAIL-induced apoptosis, lymphocyte-mediated cell lysis by the granzyme/perforin system along with EpCAMxCD3-mediated extended contact time and increased binding strengthCitation8 between tumor cells and lymphocytes. Importantly, the combined cytotoxicity of TRAIL-lymphocytes and EpCAMxCD3 even reduced the tumor-initiating potential of drug-resistant pancreatic cancer cells with CSC features. Besides direct induction of cell death, anti-angiogenic effects may have contributed to tumor eradication in our system. We observed inhibition of CD31+ tumor vessels in xenograft tumors upon treatment with EpCAMxCD3 or TRAIL-lymphocytes with a most significant effect for combination treatment. Of special interest is the observed formation of large intratumoral liqid-filled cysts in xenografts of mice, which may be mainly due to TRAIL-lymphocytes but which were even more pronounced upon combination with EpCAMxCD3. Since we did not observe cyst formation in xenografts of mice injected with EpCAMxCD3 alone,Citation3 we assume that TRAIL may have induced inflammation and attraction of host responses, since cells undergoing apoptosis release several factors that attract phagocytes, in particular macrophagesCitation9 such as observed in our study. In addition, there is strong evidence that EpCAMxCD3 leads to secretion of multiple chemokines by lymphocytes resulting in inflammation as shown recently.Citation3 In addition, disruption of vasculature due to anti-angiogenic effects of EpCAMxCD3 and TRAIL may have contributed to cyst formation.
A critical question concerns TRAIL resistance of tumor cells, which is frequently observed in around 50% of tumor cell lines. This observation matches with the situation in cell lines used for our study. Upon treatment with TRAIL-lymphocytes, only a small percentage of drug-resistant cancer stem-like cells with active caspase-3 were detected and tumor-initiating potential in mice was not abrogated completely. This suggests that TRAIL-lymphocytes alone do not completely target CSC. However, combination with EpCAMxCD3 strongly increased therapeutic efficacy as demonstrated by treatment of tumor-bearing mice, tumor take experiments in mice and by in vitro experiments.
Regarding translational relevance of local injection of TRAIL-lymphocytes and EpCAMxCD3, our model does not clearly mimic human T cell-based adoptive cancer immunotherapy, where tumor-reactive/specific T lymphocytes are usually systemically administered to patients. Our model rather reflects a proof of concept study, which demonstrates a powerful anti-tumor response by activating both innate and adaptive immune mechanisms. Another important aspect of this gene-immunotherapy may be the “opening” of the tumor microenvironment for conventional cytotoxic therapy due to induction of a sustained inflammatory response.
References
- McLaughlin PM, Harmsen MC, Dokter WH, Kroesen BJ, van der Molen H, Brinker MG, et al. The epithelial glycoprotein 2 (EGP-2) promoter-driven epithelial-specific expression of EGP-2 in transgenic mice: a new model to study carcinoma-directed immunotherapy. Cancer Res 2001; 61:4105 - 11; PMID: 11358833
- Heiss MM, Murawa P, Koralewski P, Kutarska E, Kolesnik OO, Ivanchenko VV, et al. The trifunctional antibody catumaxomab for the treatment of malignant ascites due to epithelial cancer: Results of a prospective randomized phase II/III trial. Int J Cancer 2010; 127:2209 - 21; http://dx.doi.org/10.1002/ijc.25423; PMID: 20473913
- Salnikov AV, Groth A, Apel A, Kallifatidis G, Beckermann BM, Khamidjanov A, et al. Targeting of cancer stem cell marker EpCAM by bispecific antibody EpCAMxCD3 inhibits pancreatic carcinoma. J Cell Mol Med 2009; 13:9B 4023 - 33; http://dx.doi.org/10.1111/j.1582-4934.2009.00723.x; PMID: 20196789
- Marmé A, Strauss G, Bastert G, Grischke EM, Moldenhauer G. Intraperitoneal bispecific antibody (HEA125xOKT3) therapy inhibits malignant ascites production in advanced ovarian carcinoma. Int J Cancer 2002; 101:183 - 9; http://dx.doi.org/10.1002/ijc.10562; PMID: 12209996
- Groth A, Salnikov AV, Ottinger S, Gladkich J, Kallifatidis G, Salnikova O, et al. New gene-immunotherapy combining TRAIL-lymphocytes and EpCAMxCD3 bispecific antibody for tumor targeting. Clin Cancer Res 2012; In press http://dx.doi.org/10.1158/1078-0432.CCR-11-2767; PMID: 22228630
- Cioffi M, Dorado J, Baeuerle PA, Heeschen C. EpCAM/CD3-Bispecific T-cell Engaging Antibody MT110 Eliminates Primary Human Pancreatic Cancer Stem Cells. Clin Cancer Res 2012; 18:465 - 74; PMID: 22096026
- Tolcher AW, Mita M, Meropol NJ, von Mehren M, Patnaik A, Padavic K, et al. Phase I pharmacokinetic and biologic correlative study of mapatumumab, a fully human monoclonal antibody with agonist activity to tumor necrosis factor-related apoptosis-inducing ligand receptor-1. J Clin Oncol 2007; 25:1390 - 5; http://dx.doi.org/10.1200/JCO.2006.08.8898; PMID: 17416859
- Hoffmann SC, Wabnitz GH, Samstag Y, Moldenhauer G, Ludwig T. Functional analysis of bispecific antibody (EpCAMxCD3)-mediated T-lymphocyte and cancer cell interaction by single-cell force spectroscopy. Int J Cancer 2011; 128:2096 - 104; http://dx.doi.org/10.1002/ijc.25556; PMID: 20635391
- Elliott MR, Chekeni FB, Trampont PC, Lazarowski ER, Kadl A, Walk SF, et al. Nucleotides released by apoptotic cells act as a find-me signal to promote phagocytic clearance. Nature 2009; 461:282 - 6; http://dx.doi.org/10.1038/nature08296; PMID: 19741708