Abstract
Recent findings from our laboratory provide the first indication that overexpression of Bcl-2 in Eµ-myc transgenic cells enhances tumor immunosurveillance by inducing NKG2D ligands. Initial evidence suggests that this model is relevant to human patients. Thus, antitumor therapies that target Bcl-2 harbor the risk of reducing tumor immunogenicity.
Tumors arise as a result of disturbances to homeostatic cell proliferation and cell death. B-cell lymphoma 2 (Bcl-2) is an anti-apoptotic protein that has been implicated in numerous types of cancers including melanoma and breast, prostate and lung carcinomas. Deregulation of Bcl-2 alone is not sufficient to cause neoplastic transformation,Citation1 but the overexpression of anti-apoptotic Bcl-2 in combination with oncogenes such as c-Myc may lead to tumor formation. For this reason, and because of its possible involvement in drug resistance, Bcl-2 has been identified as a prime therapeutic target. However, our recent work indicates that there is still much to learn about the role of Bcl-2 in cancer biology.
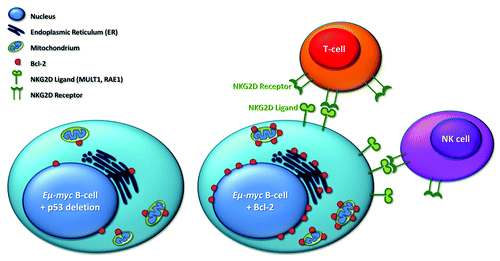
We have been investigating tumor development in a c-Myc-driven B-cell lymphoma model (Eµ-myc). In the resulting tumors, the pro-apoptotic effects of c-Myc are counteracted either by the enforced expression of Bcl-2 or by mutations within the p53 signaling cascade. We found that tumors harboring p53 deletions evolve rapidly, whereas tumors overexpressing Bcl-2 progress with increased latency.Citation2 Interestingly, the difference could be attributed to enhanced immunogenicity of Bcl-2-carrying tumors, resulting in improved tumor cell lysis mediated by natural killer (NK) and T-cells (see ). The immunogenic effect of Bcl-2 is independent of alterations in the p53 signaling cascade, as overexpression of Bcl-2 results in increased immune recognition even in the absence of an intact p53 response.
Figure 1. Eµ-myc B-lymphoid tumor cells overexpressing Bcl-2 are cleared by NK and T-cells more efficiently than Eµ-myc cells with defects in the p53 signaling cascade. This effect could be attributed to the upregulation of NKG2D ligands (RAE1 and MULT1) on lymphoma cells overexpressing Bcl-2, resulting in efficient recognition and eradication by the immune system.
We found no alterations in calreticulin expression on the cell surface, suggesting that Bcl-2 overexpression does not exert its effects via changes in the calcium flux. However, there were elevated levels of the NKG2D ligands MULT1 and RAE1 on the surface of Bcl-2-overexpressing Eµ-myc-transformed B-cell lines. Ligation of these ligands to the cognate NKG2D receptor on effector NK and T-cells induces rapid and efficient lysis of tumor cells.Citation3 The mechanism by which Bcl-2 causes this effect remains to be elucidated. How does the enforced expression of Bcl-2 change the expression of surface proteins? One may speculate that Bcl-2-induced alterations of calcium distribution are at least partially involved in the regulation of surface protein expression. Recently, the research group of D. Del Bufalo reported the involvement of the BH4 domain of Bcl-2 in the regulation of VEGF protein expression under hypoxic conditions, an effect independent of Bcl-2’s anti-apoptotic properties.Citation4
From Mitochondria to Membrane?
It has recently become clear that mitochondria are far more than mere cellular power plants: mitochondria actually have an astonishing range of functions. For example, cells can only be transformed by Ras upon mitochondrial localization of the proto-oncogene signal transducer and activator of transcription 3 (STAT3).Citation5
Bcl-2 resides primarily in membranes and acts as an endogenous inhibitor of the mitochondrial apoptotic program, which involves the permeabilization of the mitochondrial outer membrane and the release of apoptosis-inducing proteins—such as cytochrome c—into the cytosol.Citation6 Bcl-2 has also been shown to be localized to the nuclear envelope and the ER membrane,Citation8 where it is fully effective in protecting cells from apoptosis.Citation7 Bcl-2 may be experimentally targeted either to mitochondria, by increasing the COOH-terminal basicity,Citation8 or to the nuclear envelope and the ER, by knocking down FKBP38.Citation9 It would be interesting to investigate whether altering Bcl-2’s subcellular distribution interferes with its ability to regulate the surface expression of RAE1 and MULT1. If this novel effect of Bcl-2 is localization-dependent, we might have a tool to dissect the multiple functions of the protein and it can safely be expected that novel therapeutic opportunities would arise.
Concluding Remarks: What Does All This Mean?
Apart from raising a myriad of intriguing questions for basic science, our findings have clear relevance to human medicine. There are obvious difficulties in translating studies on mice to the situation in human patients and treatment of human cancer patients with cytostatic drugs is generally detrimental to the immune system. However, we found that patients suffering from lymphomas with a p53 disruption have an improved prognosis when Bcl-2 is overexpressed. It remains to be determined whether Bcl-2 inhibitors affect the immunogenicity of tumors. The outcome of such tests will be crucial in determining the usefulness of Bcl-2-inhibiting therapeutics. In the worst case, i.e., if inhibition of Bcl-2 prevents the correct immune response to tumors, Bcl-2-targeted antitumor therapies would have a serious shortcoming, as the immune response against residual micrometastases resistant to chemotherapy is indispensable for complete and sustained remission.Citation10 The future will reveal whether this novel effect of Bcl-2 is restricted to c-Myc-induced tumors of the B-lymphoid lineage and whether other anti-apoptotic members of the Bcl-2 family, such as Bcl-xL and Mcl-1, share the immunogenic property. Even if Bcl-2 inhibitors turn out not to be widely suitable for the treatment of patients, it may be possible to put our new findings to therapeutic use. For example, it is conceivable that mimicking (rather than inhibiting) Bcl-2’s effects may be beneficial for patients suffering from certain type of cancers. This notion may sound far-fetched, but there is ample evidence that the immune system contributes significantly to the elimination and control of residual tumor cells. Once we understand the molecular details of how Bcl-2 enhances immunogenic visibility we may be in a position to exploit this property for therapeutic purposes.
References
- Ogilvy S, Metcalf D, Print CG, Bath ML, Harris AW, Adams JM. Constitutive Bcl-2 expression throughout the hematopoietic compartment affects multiple lineages and enhances progenitor cell survival. Proc Natl Acad Sci U S A 1999; 96:14943 - 8; http://dx.doi.org/10.1073/pnas.96.26.14943; PMID: 10611317
- Schuster C, Berger A, Hoelzl MA, Putz EM, Frenzel A, Simma O, et al. The cooperating mutation or “second hit” determines the immunologic visibility toward MYC-induced murine lymphomas. Blood 2011; 118:4635 - 45; http://dx.doi.org/10.1182/blood-2010-10-313098; PMID: 21878673
- Champsaur M, Lanier LL. Effect of NKG2D ligand expression on host immune responses. Immunol Rev 2010; 235:267 - 85; PMID: 20536569
- Trisciuoglio D, Gabellini C, Desideri M, Ragazzoni Y, De Luca T, Ziparo E, et al. Involvement of BH4 domain of bcl-2 in the regulation of HIF-1-mediated VEGF expression in hypoxic tumor cells. Cell Death Differ 2011; 18:1024 - 35; http://dx.doi.org/10.1038/cdd.2010.175; PMID: 21233846
- Gough DJ, Corlett A, Schlessinger K, Wegrzyn J, Larner AC, Levy DE. Mitochondrial STAT3 supports Ras-dependent oncogenic transformation. Science 2009; 324:1713 - 6; http://dx.doi.org/10.1126/science.1171721; PMID: 19556508
- Green DR, Kroemer G. The pathophysiology of mitochondrial cell death. Science 2004; 305:626 - 9; http://dx.doi.org/10.1126/science.1099320; PMID: 15286356
- Häcki J, Egger L, Monney L, Conus S, Rossé T, Fellay I, et al. Apoptotic crosstalk between the endoplasmic reticulum and mitochondria controlled by Bcl-2. Oncogene 2000; 19:2286 - 95; http://dx.doi.org/10.1038/sj.onc.1203592; PMID: 10822379
- Kaufmann T, Schlipf S, Sanz J, Neubert K, Stein R, Borner C. Characterization of the signal that directs Bcl-x(L), but not Bcl-2, to the mitochondrial outer membrane. J Cell Biol 2003; 160:53 - 64; http://dx.doi.org/10.1083/jcb.200210084; PMID: 12515824
- Shirane M, Nakayama KI. Inherent calcineurin inhibitor FKBP38 targets Bcl-2 to mitochondria and inhibits apoptosis. Nat Cell Biol 2003; 5:28 - 37; http://dx.doi.org/10.1038/ncb894; PMID: 12510191
- Zitvogel L, Apetoh L, Ghiringhelli F, André F, Tesniere A, Kroemer G. The anticancer immune response: indispensable for therapeutic success?. J Clin Invest 2008; 118:1991 - 2001; http://dx.doi.org/10.1172/JCI35180; PMID: 18523649