Abstract
Lymphocyte migration into tumors remains poorly understood, despite the critical impact of these cells on cancer clinical outcome. Our recent study demonstrates the presence of blood vessels specialized in lymphocyte recruitment called high endothelial venules (HEVs) in human solid tumors and their correlation with lymphocyte infiltration and favorable prognosis in breast cancer.
The mechanisms governing infiltration of lymphocytes into tumors remain poorly characterized, despite the critical impact of these cells on patient prognosisCitation1 and therapeutic responses.Citation2 Lymphocyte migration into peripheral tissues is tightly controlled by vascular endothelium but the characteristics of tumor blood vessels which allow large scale influx of lymphocytes within human tumors were presently unknown.
High endothelial venules (HEVs) are specialized blood vessels for lymphocyte entry into peripheral lymph nodes.Citation3 HEVs are composed of plump, cuboïdal endothelial cells that selectively express sulphated sialyl-LewisX ligands for the lymphocyte homing receptor L-selectin, which mediate the initial capture and rolling interactions of lymphocytes along the vessel wall. Although HEVs are generally restricted to lymphoid organs, blood vessels with HEV characteristics develop in many chronic inflammatory diseases.Citation3
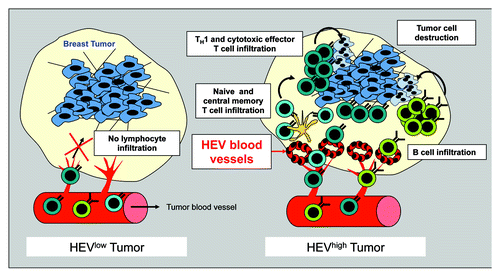
In a recent study published in Cancer Research,Citation4 we investigated the presence of HEVs within human primary solid tumors and their potential association with lymphocyte infiltration and clinical outcome. Using a panel of HEV-specific antibodies, we demonstrated that blood vessels expressing HEV classical markers can be observed in the majority of the 319 human primary tumor sections analyzed, including melanomas, breast, ovary, lung and colon carcinomas. In contrast, these HEV blood vessels were not detected in normal tissues suggesting that they are specifically induced in the tumor microenvironment.
HEVs were specifically located in lymphocyte-rich tumor areas. Within the tumor stroma, T cells were frequently seen extravasating or attached to the luminal surface of HEVs, suggesting an active role of these vessels in lymphocyte recruitment. Using a retrospective cohort of 146 primary invasive non-metastatic breast tumors, we demonstrated that the number and the density of MECA-79+ HEVs within breast tumor stroma were heterogeneous among the different patients. Interestingly, HEV number and density were both highly correlated with the density of tumor-infiltrating CD3+ T cells, CD8+ T cells and CD20+ B cells suggesting the existence of a tight link between HEV presence and lymphocyte infiltration into breast tumors (). In contrast, we found no correlation between tumor HEVs and blood vessels density indicating that differences in the density of tumor HEVs are not related to differences in tumor angiogenesis.
We further characterized immune populations associated with tumor HEVs by flow cytometry using 120 combinations of markers. We found that tumor HEVs were associated both with increased numbers of poorly differentiated populations of T cells, such as tumor infiltrating naïve and central memory T cells, and populations of activated effector memory T cells.
We used qRT-PCR to characterize the gene expression profile associated with the presence of tumor HEVs in breast cancer. Our results indicated that a high density of HEVs in breast tumors was associated with the upregulation of genes related to cytotoxic T cell and TH1 orientation. In contrast genes associated with TH2 and TH17 orientation or related to immune escape were not correlated with HEV presence.
Finally, we analyzed the clinical impact of tumor HEVs in our retrospective cohort of breast cancer patients. Univariate analysis indicated that patients with a high density of tumor HEVs had a significantly longer disease free survival, metastasis-free survival and overall survival than patients with a low density of tumor HEVs. Density of tumor HEVs also showed a significant correlation with patient’s survival in multivariate analysis after adjusting on classical prognostic factors.
Despite the ability of immune cells to kill tumor cells, the spontaneous clearance of established tumors by endogenous immune mechanisms is rare and the different immune based therapies tested so far have obtained limited results. Indeed, tumors often foster a tolerant microenvironment that counteracts effective antitumor immune responses.Citation5 In addition to these negative regulatory mechanisms, it appears that effector immune cells homing to tumors represent a major barrier to the efficiency of anti-tumor immune responses. Several mechanisms that restrict lymphocyte migration into tumors have previously been proposed.Citation6 In contrast, our study is the first to describe a mechanism which facilitates lymphocyte infiltration into tumors, the presence of HEVs within the tumor microenvironment. By their ability to recruit large numbers of circulating lymphocytes, tumor HEVs may represent new attractive targets for both cancer diagnosis and therapy.
HEVs play a critical role in immunity by allowing recirculation of naïve and memory lymphocytes throughout the different lymphoid tissues of the body, providing effective immune surveillance for pathogens.Citation7 The recruitment of naïve and central memory T cells through tumor HEVs could allow the generation in the tumor vicinity of high numbers of tumor antigen-specific effector T cells able to re-circulate throughout the body and limit the establishment of metastasis in distant organs. In agreement with this hypothesis, stimulation of naïve T cells recruitment and priming at the tumor site in mouse tumor models has been shown to promote both primary tumor and distant metastasis regression.Citation8
HEV endothelial cells exhibit a remarkable plasticity and rapidly lose their specialized characteristics outside their natural tissue microenvironment. The factors and mechanisms involved in the induction and maintenance of HEVs in human tumors remain to be determined. A better understanding of these factors may provide new opportunities to increase lymphocyte infiltration into tumors and enhance antitumor immune response. A recent report by our team demonstrates that dendritic cells maintain peripheral lymph node HEV blood vessels in steady-state conditions.Citation9 Therefore, dendritic cells may also contribute to generation and maintenance of tumor HEVs in the tumor stroma. Future work will have to address this issue.
References
- Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pagès C, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science 2006; 313:1960 - 4; http://dx.doi.org/10.1126/science.1129139; PMID: 17008531
- Denkert C, Loibl S, Noske A, Roller M, Müller BM, Komor M, et al. Tumor-associated lymphocytes as an independent predictor of response to neoadjuvant chemotherapy in breast cancer. J Clin Oncol 2010; 28:105 - 13; http://dx.doi.org/10.1200/JCO.2009.23.7370; PMID: 19917869
- Girard JP, Springer TA. High endothelial venules (HEVs): specialized endothelium for lymphocyte migration. Immunol Today 1995; 16:449 - 57; http://dx.doi.org/10.1016/0167-5699(95)80023-9; PMID: 7546210
- Martinet L, Garrido I, Filleron T, Le Guellec S, Bellard E, Fournie JJ, et al. Human solid tumors contain high endothelial venules: association with T- and B-lymphocyte infiltration and favorable prognosis in breast cancer. Cancer Res 2011; 71:5678 - 87; http://dx.doi.org/10.1158/0008-5472.CAN-11-0431; PMID: 21846823
- Zitvogel L, Tesniere A, Kroemer G. Cancer despite immunosurveillance: immunoselection and immunosubversion. Nat Rev Immunol 2006; 6:715 - 27; http://dx.doi.org/10.1038/nri1936; PMID: 16977338
- Buckanovich RJ, Facciabene A, Kim S, Benencia F, Sasaroli D, Balint K, et al. Endothelin B receptor mediates the endothelial barrier to T cell homing to tumors and disables immune therapy. Nat Med 2008; 14:28 - 36; http://dx.doi.org/10.1038/nm1699; PMID: 18157142
- von Andrian UH, Mempel TR. Homing and cellular traffic in lymph nodes. Nat Rev Immunol 2003; 3:867 - 78; http://dx.doi.org/10.1038/nri1222; PMID: 14668803
- Schrama D, thor Straten P, Fischer WH, McLellan AD, Bröcker EB, Reisfeld RA, et al. Targeting of lymphotoxin-alpha to the tumor elicits an efficient immune response associated with induction of peripheral lymphoid-like tissue. Immunity 2001; 14:111 - 21; http://dx.doi.org/10.1016/S1074-7613(01)00094-2; PMID: 11239444
- Moussion C, Girard JP. Dendritic cells control lymphocyte entry to lymph nodes through high endothelial venules. Nature 2011; 479:542 - 6; http://dx.doi.org/10.1038/nature10540; PMID: 22080953