Abstract
Adenosine deaminase (ADA) is responsible for the deamination of immunosuppressive adenosine to inosine. In human T lymphocytes, ADA is associated with dipeptidyl peptidase IV (CD26). ADA expression and activity were evaluated in regulatory T cells (Treg) and CD4+ T effector cells (Teff) of patients with head and neck squamous cell cancer (HNSCC). CD4+CD39+ and CD4+CD39neg T cells were isolated by single-cell sorting from the peripheral blood of 15 HNSCC patients and 15 healthy donors (NC). CD26/ADA expression in these cells was studied by multicolor flow cytometry, confocal microscopy, RT-PCR and immunohistochemistry in tumor tissues. ADA activity was evaluated by mass spectrometry, suppression of Teff proliferation in CFSE assays and cytokine production by Luminex. CD4+CD39+ Treg had low and CD4+CD39neg Teff high CD26/ADA expression and ADA activity in NC or HNSCC. The frequency and suppressor activity of CD39+CD26neg Treg were elevated in patients relative to NC (p < 0.01). However, ADA activity in patients’ CD4+CD39neg Teff was decreased (p < 0.05), resulting in extracellular adenosine accumulation. Also, patients’ Teff were more sensitive to inhibitory signals delivered via adenosine receptors. IL-2, IL12 and INFγ upregulated ADA expression and activity in CD4+CD39neg Teff, whereas IL-10, PGE2 and CADO downregulated it. The differentially expressed CD26/ADA can serve as surface markers for functionally-active CD39+CD26neg Treg.
Keywords: :
Introduction
The integrity of the immune system in cancer is dependent on a variety of factors, including immunosuppressive and immunoenhancing molecules generated in the tumor milieu. These factors interact with cells of the immune system determining patients’ immune competence. Chronic inflammation in diseases such as cancer or viral infections such as HIV-1, is accompanied by tissue damage and hypoxia leading to immune suppression which interferes with native and/or adaptive immunity.Citation1,Citation2 Perhaps the most ubiquitous molecular alteration in damaged tissues involves phosphohydrolysis of ATP and ADP, which is catalyzed by the ectonucleotidase CD39.Citation3 Further breakdown of AMP to adenosine by ecto-5′ nucleotidase, CD73, results in a dramatic increase of adenosine concentrations in situ, and in adenosine-mediated downregulation of immune responsiveness.Citation4-Citation6
Adenosine deaminase (ADA) is an enzyme that catabolizes adenosine to its metabolite, inosine, thus downregulating biologic effects of adenosine in situ. ADA is present on the cell surface as well as intracellularly, but it does not have its own transmembrane domain and is associated with CD26, a surface glycoprotein with dipeptidyl peptidase IV activity. CD26 serves as a binding protein for extracellular ADA in humans, anchoring it to the cell surface and thus reducing the local levels of adenosine.Citation7,Citation8 It is this surface-aligned CD26/ADA complex that by deaminating adenosine prevents its binding to A2A receptors on immune cells. The lack of this signal allows T cells to escape from adenosine-mediated suppression and to promote inflammation.Citation9 Thus, blocking of surface-bound ADA activity enhances exogenous adenosine access to A2A receptors on effector T cells (Teff) and regulates adenosine-mediated suppression in these T cells. It has been well documented that defects in the ADA gene cause an accumulation of purine metabolites, leading to an inherited form of severe combined immunodeficiency (SCID).Citation10
Adenosine exerts various biologic effects which are mediated via its four receptors (R): A1, A2A, A2B and A3.Citation11-Citation13 The immunosuppressive effects of adenosine on Teff are mainly performed via A2AR signaling.Citation14,Citation15 We and others have reported that regulatory T cells (Treg), express CD39 and CD73Citation4,Citation16-Citation18 but lack CD26 and ADA.Citation4,Citation16-Citation18 We hypothesize that these properties endow Treg with the ability to concentrate pericellular adenosine and use it for immune suppression. Treg maintain peripheral tolerance using various cell contact-dependent or contact-independent suppression mechanisms, with adenosine representing a soluble suppressive factor.Citation19,Citation20 The Treg frequency and activity are decreased in patients with autoimmune diseasesCitation21 but elevated in cancer patients,Citation22 where Treg favor tumor development and tumor escape from the host immune system.Citation23 The role of adenosine in tumor escape has been intensively investigated, and although there is evidence that human tumors can produce adenosine,Citation24,Citation25 recent attention has focused on Treg-generated adenosine.Citation4,Citation18,Citation26 Immunosuppressive activities of adenosine and its involvement in neoangiogenesis contribute to tumor progression and represent major tumor escape mechanisms.Citation27,Citation28
Extending our initial observations,Citation4 we show in this study not only that in cancer patients, CD26 and ADA expression is absent in Treg at the protein and mRNA levels, but also that ADA activity is significantly reduced in Teff in cancer patients compared with Teff in NC and that it can be altered upon exposure to different cytokines. Further, expression of CD39 in combination with the absence of CD26 defines a unique, functionally-active subset of Treg detectable by flow cytometry. This Treg subset is increased in the peripheral blood and tumor tissues of patients with HNSCC. Taken together with low ADA expression and activity in Teff of HNSCC patients, the data indicate that adenosine-mediated immune suppression is potentiated in cancer. Downregulation of ADA expression in Teff of HNSCC patients identifies yet another mechanism for increased adenosine levels in the tumor milieu.
Results
Adenosine metabolism in Treg and Teff
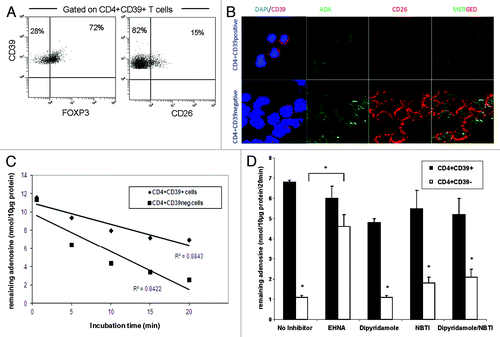
Recently, we have shown that Treg express CD39, and that CD39 is a reliable marker of human Treg suitable for their isolation.Citation29,Citation30 A large majority of CD4+CD39+ cells express FOXP3 (e.g., > 72%) and only about 15% weakly express CD26 (). In contrast, CD26 is overexpressed in most of Teff.Citation4 CD26 binds ADA at its extracellular domain and, therefore, localizes ADA to the cell surface.Citation4 We have previously reported that ADA was expressed in Teff but not in Treg.Citation4 This initial observation is extended here to studies of ADA function in T cells of patients with cancer and of NC. First, by performing confocal microscopy with single-cell sorted CD4+CD39neg Teff and CD4+CD39+ Treg we confirmed differential expression of CD26/ADA in these cells (). To determine whether decreased ADA expression in Treg translates into lower ADA activity in converting adenosine to inosine, single cell-sorted CD4+CD39+ and CD4+CD39neg cells were incubated with exogenous adenosine for different time periods. Adenosine levels remaining in the cell supernatants were then determined (). Teff cells (CD4+CD39neg) hydrolyzed more adenosine than CD4+CD39+ Treg. Upon pretreatment of these cells with EHNA, an ADA inhibitor, the ability of Teff to metabolize adenosine was greatly reduced (). EHNA itself had no effect on adenosine utilization by the cells (data not shown). To confirm that the low levels of adenosine measured in Teff cell cultures were indeed due to increased ADA activity and were independent of adenosine re-uptake by these cells, we pretreated Teff and Treg subsets with either dipyridamole and/or NBTI, agents that inhibit transport of exogenous adenosine into cells. Used alone or in combination, these inhibitors only mildly increased the adenosine levels remaining in T cell supernatants at the end of culture (), suggesting that the observed difference in adenosine levels between CD4+CD39+ and CD4+CD39neg cells was mainly due to ADA activity.
Figure 1.ADA expression and function in CD4+CD39+ Treg vs. CD4+CD39neg Teff. (A) PBMC obtained from NC were stained with relevant antibodies and analyzed by flow cytometry. The % expression of FOXP3 and CD26 on CD4+CD39+ T cells is shown. Representative dot plots were selected from 15 independent experiments performed. (B) Single cell-sorted CD4+CD39+ and CD4+CD39neg cells were stained for CD39, ADA, CD26 and DAPI. Expression and co-expression of these markers were analyzed by confocal microscopy (mag x 400). Results of one out of five representative experiments are shown. (C) Single cell-sorted CD4+CD39+ and CD4+CD39neg cells were plated in 96-well plates (25,000 cells/well) in serum-free medium with 10 µM of exogenous adenosine and were incubated for different time periods. Levels of adenosine remaining in the cell supernatant (i.e., un-metabolized adenosine) were determined by mass spectrometry. Data are from an experiment representative of three performed with cells of different donors. (D) Single cell-sorted CD4+CD39+ and CD4+CD39neg cells were plated in 96-well plates (25,000 cells/well) with 10 µM of exogenous adenosine in the presence or absence of different inhibitors. Adenosine levels remaining in the cell supernatants were determined 20 min later by mass spectrometry. Data (means ± SD) are from three indepent experiments. Asterisks indicate p < 0.05 for differences between CD4+CD39+ and CD4+CD39neg cells.
Gene expression of CD26 and ADA in Treg vs. Teff
The differences in ADA and CD26 expression levels between Treg and Teff cells were also studied at the message level. RT-PCR analysis was performed, and as shown in , gene expression for CD26 and ADA was consistently found to be higher in Teff than in Treg.
Table 1. Gene expression of CD26 and ADA in CD4+CD39+ and CD4+CD39neg T cells subsets
Increased CD39+CD26neg cell frequency in HNSCC
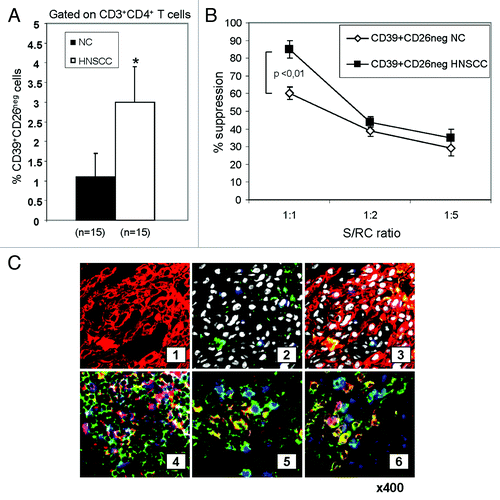
Gating on CD3+CD4+ T cells, we next determined the frequency of CD39+CD26neg Treg in the blood of HNSCC patients. As shown in , the frequency of circulating CD4+CD39+CD26neg cells was higher (p < 0.01) in HNSCC patients relative to NC. To determine whether CD4+CD39+CD26neg Treg isolated from HNSCC patients mediated higher suppression than those obtained from NC, CFSE-based proliferation assays were performed. CD4+CD39+CD26neg Treg obtained from HNSCC patients mediated suppression of RC proliferation more effectively (p < 0.01) than Treg of NC (). CD4+CD39+CD26+ T cells did not suppress RC proliferation (data not shown).
Figure 2.Frequency and activity of CD4+CD39+CD26neg in NC and HNSCC patients. (A) Freshly isolated PBMC from NC (n = 15) and HNSCC patients (n = 15) were stained and analyzed by flow cytometry. The data are means ± SD. The asterisk indicates p < 0.01. (B) PBMC obtained from NC or HNSCC patients were single-cell sorted into CD4+CD39+CD26neg and CD4+CD39neg responder T cells (RC). The latter were CFSE labeled and stimulated with plate-bound OKT-3 and soluble anti-CD28. Following addition of CD4+CD39+CD26neg suppressor cells and 150 IU/mL of IL-2, the co-cultures were incubated for 5 d. Cells were analyzed by flow cytometry as described in Materials and Methods. Suppression of RC cell proliferation mediated by CD4+CD39+CD26neg obtained from NC or cancer patients at various S/RC ratios is shown. Data represent means ± SD from three independent experiments. (C) Tumor-infiltrating T cells are shown in sections of a representative HNSCC of five specimens examined (mag. x 400) (1) tumor cells (red, stained with pancytokeratin) (2) few green CD26+ T cells are among tumor cells; tumor cell nuclei are white (DAPI); no ADA+ cells stained blue are visible. (3) Sections 1 and 2 in a merged image. Tumor cells are negative for ADA and CD26. (4) A lymphoid infiltrate into tumor is stained for CD4 (green), ADA (red) and CD26 (blue). A co-localization of ADA and CD26 in CD4+ cells (pink) is evident. (5) A tumor section containing infiltrating lymphoid cells is stained for CD4 (green), ADA (red) and FOXP3 (blue). CD4+FOXP3+ Treg (green/blue) are negative for ADA, whereas CD4+FOXP3neg Teff cells (yellow/red) are positive for ADA. (6) A lymphoid infiltrate into tumor is stained for CD4 (green), ADA (blue) and CD132 (red). CD4+CD132+ Tr1 are negative for ADA (yellow/red), whereas CD4+CD132neg Teff cells are mostly positive for ADA (blue).
In situ analysis of ADA and CD26 in the tumor microenvironment
The presence and distribution of ADA and CD26 in the tumor microenvironment was evaluated by multicolor immunoflourescence and confocal microscopy using HNSCC biopsy tissues. Tumor tissue sections were stained with labeled mAbs specific for ADA or CD26. As shown in , tumor cells were negative for ADA and CD26. Both, Teff and Treg were present in the tumor tissue. The majority of CD4+ Teff were positive for ADA and CD26 (pink, ), whereas the CD4+FOXP3+ (Treg) cells (green/blue) were negative for ADA (red, ). To determine the distribution of ADA in association with adaptive Treg (Tr1), which regularly infiltrate tumor tissue,Citation31,Citation32 we stained tumor sections with CD132, a surface-associated Tr1 marker. As shown in , CD4+CD132+ cells were mostly negative for ADA (yellow/red). CD4+ Teff cells co-expressed ADA and CD26 (green/blue). In the tumor microenviornment, similar to the peripheral blood, the presence of CD26 and ADA was largely restricted to Teff.
ADA activity in Teff of NC vs. patients with HNSCC
The restricted expression of ADA and CD26 to Teff suggests that ADA activity may be essential for preservation of their effector functions by reducing adenosine-mediated immunosuppression. To test this hypothesis, we initially compared CD26 expression in CD4+CD39neg Teff cells obtained from NC and patients with HNSCC. The frequency of CD4+CD26+ Teff was found to be significantly reduced in patients vs. NC (). Sorted CD4+CD39neg Teff cells were next incubated in the presence of exogenous adenosine to measure ADA enzymatic activity. The cells obtained from HNSCC patients deaminated adenosine less efficiently than did NC cells (), suggesting that Teff in HNSCC had decreased ADA activity. After the addition of EHNA, an ADA inhibitor, the adenosine remaining in the cell supernatants of Teff in both cohorts increased significantly (p < 0.001), as expected. The inhibitors of adenosine uptake by T cells, dipyridamole and NBTI, increased levels of adenosine remaining in supernatants of Teff in NC (p < 0.05) but not in HNSCC patients. This suggested that Teff of cancer patients not only had lower ADA activity but also that the transport of exogenous adenosine into these cells was impaired. Both events could potentially increase extracellular adenosine concentration levels, enhancing adenosine coupling to A2AR on Teff and contributing to adenosine-mediated suppression of Teff functions in HNSCC patients.
Figure 3. ADA activity in Teff of NC and patients with HNSCC. (A) Freshly isolated PBMC from NC (n = 15) and HNSCC patients (n = 15) were stained and analyzed by flow cytometry for percentages of CD39negCD4+CD26+ T cells. The data are means ± SD. The asterisk indicates p < 0.05. (B) Single cell-sorted CD4+CD39neg cells from NC and HNSCC patients were plated in 96-well plates (25,000 cells/well) with 10 µM of exogenous adenosine and in the presence or absence of different reagents. Remaining adenosine levels were determined in the cell supernatants after 20 min of addition of exogenous adenosine by mass spectrometry. Data (means ± SD) are from three independent experiments per group. Asterisks indicate p < 0.05. (C) PBMC obtained from a representative NC or HNSCC patient were single-cell sorted into CD4+CD39+ (S) and CD4+CD39neg T cells (RC). The latter were CFSE labeled and stimulated with plate-bound OKT-3 and soluble anti-CD28. Afterwards S were added to the culture and incubated with 150 IU/ml of IL-2 for 5 d. Cells were analyzed by flow cytometry as described in Materials and Methods. To selected wells CADO (20 μM) was added. The FACS plots are from an experiment representative of three independent experiments performed. (D) Results of suppression assays performed as outlined in C. The data are means ± SD. The asterisk indicates p < 0.01. (E) Single cell-sorted CD4+CD39neg cells were plated in 24-well plates (1x106 cells/well) and activated with OKT-3 (1 μg/ml) and anti-CD28 (1 μg/ml) in the presence or absence of CADO (20 μM) for 24 h. Cytokine levels in the cell supernatants were determined by LUMINEX. Data (means ± SD) are from five independent experiments. Asterisks indicate p < 0.05
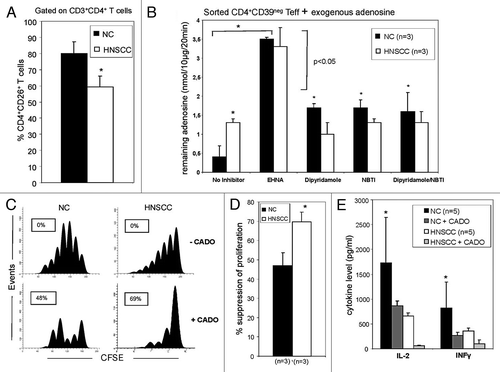
To determine whether the reduced CD26 and ADA expression in Teff of patients with HNSCC renders these cells more susceptible to adenosine-mediated immunosuppression, CFSE proliferation assays in the presence/absence of CADO, a synthetic analog of adenosine, were performed. Upon incubation of Teff isolated from NC or HNSCC with CADO the ability of these cells to proliferate in response to CD3/TCR signaling was significantly reduced. However, CADO-induced suppression was significantly greater in HNSCC patients’ Teff than in NC’s Teff (). Thus, Teff in HNSCC were more sensitive to inhibition mediated by adenosine binding to A2AR than Teff in NC. This suggests that in Teff of HNSCC patients, intrinsically increased A2AR activation upon adenosine binding as well as reduced ADA activity might be responsible for greater sensitivity of patients’ Teff to exogenous adenosine. In addition, ussing LUMINEX analysis, we showed that IL-2 and INFγ secretion by Teff in HNSCC was significantly reduced upon treatment with CADO. Again, this inhibition was more pronounced in Teff of HNSCC patients compared with NC (). In aggregate, the data indicate that CD4+ Teff in patients with cancer are less efficient in metabolizing adenosine to inosine than are CD4+ Teff in NC. Moreover, CD4+ Teff in cancer patients are more sensitive to A2AR-mediated suppression of their immune functions.
Surface and intracytoplasmic ADA expression and activity in Teff
The increased susceptibility of HNSCC patients’ Teff to adenosine-mediated immunosuppression could be due to reduced ADA expression in these cells. shows that while equal proportions of Teff in NC and patients express intracytoplasmic ADA, the frequency of Teff expressing surface ADA is lower in patients than NC. Similarly, the MFI of surface ADA was lower in Teff of HNSCC patients (). However, the MFI for intracytoplasmic ADA was also significantly lower in HNSCC patients’ Teff. Together, the data suggest that low levels of ADA in and on the surface of Teff could be responsible for the observed greater susceptibility of patients’ Teff to adenosine.
Figure 4.ADA expression in CD4+CD39neg Teff in NC and HNSCC patients. (A) PBMC obtained from NC and HNSCC patients were stained with relevant antibodies and analyzed by flow cytometry. The % surface and intracellular expression of ADA in CD4+CD39neg T cells is shown. Data represents the mean and standard deviation from five independent experiments per group performed. Asterisks indicate p < 0.05 for differences between NC and HNSCC patients. (B) The mean fluorescence intensity of surface and intracellular ADA expression in CD4+CD39neg T cells is evaluated by flow cytometry. Data represents the mean and standard deviation from five independent experiments performed. Asterisks indicate p < 0.05 for differences between NC and HNSCC patients. (C) CD4+CD39neg T cells were treated with various cytokines and factors for 48 h and afterwards evaluated by flow cytometry for their % surface expression of ADA. Data represents the mean and standard deviation from five independent experiments performed. Asterisks indicate p < 0.05 for differences compared with the baseline value.
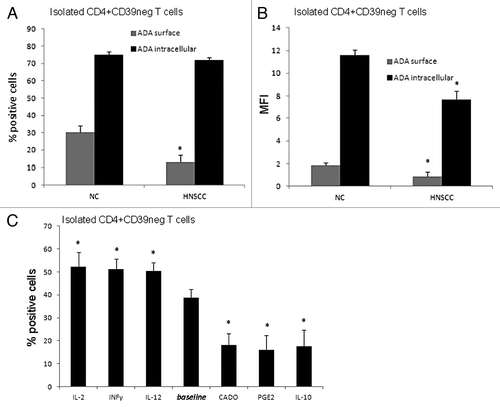
To further explore mechanisms responsible for reduced ADA expression and activity in patients with cancer, Teff were pretreated with various cytokines or inhibitory factors and tested for ADA surface expression. IL-2, IFNγ and IL-12 increased the percentages of Teff expressing surface ADA, whereas IL-10, CADO and PGE2 decreased the percentages of Teff with ADA surface expression relative to the baseline after 48 h of incubation. These alterations in the frequency of ADA+ Teff paralleled increases or decreases in ADA activity measured as consumption of exogenous adenosine by mass spectrometry after treating the cells with the various cytokines or factors (). These results confirm that ADA surface expression and its activity in Teff are modulated by factors present in the microenvironment.
Table 2. Influence of different cytokines on adenosine deaminase activity of Teff cells
ADA and CD26 as negative markers for human Treg
In addition to the importance of the CD26/ADA complex for susceptibility of human Teff to adenosine, its absence from the CD4+CD39+ Treg surface could facilitate Treg identification and isolation from PBMC. Our initial data showed that most (86 ± 4%) of CD4+CD39+ Treg were CD26neg.Citation4 Using sorted cells from PBMC of 15 NC, expression of various Treg-associated markers in CD4+CD39+ or CD4+CD39+CD26neg T cells was studied by flow cytometry. The percentages of FOXP3, GITR and CD25high were significantly higher in the CD4+CD39+CD26neg subset relative to the CD4+CD39+ Treg subset (). No difference was evident in CTLA4 expression between the two subsets. The data suggest that this combination of surface markers on Treg may be useful for the isolation of Treg populations with a greater purity from PBMC.
Figure 5.The phenotype and suppression mediated by CD4+CD39+ Treg vs. CD3+CD39+CD26neg Treg. (A) Freshly sorted PBMC obtained from 15 NC were stained and analyzed by flow cytometry. Expression of conventional Treg markers in the CD4+CD39+ and CD4+CD39+CD26neg T cell subsets was determined. Data are means ± SD from 15 independent experiments. (B) Single-cell sorted CD4+CD39neg cells were CFSE-labeled and stimulated with plate-bound OKT-3 and soluble anti-CD28 in the presence of CD4+CD39+ or CD4+CD39+CD26neg suppressor cells and 150 IU/ml of IL-2 for 5 d. The % inhibition of RC proliferation was determined by flow cytometry and analyzed using the Modfit software. (C) An inhibitor of CD39 activity (ARL67156) or an antagonist of the A2AR (ZM 241385) were added to the suppression assays at the beginning of the co-cultures set up as described in (B). Suppression of CD4+CD39neg cell proliferation mediated by CD4+CD39+ cells or CD4+CD39+CD26neg cells at various S/RC ratios was determined as described in Materials and Methods. The data are means ± SD from three independent experiments.
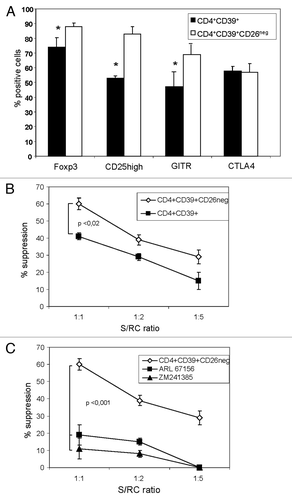
The ability of CD4+CD39+ and CD4+CD39+CD26neg Treg subsets to suppress responder cell (RC) proliferation was also compared. Single cell-sorted CD4+CD39+ or CD4+CD39+CD26neg suppressor cells (S) obtained from freshly-isolated PBMC of NC were co-incubated with autologous CD4+CD25neg RC at different RC/S ratios. After a five-day culture, the mean suppressor activity of CD4+CD39+ cells at the 1S:1RC ratio was 41% ± 3, whereas the mean suppressor activity of CD4+CD39+CD26neg was 61% ± 3 (p < 0.002; ). The suppression of proliferation linearly decreased upon further dilution of S (). To examine the involvement of the adenosinergic pathway in Treg-mediated suppression, ARL67156, a CD39 inhibitor, or ZM241385, a selective A2AR antagonist was added, to some culture wells. As shown in , Treg-mediated suppression was reduced (p < 0.01) using either of these reagents. CD4+CD39+CD26neg Treg not only generate immunosuppressive adenosine in co-cultures with RC but due to the absence of ADA are also able to maintain high adenosine levels in their microenvironment. Pericellular accumulations of adenosine could contribute to the increased levels of suppression mediated by these cells relative to that mediated by CD4+CD39+ cells.
Discussion
ADA is the key enzyme catalyzing the irreversible deamination of extracellular immunosuppressive adenosine into inosine and is, therefore, an important modulator of immune responses. ADA activity is necessary for sustaining Teff proliferation and cytokine production.Citation33 ADA deficiency results in abnormalities in the development of the immune system (SCID), which are fatal if left untreated.Citation10 CD26 is a lymphocyte marker that anchors ADA on the T cell surface.Citation9 We demonstrate here that Treg, which suppress immune responses, lack ADA as well as ADA-associated CD26 expression at the mRNA and protein levels, and that the lack of surface CD26 in combination with CD39 expression can be considered as a useful biomarker for the definition and isolation of functionally-active human Treg. The identification of reliable surface markers specific for human Treg has been difficult. FOXP3, a transcription factor, considered to be a specific marker for these cells can be also expressed albeit transiently on activated T cells.Citation34 Further, because of its intracellular location, FOXP3 cannot be used for Treg isolation. The CD4+CD39+CD26neg Treg subset is highly suppressive and capable of not only generating but maintaining high levels of adenosine. CD39+CD26neg Treg are present in a higher frequency in the peripheral blood of patients with cancer relative to NC, and they also accumulate at tumor sites.
The tumor microenvironment is characterized by high levels of adenosine production which can originate from tumor cells, stromal cells and/or infiltrating Treg.Citation25 The survival of Teff in this microenvironment appears to be dependent on their ability to reduce adenosine concentrations. In this respect, the ADA/CD26 complex plays a critical role. By deaminating exogenous adenosine, this cell surface-localized enzymatic complex protects Teff from suppression and thus plays a key role in preserving anti-tumor immunity in situ. On the other hand, human Treg which produce adenosine in order to mediate suppression of Teff functionsCitation16-Citation18 appear to be resistant to its effects, although they express A2AR on their cell surface (our unpublished data). In fact, adenosine seems to be necessary for Treg expansion.Citation35 Thus, Treg have little need for ADA and consequently, in contrast to Teff, express minimal levels of ADA/CD26. It is, of course, possible that the ADA/CD26 expression is upregulated on Treg when it becomes necessary to limit or stop suppression. Specifically, it has been reported that Th1 cytokines, IL-2 and IL-12, upregulate ADA and CD26 expression on the T cell surface by increasing translocation of ADA to this site.Citation36 This suggests that during cytokine-promoted immune responses, ADA expression/activity increases to deaminate immunosuppressive adenosine.
Teff present in the tumor microenvironment find themselves in the position to eliminate exogenous adenosine utilizing ADA in order to function. If exogenous adenosine levels are elevated, surface expression of the ADA/CD26 complex on Teff could decrease as a result of its rapid utilization. For example, CD26 cell surface expression is downregulated independently of adenosine receptor expression in tumor cells exposed to adenosine and on lymphocytes in breast cancer.Citation37,Citation38 It has been reported that a prolonged pulmonary hypoxia results in a transcriptional induction of ADA.Citation39 With chronically elevated adenosine levels, as is the case in the tumor microenvironment, such upregulation of ADA in response to hypoxia might exhaust its cellular stores resulting in its lower expression on the surface of Teff, decreased enzymatic activity, increased adenosine levels and thus enhanced suppression of Teff functions. Here, we show for the first time that in cancer patients, the ADA activity in CD4+ Teff is significantly reduced compared with CD4+ Teff in NC. Further, cellular up-take of exogenous adenosine by Teff of cancer patients is reduced relative to that in Teff of NC. Greater sensitivity of patients’ Teff to adenosine-mediated suppression possibly reflects intrinsically increased A2AR activation upon adenosine coupling. While speculative at this time, this scenario provides a partial explanation of why immunosuppressive adenosine is less efficiently cleared and thus more inhibitory for Teff in the tumor microenvironment compared with Teff in NC.
In summary, increased adenosine production by CD4+CD39+ TregCitation18 and reduced ADA activity in CD4+ Teff of HNSCC patients as shown in this study, contribute to tumor escape by increasing levels of immunosuppressive adenosine. An improvement in immunotherapy for these patients could potentially be achieved by enhancing ADA activity and preventing its downregulation in CD4+ Teff in addition to controlling the generation of immunosuppressive adenosine by Treg and/or tumor cells. These strategies based on pharmacologic interventions with drugs targeting the adenosinergic pathway are available for clinical use today.Citation40,Citation41
Materials and Methods
HNSCC patients and healthy volunteers
Peripheral venous blood samples were obtained from 15 HNSCC patients and 15 age-matched NC. All patients were seen in the Outpatient Clinic of the Department of Otolaryngology at the University of Pittsburgh Medical Center (UPMC) between December 2007 and September 2008. All subjects signed an informed consent approved by the Institutional Review Board of the University of Pittsburgh. At the time of blood draws the patients had an active disease prior to any form of therapy.
Collection of peripheral blood mononuclear cells
Blood samples (20–30 mL) were drawn into heparinized tubes and centrifuged on Ficoll-Hypaque gradients (GE Healthcare Bioscience). PBMC were recovered, washed in AIM-V medium (Invitrogen), counted in a trypan blue dye, and immediately used for experiments.
Separation of Treg
CD4+CD39neg T cells, CD4+CD39+ und CD4+CD39+CD26neg Treg were single cell-sorted from freshly-obtained PBMC of NC and HNSCC patients using a Cytomation MoFlo® high speed sorter after staining of lymphocytes with the relevant antibodies.
Antibodies
The following anti-human monoclonal antibodies (MoAbs) were used for flow-cytometry: anti-CD4-ECD, anti-CD26-PC5, anti-CD25-FITC, anti-FOXP3-FITC, anti-CD39-PE, anti-GITR, anti-CTLA4-PE and anti-ADA. Anti-CD4+, anti-CD25 Abs and their respective isotypes, were purchased from Beckman Coulter. The anti-FOXP3 (clone PCH101), anti-CD39-PE Abs and secondary PE-labeled goat anti-mouse for ADA staining were purchased from eBioscience. The anti-CTLA4 and anti-GITR-Abs were purchased from R&D Systems, anti-CD26 Ab from BioLegend, and anti-ADA Ab from Abcam. Isotype controls, which served as negative controls for surface as well as intracellular staining, were purchased from Beckman Coulter. Before use, all Abs were titrated using activated as well as non-activated PBMC to determine the optimal staining dilution for each.
Surface and intracellular staining
Freshly isolated cells were stained for flow cytometry as previously described.Citation42 Briefly, cells were incubated with the antibodies for surface markers for 30 min at 4°C in the dark and then fixed with 2% (w/v) paraformaldehyde in PBS for 15 min. Afterwards, the cells were permeabilized with 0.1% (w/v) saponin in PBS for 30 min and stained with Abs specific for intracellular markers for 30 min at 4°C in the dark. Cells were washed twice with 0.1% saponin in PBS, resuspended in a flow solution and immediately analyzed by flow cytometry. Appropriate isotype controls were included for each sample.
Flow cytometry
Flow cytometry was performed using a EPICS® XL-MCL flow cytometer equipped with Expo32 software (Beckman Coulter). The acquisition and analysis were restricted to the lymphocyte gate based on characteristic properties of the cells in the forward (FSC) and side scatter (SSC). FSC and SSC were set in a linear scale, and at least 105 cells were acquired for analysis, which was performed using the Coulter EXPO 32vl.2 analysis program. For additional analyses, gates were restricted to the CD4+CD39+ or CD4+CD39+CD26neg subsets.
Immunostaining
Single cell-sorted CD4+CD39+ and CD4+CD39neg cells were cytocentrifuged onto glass slides and stained using a standard immunoperoxidase method. Cells were first fixed using a 1:1 methanol/acetone solution and then dried at room temperature for 4 h. Afterwards, cells were treated with a serum-free protein block (Dako) for 1 h at room temperature, followed by washing with PBS and an overnight incubation at 4°C in the dark with the primary Ab. The following Abs were used: unconjugated anti-human ADA antibody (1:100 dilution, Santa Cruz Biotechnology) or appropriate isotype controls. Slides were then washed and incubated with a donkey anti-mouse-Cy3 (1:500, Jackson ImmunoResearch). Next, slides were washed, fixed, and evaluated in an inverted Olympus FluoView 1000 laser scanning confocal microscope under an oil immersion objective (Center for Biology Imaging Core Facility, University of Pittsburgh). For digital image analysis, the software Adobe Photoshop version 7.0 was used.
Mass spectometry for adenosine
CD4+CD39neg or CD4+CD39+ T cells (25,000 cells/well) obtained from NC and HNSCC were incubated with 10 µM exogenous adenosine in either the presence or absence of erythro-9-(2-hydroxy-3-nonyl) adenine (EHNA, 2µM, Sigma Aldrich), dipyridamole (5µM, Tocris Bioscience) and/or NBTI (5µM, Tocris Bioscience) or IL2 (50U/ml, Peprotech), IL10 (50U/ml, Peprotech), IL-12 (50U/ml, Peprotech), IFNγ (50U/ml, eBioscience), PGE2 (1μM, Sigma Aldrich) or 2 chloro-adenosine (CADO) (5μM, Sigma Aldrich), in wells of 96-well flat bottom plates. Cell supernatants were collected after 30 min of incubation with the reagents. Samples were centrifuged and boiled for 2 min and stored in an ultra-low freezer until analysis. Adenosine levels were measured by high performance liquid chromatography-tandem mass spectrometry using a triple quadrupole mass spectrometer (TSO Quantum-Ultra, ThermoFisher Scientific) operating in the selective reaction monitoring mode with a heated electrospray ionization source as previously described.Citation43
Real-time PCR analysis
Total RNA was isolated using the QiagenRNeasy Mini Kit (Qiagen®). 300–500 ng RNA in a final volume of 20 μL was converted into cDNA using the SABioscience RTCitation2 First strand Kit (SABioscience) according to the manufacturer’s instructions. Using the SABiosciences RTCitation2 Real-TimeTM SYBR Green PCR master mix, the real-time quantitative PCR analysis was performed on an ABI Prism 7900HT sequence detector system (AB Applied Biosystems). The PCR thermal cycle conditions were as follows: denaturation at 95°C for 10 min, follow with 40 cycles at 95°C for 15 sec and annealing at 60°C for 1 min. The expression levels of the housekeeping gene GAPDH was measured as an internal reference with a standard curve to determine the integrity of template RNA for all specimens. The primer for ADA, CD26 and GAPDH were as follows: Primer sequence each was for ADA forward, 5′-TTCCTTCCAAG AAGACCATGA-3′, and reverse, 5′-GGTTTCAGATTCAACCATGC-3′; for CD26 forward, 5′-AGACTGGCACAGTTTTCTGAG-3′, and reverse, 5′-CTTTCCCATCACCCTTGCTGT-3′; and for GAPDH forward, 5′-GGAGTCCACTGGCGTCTTCAC-3′, and reverse 5′-GAGGCTGTTGTCATACTTCTCATG-3′.
Suppression assays
Single cell-sorted CD4+CD39+ or CD4+CD39+CD26neg cells obtained from NC or patients by single-cell sorting were tested for suppression of proliferative activity in co-cultures with autologous CD4+CD25neg responder cells (RC) as previously described.Citation42 Aliquots (105cells/well) of CFSE-labeled autologous CD4+CD25neg cells were incubated in wells of flat-bottom 96-well plates at the responder cell (RC)/suppressor (S) ratios of 1:1, 2:1, 5:1 and 10:1. Using the same assay format, either ARL67165 (250µM, Sigma Aldrich), ZM241385 (0.3µM, Tocris Bioscience) or 2 chloro-adenosine (CADO; 20 μM, Sigma Aldrich) were added to selected wells 30 min prior to the addition of S cells. To induce proliferation, RC were stimulated with plate-bound OKT-3 (2 µg/ml) and soluble anti-CD28 mAb (2 µg/ml) (Miltenyi) in the presence of 150 IU/ml IL-2 for 5 d. All CFSE data were analyzed using the ModFit software provided by Vertity Software (Topsham) as previously described.Citation42
Immunoflourescence
HNSCC tissue samples were embedded in OCT, and 5 mm frozen sections were cut in a cryostat, fixed for 10 min in cold acetone/ethanol (1:1) and dried at room temperature. The following anti-human Abs were used for staining: anti-CD4-FITC, anti-CD132-PE, anti-ADA, anti-FOXP3 and anti-CD26 (BD PharMingen). The secondary Ab was Cy5-labeled donkey anti-rabbit (Jackson Immuno Research). To eliminate non-specific staining, tissue sections were incubated with 10% donkey serum for 1 h and then washed in PBS. Sections were incubated with the primary Abs for 1 h in a moist chamber at room temperature. Next, slides were washed and incubated with the secondary Abs under the same conditions. Primary Abs were omitted in all negative controls. Sections were mounted in a mounting medium with DAPI (Vector Laboratories) in order to trace cell nuclei. Slides were evaluated in the Olympus Provis (Olympus) fluorescence microscope under 400 x mag. For digital image analysis the software Adobe Photoshop 6.0 version was used.
LUMINEX
Cytokine levels in supernatants of Teff cell cultures were analyzed by LUMINEX, using a 10-plex Ab bead kit (Biosource/Invitrogen). After 24 h of stimulation with OKT-3 (1μg/ml) and anti-CD28 (1μg/ml), supernatant were harvested and stored frozen until analyzed.
Statistical analysis
All data are presented as means of at least three experiments ± 1 standard deviation (SD). The data were analyzed using the student t-test. P values < 0.05 were considered to be significant.
| Abbreviations: | ||
| HNSCC | = | head and neck squamous cell carcinoma |
| Treg | = | regulatory T cells |
| Teff | = | effector T cells |
| ADA | = | adenosine deaminase |
| CADO | = | 2 chloro-adenosine |
| PGE2 | = | prostaglandin E2 |
| EHNA | = | erythro-9-(2-hydroxy-3-nonyl) adenine |
| CFSE | = | carboxyfluorescein sccinimidyl ester |
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
Supported in part by NIH grant PO1 CA109688 to TLW; NIH grants DK079307, DK068575 and HL069846 to EKJ; Polish Ministry of Science and Higher Education grant NN401047738 to MJS; and by the Interdisciplinary Grant from the University of Essen (IFORES) to MM.
References
- Kim PS, Ahmed R. Features of responding T cells in cancer and chronic infection. Curr Opin Immunol 2010; 22:223 - 30; http://dx.doi.org/10.1016/j.coi.2010.02.005; PMID: 20207527
- Chinen J, Shearer WT. Secondary immunodeficiencies, including HIV infection. J Allergy Clin Immunol 2010; 125:Suppl 2 S195 - 203; http://dx.doi.org/10.1016/j.jaci.2009.08.040; PMID: 20042227
- Schetinger MR, Morsch VM, Bonan CD, Wyse AT. NTPDase and 5′-nucleotidase activities in physiological and disease conditions: new perspectives for human health. Biofactors 2007; 31:77 - 98; http://dx.doi.org/10.1002/biof.5520310205; PMID: 18806312
- Mandapathil M, Hilldorfer B, Szczepanski MJ, Czystowska M, Szajnik M, Ren J, et al. Generation and accumulation of immunosuppressive adenosine by human CD4+CD25highFOXP3+ regulatory T cells. J Biol Chem 2010; 285:7176 - 86; http://dx.doi.org/10.1074/jbc.M109.047423; PMID: 19858205
- Raskovalova T, Lokshin A, Huang X, Su Y, Mandic M, Zarour HM, et al. Inhibition of cytokine production and cytotoxic activity of human antimelanoma specific CD8+ and CD4+ T lymphocytes by adenosine-protein kinase A type I signaling. Cancer Res 2007; 67:5949 - 56; http://dx.doi.org/10.1158/0008-5472.CAN-06-4249; PMID: 17575165
- Lokshin A, Raskovalova T, Huang X, Zacharia LC, Jackson EK, Gorelik E. Adenosine-mediated inhibition of the cytotoxic activity and cytokine production by activated natural killer cells. Cancer Res 2006; 66:7758 - 65; http://dx.doi.org/10.1158/0008-5472.CAN-06-0478; PMID: 16885379
- Dong RP, Kameoka J, Hegen M, Tanaka T, Xu Y, Schlossman SF, et al. Characterization of adenosine deaminase binding to human CD26 on T cells and its biologic role in immune response. J Immunol 1996; 156:1349 - 55; PMID: 8568233
- Richard E, Arredondo-Vega FX, Santisteban I, Kelly SJ, Patel DD, Hershfield MS. The binding site of human adenosine deaminase for CD26/Dipeptidyl peptidase IV: the Arg142Gln mutation impairs binding to cd26 but does not cause immune deficiency. J Exp Med 2000; 192:1223 - 36; http://dx.doi.org/10.1084/jem.192.9.1223; PMID: 11067872
- Morimoto C, Schlossman SF. The structure and function of CD26 in the T-cell immune response. Immunol Rev 1998; 161:55 - 70; http://dx.doi.org/10.1111/j.1600-065X.1998.tb01571.x; PMID: 9553764
- Hirschhorn R. Adenosine deaminase deficiency. Immunodefic Rev 1990; 2:175 - 98; PMID: 2078332
- Hershfield MS. New insights into adenosine-receptor-mediated immunosuppression and the role of adenosine in causing the immunodeficiency associated with adenosine deaminase deficiency. Eur J Immunol 2005; 35:25 - 30; http://dx.doi.org/10.1002/eji.200425738; PMID: 15580654
- Klinger M, Freissmuth M, Nanoff C. Adenosine receptors: G protein-mediated signalling and the role of accessory proteins. Cell Signal 2002; 14:99 - 108; http://dx.doi.org/10.1016/S0898-6568(01)00235-2; PMID: 11781133
- Ohta A, Sitkovsky M. Role of G-protein-coupled adenosine receptors in downregulation of inflammation and protection from tissue damage. Nature 2001; 414:916 - 20; http://dx.doi.org/10.1038/414916a; PMID: 11780065
- Raskovalova T, Huang X, Sitkovsky M, Zacharia LC, Jackson EK, Gorelik E. Gs protein-coupled adenosine receptor signaling and lytic function of activated NK cells. J Immunol 2005; 175:4383 - 91; PMID: 16177079
- Sitkovsky MV, Ohta A. The ‘danger’ sensors that STOP the immune response: the A2 adenosine receptors?. Trends Immunol 2005; 26:299 - 304; http://dx.doi.org/10.1016/j.it.2005.04.004; PMID: 15922945
- Borsellino G, Kleinewietfeld M, Di Mitri D, Sternjak A, Diamantini A, Giometto R, et al. Expression of ectonucleotidase CD39 by Foxp3+ Treg cells: hydrolysis of extracellular ATP and immune suppression. Blood 2007; 110:1225 - 32; http://dx.doi.org/10.1182/blood-2006-12-064527; PMID: 17449799
- Deaglio S, Dwyer KM, Gao W, Friedman D, Usheva A, Erat A, et al. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J Exp Med 2007; 204:1257 - 65; http://dx.doi.org/10.1084/jem.20062512; PMID: 17502665
- Mandapathil M, Szczepanski MJ, Szajnik M, Ren J, Lenzner DE, Jackson EK, et al. Increased ectonucleotidase expression and activity in regulatory T cells of patients with head and neck cancer. Clin Cancer Res 2009; 15:6348 - 57; http://dx.doi.org/10.1158/1078-0432.CCR-09-1143; PMID: 19825957
- Dieckmann D, Plottner H, Berchtold S, Berger T, Schuler G. Ex vivo isolation and characterization of CD4(+)CD25(+) T cells with regulatory properties from human blood. J Exp Med 2001; 193:1303 - 10; http://dx.doi.org/10.1084/jem.193.11.1303; PMID: 11390437
- Roncarolo MG, Bacchetta R, Bordignon C, Narula S, Levings MK. Type 1 T regulatory cells. Immunol Rev 2001; 182:68 - 79; http://dx.doi.org/10.1034/j.1600-065X.2001.1820105.x; PMID: 11722624
- Vila J, Isaacs JD, Anderson AE. Regulatory T cells and autoimmunity. Curr Opin Hematol 2009; 16:274 - 9; http://dx.doi.org/10.1097/MOH.0b013e32832a9a01; PMID: 19417650
- Beyer M, Schultze JL. Immunoregulatory T cells: role and potential as a target in malignancy. Curr Oncol Rep 2008; 10:130 - 6; http://dx.doi.org/10.1007/s11912-008-0021-z; PMID: 18377826
- Whiteside TL. The tumor microenvironment and its role in promoting tumor growth. Oncogene 2008; 27:5904 - 12; http://dx.doi.org/10.1038/onc.2008.271; PMID: 18836471
- Sitkovsky MV, Kjaergaard J, Lukashev D, Ohta A. Hypoxia-adenosinergic immunosuppression: tumor protection by T regulatory cells and cancerous tissue hypoxia. Clin Cancer Res 2008; 14:5947 - 52; http://dx.doi.org/10.1158/1078-0432.CCR-08-0229; PMID: 18829471
- Stagg J, Smyth MJ. Extracellular adenosine triphosphate and adenosine in cancer. Oncogene 2010; 29:5346 - 58; http://dx.doi.org/10.1038/onc.2010.292; PMID: 20661219
- Bynoe MS, Viret C. Foxp3+CD4+ T cell-mediated immunosuppression involves extracellular nucleotide catabolism. Trends Immunol 2008; 29:99 - 102; http://dx.doi.org/10.1016/j.it.2007.12.005; PMID: 18258482
- Komi Y, Ohno O, Suzuki Y, Shimamura M, Shimokado K, Umezawa K, et al. Inhibition of tumor angiogenesis by targeting endothelial surface ATP synthase with sangivamycin. Jpn J Clin Oncol 2007; 37:867 - 73; http://dx.doi.org/10.1093/jjco/hym115; PMID: 17956898
- Hoskin DW, Mader JS, Furlong SJ, Conrad DM, Blay J. Inhibition of T cell and natural killer cell function by adenosine and its contribution to immune evasion by tumor cells. Int J Oncol 2008; 32:527 - 35; PMID: 18292929
- Mandapathil M, Lang S, Gorelik E, Whiteside TL. Isolation of functional human regulatory T cells (Treg) from the peripheral blood based on the CD39 expression. J Immunol Methods 2009; 346:55 - 63; http://dx.doi.org/10.1016/j.jim.2009.05.004; PMID: 19450601
- Schuler PJ, Harasymczuk M, Schilling B, Lang S, Whiteside TL. Separation of human CD4+CD39+ T cells by magnetic beads reveals two phenotypically and functionally different subsets. J Immunol Methods 2011; 369:59 - 68; http://dx.doi.org/10.1016/j.jim.2011.04.004; PMID: 21513715
- Nagorsen D, Voigt S, Berg E, Stein H, Thiel E, Loddenkemper C. Tumor-infiltrating macrophages and dendritic cells in human colorectal cancer: relation to local regulatory T cells, systemic T-cell response against tumor-associated antigens and survival. J Transl Med 2007; 5:62; http://dx.doi.org/10.1186/1479-5876-5-62; PMID: 18047662
- Bergmann C, Strauss L, Wang Y, Szczepanski MJ, Lang S, Johnson JT, et al. T regulatory type 1 cells in squamous cell carcinoma of the head and neck: mechanisms of suppression and expansion in advanced disease. Clin Cancer Res 2008; 14:3706 - 15; http://dx.doi.org/10.1158/1078-0432.CCR-07-5126; PMID: 18559587
- Climent N, Martinez-Navio JM, Gil C, Garcia F, Rovira C, Hurtado C, et al. Adenosine deaminase enhances T-cell response elicited by dendritic cells loaded with inactivated HIV. Immunol Cell Biol 2009; 87:634 - 9; http://dx.doi.org/10.1038/icb.2009.53; PMID: 19668260
- Roncarolo MG, Gregori S. Is FOXP3 a bona fide marker for human regulatory T cells?. Eur J Immunol 2008; 38:925 - 7; http://dx.doi.org/10.1002/eji.200838168; PMID: 18395862
- Ehrentraut H, Westrich JA, Eltzschig HK, Clambey ET. Adora2b adenosine receptor engagement enhances regulatory T cell abundance during endotoxin-induced pulmonary inflammation. PLoS One 2012; 7 12; http://dx.doi.org/10.1371/journal.pone.0032416; PMID: 22389701
- Cordero OJ, Salgado FJ, Fernández-Alonso CM, Herrera C, Lluis C, Franco R, et al. Cytokines regulate membrane adenosine deaminase on human activated lymphocytes. J Leukoc Biol 2001; 70:920 - 30; PMID: 11739555
- Linden J. Adenosine metabolism and cancer. Focus on “Adenosine downregulates DPPIV on HT-29 colon cancer cells by stimulating protein tyrosine phosphatases and reducing ERK1/2 activity via a novel pathway”. Am J Physiol Cell Physiol 2006; 291:C405 - 6; http://dx.doi.org/10.1152/ajpcell.00242.2006; PMID: 16707553
- Erić-Nikolić A, Matić IZ, Dorđević M, Milovanović Z, Marković I, Džodić R, et al. Serum DPPIV activity and CD26 expression on lymphocytes in patients with benign or malignant breast tumors. Immunobiology 2011; 216:942 - 6; http://dx.doi.org/10.1016/j.imbio.2011.01.005; PMID: 21281985
- Van Linden A, Eltzschig HK. Role of pulmonary adenosine during hypoxia: extracellular generation, signaling and metabolism by surface adenosine deaminase/CD26. Expert Opin Biol Ther 2007; 7:1437 - 47; http://dx.doi.org/10.1517/14712598.7.9.1437; PMID: 17727332
- Salamone JD. Preladenant, a novel adenosine A(2A) receptor antagonist for the potential treatment of parkinsonism and other disorders. IDrugs 2010; 13:723 - 31; PMID: 20878595
- Hocher B. Adenosine A1 receptor antagonists in clinical research and development. Kidney Int 2010; 78:438 - 45; http://dx.doi.org/10.1038/ki.2010.204; PMID: 20592713
- Strauss L, Bergmann C, Gooding W, Johnson JT, Whiteside TL. The frequency and suppressor function of CD4+CD25highFoxp3+ T cells in the circulation of patients with squamous cell carcinoma of the head and neck. Clin Cancer Res 2007; 13:6301 - 11; http://dx.doi.org/10.1158/1078-0432.CCR-07-1403; PMID: 17975141
- Jackson EK, Ren J, Mi Z. Extracellular 2′,3′-cAMP is a source of adenosine. J Biol Chem 2009; 284:33097 - 106; http://dx.doi.org/10.1074/jbc.M109.053876; PMID: 19801686