Abstract
To gain further insights into the role of T lymphocytes in immune responses against bladder tumors, we developed a method that monitors the presence of functional antigen-specific T cells in the urine of non-muscle invasive bladder cancer patients. As relatively few immune cells can usually be recovered from urine, we examined different isolation/amplification protocols and took advantage of patients treated with weekly intravesical instillations of Bacillus Calmette-Guérin, resulting in large amounts of immune cells into urine. Our findings demonstrate that, upon in vitro amplification, antigen-specific T cells can be detected by an interferon γ (IFNγ)-specific ELISPOT assay.
Keywords: :
Introduction
Bladder cancer is the fourth and eight most common malignancy in men and women, respectively. Seventy percent of bladder cancers are diagnosed as non-muscle invasive, i.e., they are confined to the mucosal or submucosal layer.Citation1 However, 60–70% of the tumors will recur, and according to stage and grade, 10 to 40% will progress to an invasive bladder cancer. Treatment of advanced disease (i.e., muscle invasive bladder cancer) is based on the complete resection of the bladder (cystectomy) combined with chemotherapy or radiotherapy.Citation1,Citation2 The transurethral resection of bladder tumors (TURB) in combination with intravesical instillations of Bacillus Calmette-Guérin (BCG), an attenuated strain of Mycobacterium bovis, has been the standard of care for non-muscle invasive bladder cancer (NMIBC) patients since the work of Morales et al.Citation3 Intravesical BCG therapy has indeed shown to reduce both recurrence and progression.Citation4 BCG causes a strong local inflammation, which generates a robust infiltration of immune cells into the bladder wall and their accumulation in the urine. An influx of granulocytes and mononuclear cells is induced together with a Th1 cytokine profile, maturation of dendritic cell (DC) and activation of natural killer cells.Citation4,Citation5 This robust influx of cells involves not only cells from the innate immune system but also lymphocyte populations, which appear to contribute to the efficacy of BCG immunotherapy.Citation6-Citation8 Although BCG instillations for superficial bladder cancer treatment constitute the most successful immunotherapy on a large scale and are considered as a standard treatment for this indication, repeated BCG administration are associated with significant toxicity. Moreover, resistance occurs in 30 to 50% of cases, underlying the necessity for alternative or complementary immunotherapy. Tumor associated antigens (TAA) used as a vaccine to induce antitumor immune responses are a promising strategy for treating different neoplasms including bladder cancer, though with inconsistent clinical efficacy to date. Vaccine responses are usually measured in the blood, which may not reflect local antitumor T cells responses, de facto hindering the optimization of immunotherapies.
Monitoring cellular immune responses against a broad array of epitopes is complex, especially when limited testing material is available and when patients are not selected for particular HLA-types. In the case of bladder cancer, in order to better characterize antitumor T-cell responses, it would be interesting to analyze the specificity and the functionality of T cells present in patient’s urine, which may reflect the T lymphocytes infiltrating bladder tissue. One of the problem in this setting is that urine is a toxic environment that influences T cells viability and functionality.Citation9 To address these issues, we adapted techniques used to examine lymphocytes in other tissue locations. We took advantage of urine from NMIBC patients undergoing BCG treatment to maximize the recovery of immune cells. Using in vitro expanded CD3+ lymphocytes or total cells purified from freshly recovered urine in combination with an interferon γ (IFNγ)-specific ELISPOT assay, we were able to detect, for the first time, functional antigen-specific T lymphocyte responses from NMIBC patients. These techniques will be useful tools to monitor bladder cancer immunotherapy trials.
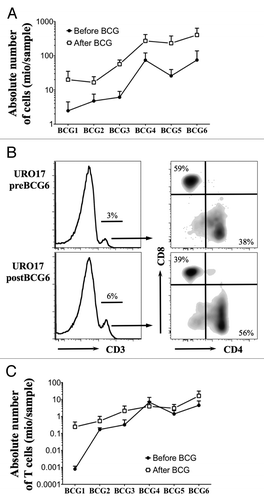
BCG therapy increased the prevalence of T cells in the urine of NMIBC patients. Although innate immune cells represent the main type of effector cells infiltrating the bladder mucosa after BCG instillations, some cells from the adaptive immune system, notably T cells, can also invade bladder tissue and therefore be found in the urine of patients throughout BCG therapy.Citation6,Citation10,Citation11 We took advantage of samples obtained from patients with different bladder cancer stages during an ongoing exploratory study (named URO) approved by the local ethical committee, and selected a subset of patients undergoing BCG instillations and analyzed the presence of T cells in urine by flow cytometry. Eleven patients diagnosed with NMIBC upon histological analysis were recommended to receive BCG treatment according to European guidelines. Most patients received six weekly intravesical instillations of OncoTICE® (MSD Merck Sharp and Dohme AG). Urine was collected before and 4 h after BCG treatment. In order to increase cell viability, urine samples (10–500 ml) were processed within 2 h after urine collection as follows. Samples were promptly supplemented with FCS (30% v/v),Citation9 then centrifuged and cells were resuspended in RPMI 1640 medium supplemented with 10% FCS and counted. As expected, we invariably observed a higher number of total cells in the urine after BCG instillations as compared with before the treatment (). We also found an increase in the total amount of cells recovered beyond the third BCG instillation. Samples from nine patients were available for cell cytometry analysis. To this aim, cells were stained with anti-CD3 AF700 (Biolegend), anti-CD8 PE/AF610 (Invitrogen) and anti-CD4 APC/H7 (PharMingen), followed by live/dead fixable aqua stain (Invitrogen).Citation12 As shown in , upon the exclusion of dead cells and doublets, we determined the percentage of ex vivo CD3+ cells and subsequently of CD8+ and CD4+ cells among CD3+ cells. While the proportion of CD3+ T cells remained stable throughout BCG therapy (data not shown), a regular increase in the absolute number of those cells was observed both before and after BCG instillations (). Similar results were found when results were normalized to the volume of each urine sample (data not shown), suggesting that T cells progressively infiltrate bladder tissue and are subsequently found in the urine of NMIBC patients during BCG therapy.
Figure 1. Ex vivo analysis of urine T cells during BCG therapy. (A) Mean +/− SEM of the absolute number of total cells recovered from urine samples before (white square) and after (black square) each BCG instillation (n = 11 patients). (B) Representative example of ex vivo CD3, CD8 and CD4 staining of urine cells from one NMIBC patient before and after the sixth BCG treatment. CD8 and CD4 dot plots are gated on CD3+ population. (C) Mean +/− SEM of absolute number of CD3 T cells from the analyzed urine samples before (white square) and after (black square) each BCG instillation (n = 9 patients).
In Vitro Expansion of Urine-Isolated T Cells
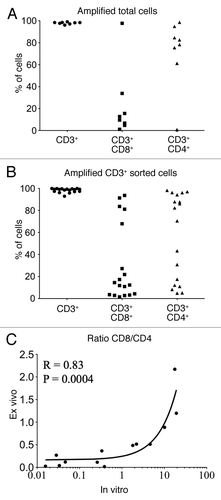
Since a low number of T cell was detected in the urine in absence of BCG or at early time points of BCG therapy, we decided to amplify them in vitro. Therefore, cells from the available urine sample were directly stimulated in vitro (5 × 104 to 4 × 106 total cells) in presence of irradiated (3,000 rad) allogenic PBMC (ratio: 1/1), and PHA (1 µg/ml) in RPMI 1640 medium supplemented with 8% human serum, 1% penicillin/streptomycin, 1% non-essential amino acids, 1% sodium pyruvate, β-mercaptoethanol and IL-2 (150 U/ml).Citation13 Medium was changed every 2 d for 2 to 4 weeks, when functional tests were performed. As shown in , out of 32 samples tested from seven patients, only about one third was able to grow in culture when total urine cells were directly stimulated. This poor rate of expansion was most likely due to the presence of high number of granulocytes in the urine upon BCG treatment,Citation6,Citation14,Citation15 which rapidly die and release toxic compounds during culture. In an attempt to remove these granulocytes, we performed Percoll or Ficoll gradients. However, we were unable to separate granulocytes from mononuclear cells (MNC), since surprisingly almost all granulocytes remained in the MNC layer after centrifugation. This is reminiscent of recently reported “low-density” neutrophils that can be found among the PBMCs from bladder, renal and lung cancer patients.Citation16 As an alternative purification method, we tested CD3+ magnetic sorting kit. CD3+ cells were primarily isolated from urine cells by magnetic sorting according to manufacturer’s instructions (Dynabeads FlowComp human CD3, 113–65D, Life Technologies) and subsequently stimulated as described above. Between 14 × 106 and 300 × 106 total urine cells were sorted and 3 × 103 to 2 × 106 CD3+ cells were obtained. Almost 82% of the 22 magnetically sorted samples from six patients were expanded (). These results show that T cells from the urine of bladder cancer patients may be reliably expanded in vitro using ex vivo magnetic sorting prior to culture. In contrast, only 31% of the urine samples cultured without prior purification were amplified upon stimulation.
Table 1. Expansion of cells from urine of NMIBC patients. Cells from available urine samples were stimulated in presence of allogenic irradiated PBMC, 150 U/ml IL-2 and 1 µg/ml PHA, either directly after washing (n = 7 patients) or after CD3+ magnetic sorting (n = 6 patients)
Next, we studied by flow cytometry the expression of CD3, CD8 and CD4 in all samples that were successfully amplified in culture from total cells () or from CD3+ sorted cells (). We found that all obtained populations are exclusively CD3+, with a varying proportion of CD8 and CD4 cells among them. Some samples had a predominance of CD4 T cells, while others contained a higher proportion of CD8 T cells (). To examine whether the permanence in culture influenced the T cell type obtained, we plotted the ratio of CD8 and CD4 T cells measured in the amplified CD3+ sorted populations against the ratio measured in the corresponding ex vivo samples prior to amplification (). Our data shows that there is a significant correlation between the CD8/CD4 ratio observed ex vivo and in vitro (Spearman R = 0.83, p = 0.0004). However, we observed a bias of in vitro expansion toward the population that was initially outnumbered ex vivo (). This suggests that despite in vitro amplification was performed in the absence of specific antigens, the frequency of antigen-specific T cells measured in amplified urine T cells may not faithfully reflect the ex vivo frequency.
Figure 2. Analysis of urine T cells expanded in vitro. (A and B) Percentage of CD3+, CD3+CD8+ and CD3+CD4+ T cells after in vitro amplification from total urine cells (A) or magnetically isolated T lymphocytes (B). (C) CD8/CD4 ratio among CD3+ T cells from ex vivo samples (vertical axis) were plotted against those obtained after in vitro amplification of CD3+ sorted T cells (horizontal axis). Spearman correlation R and p values are indicated.
Detection of Pathogen-Specific T Lymphocytes from Urine of Bladder Cancer Patients
Finally, we tried to detect the presence of antigen-specific T cells in the expanded population from patient’s urine by an IFNγ-specific ELISPOT assay (human IFNγ ELISPOT Kit, 856.051.020, Gen-Probe). In the absence of TAA vaccination, we examined the T cell responses to pathogens to which most of the patient have been exposed. As stimulating antigens, we used a pool of 27 HLA Class I and II restricted epitopes derived from different pathogens: Cytomegalovirus, Epstein Barr virus, influenza virus and Clostridium tetani (CEFT pool PepMix, PM-CEFT, JPT Technologies, see http://shop.jpt.com/images/Bild_5/3_UK_datasheet%2Bceft%2Bpool.pdf). The test was performed in nitrocellulose-lined 96-well plates (MultiScreen-HA sterile plate, MAHAS4510, Millipore). Briefly, plates were coated overnight with antibody to human IFNγ and washed eight times with PBS 0.05% Tween-20. Then, 2 × 105, 1 × 105 and 0.5 × 105 urine-isolated cells were added in 200 µL RPMI 1640 medium supplemented with 10% FCS with or without 10 µg/mL peptide CEFT pool mix and incubated for 18 h at 37°C. Assays were performed in duplicates. Then, cells were removed and plates incubated with a second biotinylated antibody to human IFNγ and streptavidin-alkaline phosphatase. The spots were revealed with BCIP/NBT substrate and counted. Results with more than 30 background spots per 105 T cells were considered invalid. In order to exclude false-positive results, statistical analyses of IFNγ ELISPOT responses were done using the free website tool (http://www.scharp.org/zoe/runDFR) developed by Moodie et al.Citation17 Antigen presenting cells (APC) were not added in this assay, because preliminary data using autologous monocytic derived dendritic cells showed a high background signal. Noteworthy, all T cells express HLA molecules at their surface, allowing them to present exogenous peptides and therefore serve as APC to the surrounding CD4 or CD8 T cells.Citation18
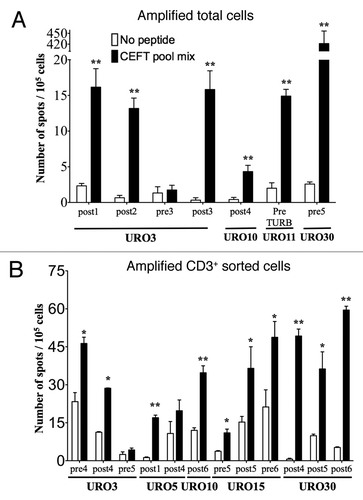
In vitro amplified samples obtained from NMIBC pre- and/or post-BCG or pre-TURB, from total and CD3+ cells obtained from four (URO3, 10, 11 and 30) and five patients (URO3, 5, 10, 15 and 30), respectively, were tested. Six out of seven samples (two pre-BCG, one pre-TURB and four post-BCG) from amplified total cells () and ten out of 12 samples (four pre-BCG and eight post-BCG) from amplified CD3+ sorted cells were specific for pathogen epitopes (). Of note, three samples of amplified total cells from patient URO30 and six samples of CD3+ sorted cells from patient URO15 and URO17 were removed from the analysis due to high background (mean +/− SEM of 157.4 +/− 44.8 spot / 105 cells in wells with medium alone, data not shown). Thus, with both amplification methods, about 30% of the CD3 cells yielded a too high background to be analyzable by ELISPOT. Preliminary experiments suggest that keeping amplified cells in culture for long time (> 4 weeks) may help in decreasing IFNγ secretion in the absence of peptides. In two patients for whom multiple urine samples were analyzed by ELISPOT, significant pathogen-specific T cell responses were not always measured (i.e., 5/7 in URO3 and 1/2 in URO5, ). This may reflect local, intrabladder variations in the T cell responses, highlighting the necessity and relevance to determine immune responses at the site of the tumor.Citation19-Citation21 However, a more comprehensive and longitudinal analysis is needed in order to determine the variation of T cell immune responses in the bladder upon BCG therapy and possibly its correlation with systemic responses.
Figure 3. Detection of antigen-specific T cells in the urine of NMIBC patients. The presence of functional antigen-specific T lymphocytes was assessed using an IFNγ- specific ELISPOT assay on amplified total cells (A) or amplified CD3+ sorted cells (B) as isolated from the urine of NMIBC patients. Samples from each of the indicated URO patient correspond to urines obtained at the specified pre- and/or post-BCG instillation or pre-TURB time, after which T cells were expanded in vitro and tested by ELISPOT. Number of IFNγ secreting cells (spot)/105 cells are indicated in presence of peptide (black bars) or medium alone (white bars). Significant differences were calculated with the free website tool (http://www.scharp.org/zoe/runDFR).Citation17 p < 0.05 and p < 0.01 are indicated by * and **, respectively.
Altogether, our data demonstrate that antigen-specific T cells isolated from the urine may be detected by IFNγ-specific ELISPOT assay, after in vitro amplification. However, our data showed that total urine cells were hardly expanded in vitro as compared with purified CD3+ T cells (). In addition, when a low amount of cells (< 107 cells) is recovered, which is often the case in early time points after BCG therapy or in pre-BCG samples, magnetic isolation is not possible due to the low proportion of CD3+ T cells. This limitation has to be considered when trying to monitor antigen-specific T cells from the urine in the absence of BCG treatment or at early time points after the initiation of therapy. A solution may be to gather multiple urine samples from a given patient to augment the probability to achieve T cell amplification and thus IFNγ ELISPOT analysis. Finally, the massive accumulation of immune cells in urine due to BCG is a unique opportunity to recover, without any invasive surgery, cells that have potentially infiltrated tumor sites. This has interesting implications for the monitoring of antigen-specific therapies targeting T cells in superficial bladder cancer patients.
| Abbreviations: | ||
| BCG | = | Bacillus Calmette-Guérin |
| ELISPOT | = | enzyme-linked immunosorbent spot |
| IFNγ | = | interferon γ |
| NMIBC | = | non-muscle invasive bladder cancer |
| PHA | = | phytohaemagglutinin |
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We are obliged to the patients for their dedicated collaboration. This work was supported by institutional funds of the Department of Urology.
References
- Metts MC, Metts JC, Milito SJ, Thomas CR Jr. Bladder cancer: a review of diagnosis and management. J Natl Med Assoc 2000; 92:285 - 94; PMID: 10918764
- Utz DC, Farrow GM. Management of carcinoma in situ of the bladder: the case for surgical management. Urol Clin North Am 1980; 7:533 - 41; PMID: 7456165
- Morales A, Eidinger D, Bruce AW. Intracavitary Bacillus Calmette-Guerin in the treatment of superficial bladder tumors. J Urol 1976; 116:180 - 3; PMID: 820877
- Schenk-Braat EA, Bangma CH. Immunotherapy for superficial bladder cancer. Cancer Immunol Immunother 2005; 54:414 - 23; http://dx.doi.org/10.1007/s00262-004-0621-x; PMID: 15565330
- Totterman TH, Loskog A, Essand M. The immunotherapy of prostate and bladder cancer. BJU Int 2005; 96:728 - 35; http://dx.doi.org/10.1111/j.1464-410X.2005.05772.x; PMID: 16144528
- Bisiaux A, Thiounn N, Timsit MO, Eladaoui A, Chang HH, Mapes J, et al. Molecular analyte profiling of the early events and tissue conditioning following intravesical bacillus calmette-guerin therapy in patients with superficial bladder cancer. J Urol 2009; 181:1571 - 80; http://dx.doi.org/10.1016/j.juro.2008.11.124; PMID: 19230924
- Bredan R, Bisiaux A, Biot C, Rentsch CA, Bousso P, Albert ML. Mathematical model of tumor immunotherapy for bladder carcinoma identifies the limitations of the innate immune response. OncoImmunology 2012; 1:9 - 17; http://dx.doi.org/10.4161/onci.1.1.17884
- Ratliff TL. Role of the immune response in BCG for bladder cancer. Eur Urol 1992; 21:Suppl 2 17 - 21; PMID: 1396942
- Stachowski J, Barth C, Lewandowska-Stachowiak M, Lammerding P, Runowski D, Baldamus CA. Flow cytometric analysis of urine lymphocytes isolated from patients with renal transplants--purification of urine lymphocytes. J Immunol Methods 1998; 213:145 - 55; http://dx.doi.org/10.1016/S0022-1759(98)00013-1; PMID: 9692847
- de Boer EC, de Jong WH, van der Meijden AP, Steerenberg PA, Witjes F, Vegt PD, et al. Leukocytes in the urine after intravesical BCG treatment for superficial bladder cancer. A flow cytofluorometric analysis. Urol Res 1991; 19:45 - 50; http://dx.doi.org/10.1007/BF00294021; PMID: 2028562
- De Boer EC, De Jong WH, Van Der Meijden AP, Steerenberg PA, Witjes JA, Vegt PD, et al. Presence of activated lymphocytes in the urine of patients with superficial bladder cancer after intravesical immunotherapy with bacillus Calmette-Guérin. Cancer Immunol Immunother 1991; 33:411 - 6; http://dx.doi.org/10.1007/BF01741603; PMID: 1878894
- Derré L, Jandus C, Baumgaertner P, Posevitz V, Devêvre E, Romero P, et al. Quantitative multiparameter assays to measure the effect of adjuvants on human antigen-specific CD8 T-cell responses. Methods Mol Biol 2010; 626:231 - 49; http://dx.doi.org/10.1007/978-1-60761-585-9_16; PMID: 20099132
- Brunst M, Shahmanesh M, Sukthankar A, Pearce JH, Gaston JS. Isolation and characterisation of T lymphocytes from the urethra of patients with acute urethritis. Sex Transm Infect 1998; 74:279 - 83; http://dx.doi.org/10.1136/sti.74.4.279; PMID: 9924470
- Siracusano S, Vita F, Abbate R, Ciciliato S, Borelli V, Bernabei M, et al. The role of granulocytes following intravesical BCG prophylaxis. Eur Urol 2007; 51:1589 - 97, discussion 1597-9; http://dx.doi.org/10.1016/j.eururo.2006.11.045; PMID: 17222501
- Suttmann H, Riemensberger J, Bentien G, Schmaltz D, Stöckle M, Jocham D, et al. Neutrophil granulocytes are required for effective Bacillus Calmette-Guérin immunotherapy of bladder cancer and orchestrate local immune responses. Cancer Res 2006; 66:8250 - 7; http://dx.doi.org/10.1158/0008-5472.CAN-06-1416; PMID: 16912205
- Brandau S, Trellakis S, Bruderek K, Schmaltz D, Steller G, Elian M, et al. Myeloid-derived suppressor cells in the peripheral blood of cancer patients contain a subset of immature neutrophils with impaired migratory properties. J Leukoc Biol 2011; 89:311 - 7; http://dx.doi.org/10.1189/jlb.0310162; PMID: 21106641
- Moodie Z, Price L, Gouttefangeas C, Mander A, Janetzki S, Löwer M, et al. Response definition criteria for ELISPOT assays revisited. Cancer Immunol Immunother 2010; 59:1489 - 501; http://dx.doi.org/10.1007/s00262-010-0875-4; PMID: 20549207
- Lanzavecchia A, Roosnek E, Gregory T, Berman P, Abrignani S. T cells can present antigens such as HIV gp120 targeted to their own surface molecules. Nature 1988; 334:530 - 2; http://dx.doi.org/10.1038/334530a0; PMID: 2841610
- Decrausaz L, Revaz V, Bobst M, Corthésy B, Romero P, Nardelli-Haefliger D. Induction of human papillomavirus oncogene-specific CD8 T-cell effector responses in the genital mucosa of vaccinated mice. Int J Cancer 2010; 126:2469 - 78; PMID: 19816937
- Piersma SJ, Jordanova ES, van Poelgeest MI, Kwappenberg KM, van der Hulst JM, Drijfhout JW, et al. High number of intraepithelial CD8+ tumor-infiltrating lymphocytes is associated with the absence of lymph node metastases in patients with large early-stage cervical cancer. Cancer Res 2007; 67:354 - 61; http://dx.doi.org/10.1158/0008-5472.CAN-06-3388; PMID: 17210718
- Revaz V, Debonneville A, Bobst M, Nardelli-Haefliger D. Monitoring of vaccine-specific gamma interferon induction in genital mucosa of mice by real-time reverse transcription-PCR. Clin Vaccine Immunol 2008; 15:757 - 64; http://dx.doi.org/10.1128/CVI.00392-07; PMID: 18367582