Abstract
This commentary provides the authors’ perspective on the article “Ipilimumab and a poxviral vaccine targeting prostate-specific antigen in metastatic castration-resistant prostate cancer: a phase 1 dose-escalation trial,” which has recently been published in The Lancet Oncology.
We recently published the results of a Phase I dose-escalation trial combining ipilimumab (anti-CTLA-4) and the PSA-TRICOM (PROSTVAC) vaccine.Citation1 Sipuleucel-T was the first therapeutic cancer vaccine to receive US Food and Drug Administration (FDA) approval after demonstrating an overall survival (OS) benefit compared with placebo control in patients with metastatic castration-resistant prostate cancer (mCRPC). The promise of clinical benefit with cancer vaccines was bolstered by this positive result and subsequent approval,Citation2 and indicated that endpoints commonly used in standard cancer therapies, such as progression-free survival (PFS), may not be ideal for evaluating cancer vaccines.Citation3 Indeed, it appears that the immune response develops over time and is sustained after treatment is completed, resulting in prolonged OS but not improved PFS. Similar results were seen in a multicenter Phase II randomized trial of PSA-TRICOM, which demonstrated an 8.5-mo improvement in median OS, as compared with placebo (p = 0.006), despite a lack of improvement in PFS.Citation4 PSA-TRICOM is now being investigated in a Phase III trial.
CTLA-4 on T cells binds to CD80 (B7) on antigen-presenting cells, and attenuates the immune response after T-cell activation as part of the body’s own means of regulating immune responses.Citation5 Ipilimumab, a monoclonal antibody directed against CTLA-4, can inhibit such a negative regulatory signal, and potentially create more robust T-cell responses and higher-avidity antitumor T cells.Citation6 Ipilimumab was approved for treatment of metastatic melanoma by the FDA, based on improved OS for patients receiving this treatment compared with an active control.Citation7 Combining anti-CTLA-4 with PSA-TRICOM potentially provides directed T-cell activation, while allowing those activated T cells to kill tumor cells owing to diminished inhibitory signals.
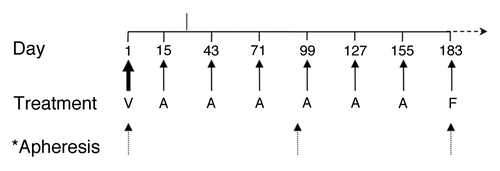
The recently reported Phase I trialCitation1 was undertaken to determine if the combination of PSA-TRICOM (which induces positive co-stimulation) and ipilimumab (which blocks negative co-stimulation) in patients with mCRPC would lead to increased adverse events over what was expected from ipilimumab alone, whose known toxicities include autoimmune colitis, rash, hypophysitis, hypothyroidism, and adrenal insufficiency.Citation7 A priming dose of 2 × 108 plaque-forming units (PFU) of recombinant vaccinia (rV)-PSA-TRICOM was given to all patients on day 1. Cohorts 1 to 4 received ipilimumab as a 90 min intravenous infusion at 1, 3, 5, and 10 mg/kg respectively, in combination with 1 × 109 PFU of recombinant fowlpox (rF)-PSA-TRICOM on day 15 of cycle 1, then every 28 d (). The trial was initially designed to give 6 doses of ipilimumab and then continue with monthly vaccine courses if patients had stable disease, but an amendment allowed for 4 additional doses of ipilimumab.
Figure 1. Schematic representation of dosing schedule on trial. V, PROSTVAC-V/TRICOM (vaccinia) × 1 dose + sargramostim daily × 4 doses (no ipilimumab); A, PROSTVAC-F/TRICOM (fowlpox) × 1 dose + ipilimumab × 1 dose + sargramostim daily × 4 doses; F, PROSTVAC-F/TRICOM (fowlpox) × 1 dose + sargramostim daily × 4 doses (no ipilimumab).
For the 30 patients enrolled, the combination of PSA-TRICOM and ipilimumab had a similar toxicity profile to that described with ipilimumab alone, with increased incidence of autoimmune endocrinopathies at the 5 and 10 mg/kg doses and colitis at all doses higher than 1 mg/kg. Rash was also common, in particular at the dose of 10 mg/kg. The maximum tolerated dose was not exceeded. Additional hypothesis-generating data were presented.
At the midpoint of dose level 2, the protocol was amended to enroll only chemotherapy-naïve patients to maximize the immune effect.Citation8 Only 1 out of 6 patients who had previous chemotherapy had a PSA decline. Of the remaining 24 chemotherapy-naïve patients, 58% (n = 14) had PSA declines and 25% (n = 6) had PSA declines ≥ 50%. Both these findings represent substantial ameliorations as compared with previous studies employing PSA-TRICOM alone.Citation4,Citation9 There was no evidence of anti-PSA antibodies to explain the decrease in PSA values. Autoimmune endocrinopathy (e.g., adrenal insufficiency) was a potential confounder that may have been responsible for PSA declines, but 2 patients with PSA declines ≥ 50% experienced no autoimmune endocrinopathy. Of 12 patients with measurable disease, 3 had unconfirmed partial responses. There was no significant difference in OS among the cohorts attributable to the dose of ipilimumab. Median OS for all patients was 34.4 mo (95% CI 29.6 to > 41), with a 2-y OS of 73% (95% CI 55.6 to 85.8). In the 24 chemotherapy-naïve patients, 3-y OS was 52.6% (95% CI 31.4 to 72.9). After a median potential follow-up of 48.5 mo, median OS has not yet been reached.
Peripheral blood mononuclear cells from HLA-A2+ patients were tested by an enzyme-linked immunosorbent spot (ELISPOT) assay to measure immunologic responses (PSA-specific T cells). Of the 3 patients evaluable at the highest dose level, 2 had increased responses to PSA post-treatment and 1 had PSA-specific T cells pre- and post-treatment. Although not explicitly targeted by the vaccine, some patients generated T cells specific for additional tumor-associated antigens post-treatment. This finding is likely attributable to the previously described phenomenon of the so-called “antigen cascade,” in which patients develop immune responses to antigens found on tumor cells, but not found in the vaccine, through cross-priming during T-cell killing.Citation10 Overall, 6 of 9 evaluable patients had enhanced antigen-specific T-cell responses.
This trial demonstrated that the combination of 10 mg/kg of ipilimumab plus a vaccine with substantial positive costimulation (PSA-TRICOM) does not worsen toxicity compared with ipilimumab alone. Additionally, the secondary data suggest that the median OS of patients treated with this combination (34.4 mo) may be superior to vaccine therapy alone (26 mo).Citation2,Citation4 While this trial was not randomized, OS in chemotherapy-naïve patients is greater than would be expected in a historically similar patient population and provides the rationale for a larger randomized clinical trial to determine the efficacy of combining checkpoint inhibition with PSA-TRICOM vaccine.
References
- Madan RA, Mohebtash M, Arlen PM, Vergati M, Rauckhorst M, Steinberg SM, et al. Ipilimumab and a poxviral vaccine targeting prostate-specific antigen in metastatic castration-resistant prostate cancer: a phase 1 dose-escalation trial. [Epub ahead of print] Lancet Oncol 2012; 13:501 - 8; http://dx.doi.org/10.1016/S1470-2045(12)70006-2; PMID: 22326924
- Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF, et al, IMPACT Study Investigators. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med 2010; 363:411 - 22; http://dx.doi.org/10.1056/NEJMoa1001294; PMID: 20818862
- Gulley JL, Drake CG. Immunotherapy for prostate cancer: recent advances, lessons learned, and areas for further research. Clin Cancer Res 2011; 17:3884 - 91; http://dx.doi.org/10.1158/1078-0432.CCR-10-2656; PMID: 21680544
- Kantoff PW, Schuetz TJ, Blumenstein BA, Glode LM, Bilhartz DL, Wyand M, et al. Overall survival analysis of a phase II randomized controlled trial of a Poxviral-based PSA-targeted immunotherapy in metastatic castration-resistant prostate cancer. J Clin Oncol 2010; 28:1099 - 105; http://dx.doi.org/10.1200/JCO.2009.25.0597; PMID: 20100959
- Egen JG, Kuhns MS, Allison JP. CTLA-4: new insights into its biological function and use in tumor immunotherapy. Nat Immunol 2002; 3:611 - 8; http://dx.doi.org/10.1038/ni0702-611; PMID: 12087419
- Hodge JW, Chakraborty M, Kudo-Saito C, Garnett CT, Schlom J. Multiple costimulatory modalities enhance CTL avidity. J Immunol 2005; 174:5994 - 6004; PMID: 15879092
- Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010; 363:711 - 23; http://dx.doi.org/10.1056/NEJMoa1003466; PMID: 20525992
- von Mehren M, Arlen P, Tsang KY, Rogatko A, Meropol N, Cooper HS, et al. Pilot study of a dual gene recombinant avipox vaccine containing both carcinoembryonic antigen (CEA) and B7.1 transgenes in patients with recurrent CEA-expressing adenocarcinomas. Clin Cancer Res 2000; 6:2219 - 28; PMID: 10873071
- Gulley JL, Arlen PM, Madan RA, Tsang KY, Pazdur MP, Skarupa L, et al. Immunologic and prognostic factors associated with overall survival employing a poxviral-based PSA vaccine in metastatic castrate-resistant prostate cancer. Cancer Immunol Immunother 2010; 59:663 - 74; http://dx.doi.org/10.1007/s00262-009-0782-8; PMID: 19890632
- Kudo-Saito C, Schlom J, Hodge JW. Induction of an antigen cascade by diversified subcutaneous/intratumoral vaccination is associated with antitumor responses. Clin Cancer Res 2005; 11:2416 - 26; http://dx.doi.org/10.1158/1078-0432.CCR-04-1380; PMID: 15788693