Abstract
Cancer immunotherapy attempts to harness the immune system by breaking tolerance and generating a robust anticancer response. We have recently demonstrated a synergistic enhancement in tumor clearance following therapeutic, dual PD-1 and LAG-3 blockade. Here, we discuss the implications of these findings in the context of future combinatorial immunotherapeutic approaches.
Cancer immunotherapy attempts to harness the body’s own immune system to generate a robust anticancer response. However, preservation of homeostasis requires the presence of multiple inhibitory checkpoints. Thus, negative receptors on immune cells can serve as pivotal regulators of immune escape in cancer. In a report that we have recently published in Cancer Research,Citation1 we explored the extent of synergy and cooperative interactions between the inhibitory receptors LAG-3 and PD-1 in several cancer models.
PD-1 (programmed death 1, CD279, gene name PCDC1) is a negative co-stimulatory molecule that play san important role in restraining adaptive immune responses. Its ligand, PD-L1, is expressed on multiple cell types including B cells, T cells, dendritic cells (DCs), macrophages and tumor cells. Engagement of the PD-1/PD-L1 pathway results in bidirectional activity, including inhibition of T-cell effector function, cytokine secretion, and proliferation. High levels of PD-1 are associated with exhausted or chronically stimulated T cells. Moreover, increased PD-1 expression correlates with reduced survival in cancer patients.Citation2
LAG-3 (lymphocyte activation gene, CD223, gene name LAG3) is a negative regulatory molecule expressed upon activation of various lymphoid cell types. Its high-affinity binding to MHC Class II leads to negative regulation of T cell proliferation and homeostasis. In addition, LAG-3 is required for the optimal function of both natural and induced immunosoppressive Treg cells.Citation3,Citation4 We and others have demonstrated the co-expression of LAG-3 and PD-1 by tumor-infiltrating lymphocytes (TILs).Citation5,Citation6 This dual expression is enriched in lymphocytes infiltrating certain human malignancies and correlates with impaired CD8+ effector function. These data, along with other preclinical findings, suggest that dual inhibition of the negative regulators LAG-3 and PD-1 could enhance T effector activity and improve antitumor immunity.
In our recent Cancer Research article,Citation1 we demonstrated that combinatorial blockade of PD-1 and LAG-3 (with monoclonal antibodies) synergistically limited the growth of established tumors, using multiple transplantable cancer models that were largely resistant to either antibody-based monotherapy. Surprisingly, over three-quarters of mice bearing MC38 carcinoma or Sa1N fibrosarcoma and co-treated with anti-PD-1 and anti-LAG-3 antibodies cleared tumors completely and survived for long periods with no evidence of disease. Importantly, mice receiving combinatorial anti–LAG-3/anti–PD-1 immunotherapy displayed no evidence of systemic or organ-specific autoimmunity, despite efficient tumor clearance.
We also generated mice harboring null mutations in both the Lag3 and Pdcd1 genes. The spontaneous autoimmune phenotype and lethality caused by these mutations,Citation1 not seen in either single knockout mouse, demonstrates clear synergy between LAG-3 and PD-1. Importantly, Lag3─/─Pdcd1─/─ mice were able to resist the growth of multiple transplantable tumors including B16 and MC38-derived neoplasms. Molecular analyses of tumor-bearing mice revealed enhanced interferon γ (IFNγ) production in tumor-draining lymph nodes and TILs, phenotypically similar to the dual antibody-treated mice (). These findings again demonstrate the clear synergy between the PD-1 and LAG-3 inhibitory pathways in tolerance to both self and tumor-associated antigens, suggesting that the dual blockade of these signaling pathways may represent a promising therapeutic strategy for cancer patients.
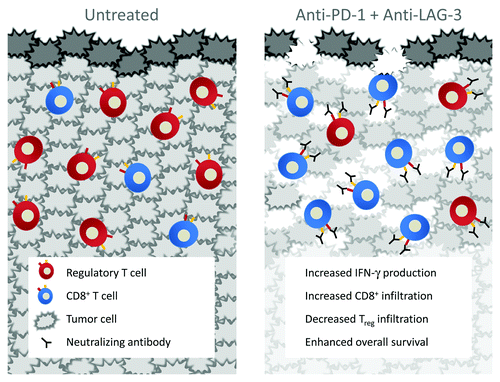
Figure 1. Untreated tumors are infiltrated with a high ratio of regulatory T-cells (Treg) to effector T-cells. Dual inhibition of the negative regulatory molecules PD-1 and LAG-3 leads to enhanced CD8:Treg infiltration, proinflammatory cytokine production, and increased survival of tumor-bearing animals.
There is considerable interest in the therapeutic potential of targeting inhibitory molecules in cancer, which has seen renewed impetus following the recent approval of ipilimumab (anti-CTLA-4; cytotoxic T-lymphocyte-associated protein 4; CD152) for the treatment of metastatic melanoma. Although results from Phase III clinical trials demonstrated a clear survival benefit,Citation7 notable immune toxicity was observed, raising concerns that combinatorial therapy with ipilimumab may result in clinically significant levels of side effects. Thus a key challenge for future combinatorial immunotherapy approaches is whether it will be possible to achieve increased efficacy without enhanced toxicity.
CTLA-4, PD-1 and LAG-3 are inhibitory molecules expressed during T-cell activation. However, high LAG-3/PD-1 expression is mostly restricted to infiltrating TILs. Thus, LAG-3/PD-1 combinatorial immunotherapy may exhibit less systemic toxicity than CTLA-4 blockade while promoting potent tumor-specific responses. BMS 936,558 (MDX-1106), a blocking antibody that targets PD-1, has been evaluated in a Phase I clinical trial in refractory solid tumors.Citation9 Blockade of PD-1 was well tolerated, with only one serious adverse event reported in this first-in-man trial. Thus, it is possible that BMS 936,558 will show less toxicity than ipilimumab despite comparable efficacy. While BMS 936,558 may prove effective as a standalone therapy, combinations with other immunotherapeutic, chemotherapeutic or radiotherapeutic agents are likely to increase efficacy in a substantial fashion. Indeed, a trial combining anti-PD-1 with CTLA-4 blockade is currently accruing (NCT01024231).
Our demonstration of potent synergy between LAG-3 and PD-1 highlights a promising immunotherapeutic combination for future trials. Nevertheless, the spontaneous autoimmune phenotype observed in Lag3─/─Pdcd1─/─ mice suggest the existence of a potentially thin borderline between efficacy and toxicity. Our study has also highlighted the difficulty in predicting the outcome of combinatorial treatments. As a monotherapy, anti-LAG-3 appeared to have modest effects depending on the tumor model, but was highly effective when combined with anti-PD-1. So, although anti-LAG-3 has not yet been evaluated in the clinic, one might predict that it will result in improved efficacy when combined with approaches such as anti-PD-1 therapies.
Future therapeutic strategies may also combine dual PD-1/LAG3 blockade with other modalities that target different effector or regulatory populations or pathways. These might include monoclonal antibodies against other immune checkpoints or inhibitory molecules such as the recently identified inhibitory cytokine IL-35,Citation10 targeted therapies including tyrosine kinase inhibitors, focal radiotherapy, therapeutic vaccination and/or cytotoxic chemotherapy. Given the dangers of enhanced toxicity inherent in combination regimens, the most efficient combinations may be those that target disparate cell populations and mechanisms, or those that exhibit minimal toxicity as monotherapies.
| Abbreviations: | ||
| PD-1 | = | programmed death 1 |
| LAG-3 | = | lymphocyte activation gene 3 |
| CTLA-4 | = | cytotoxic T-lymphocyte-associated protein 4 |
References
- Woo SR, Turnis ME, Goldberg MV, Bankoti J, Selby M, Nirschl CJ, et al. Immune inhibitory molecules LAG-3 and PD-1 synergistically regulate T-cell function to promote tumoral immune escape. Cancer Res 2012; 72:917 - 27; http://dx.doi.org/10.1158/0008-5472.CAN-11-1620; PMID: 22186141
- Wang W, Lau R, Yu D, Zhu W, Korman A, Weber J. PD1 blockade reverses the suppression of melanoma antigen-specific CTL by CD4+ CD25(Hi) regulatory T cells. Int Immunol 2009; 21:1065 - 77; http://dx.doi.org/10.1093/intimm/dxp072; PMID: 19651643
- Huang CT, Workman CJ, Flies D, Pan X, Marson AL, Zhou G, et al. Role of LAG-3 in regulatory T cells. Immunity 2004; 21:503 - 13; http://dx.doi.org/10.1016/j.immuni.2004.08.010; PMID: 15485628
- Workman CJ, Vignali DA. Negative regulation of T cell homeostasis by lymphocyte activation gene-3 (CD223). J Immunol 2005; 174:688 - 95; PMID: 15634887
- Grosso JF, Goldberg MV, Getnet D, Bruno TC, Yen HR, Pyle KJ, et al. Functionally distinct LAG-3 and PD-1 subsets on activated and chronically stimulated CD8 T cells. J Immunol 2009; 182:6659 - 69; http://dx.doi.org/10.4049/jimmunol.0804211; PMID: 19454660
- Baitsch L, Legat A, Barba L, Fuertes Marraco SA, Rivals JP, Baumgaertner P, et al. Extended co-expression of inhibitory receptors by human CD8 T-cells depending on differentiation, antigen-specificity and anatomical localization. PLoS One 2012; 7:e30852; http://dx.doi.org/10.1371/journal.pone.0030852; PMID: 22347406
- Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010; 363:711 - 23; http://dx.doi.org/10.1056/NEJMoa1003466; PMID: 20525992
- Curran MA, Montalvo W, Yagita H, Allison JP. PD-1 and CTLA-4 combination blockade expands infiltrating T cells and reduces regulatory T and myeloid cells within B16 melanoma tumors. Proc Natl Acad Sci U S A 2010; 107:4275 - 80; http://dx.doi.org/10.1073/pnas.0915174107; PMID: 20160101
- Brahmer JR, Drake CG, Wollner I, Powderly JD, Picus J, Sharfman WH, et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol 2010; 28:3167 - 75; http://dx.doi.org/10.1200/JCO.2009.26.7609; PMID: 20516446
- Collison LW, Workman CJ, Kuo TT, Boyd K, Wang Y, Vignali KM, et al. The inhibitory cytokine IL-35 contributes to regulatory T-cell function. Nature 2007; 450:566 - 9; http://dx.doi.org/10.1038/nature06306; PMID: 18033300