Abstract
Vaccines that generate Ag-specific CD8+ T-cell responses of appropriate quality, magnitude and duration are highly desirable. The ability of mTOR to regulate CD8+ T-cell functional differentiation must be exploited for clinical benefit. In a recent paper, we report that varying the regimen of rapamycin administration regulates viral vaccine-induced CD8+ T-cell responses for tumor immunity. These observations validate the use of rapamycin in vaccination strategies and demonstrate the efficacy of memory CD8+ T-cell responses for tumor immunity.
Keywords: :
The ability of CD8+ T cells to induce tumor regression makes them an attractive target for vaccination strategies.Citation1 Emerging information demonstrates the importance of memory CD8+ T cells in tumor immunity.Citation2 Inducing effective memory T-cell responses is a major goal for vaccines protecting against various infectious agents and cancer. Hence, new vaccine strategies that exploit mechanisms regulating memory CD8+ T cell generation are urgently needed. The striking discovery that rapamycin-mediated inhibition of the mechanistic target of rapamycin (mTOR) augments CD8+ T-cell memory,Citation3 has fuelled new research to understand rapamycin-mediated modulation of CD8+ T cell functional maturation. The rapamycin-dependent transition of effector CD8+ T cells to their memory counterparts is mediated by a switch in the expression of the T-box family of transcriptional factor T-bet to Eomesodermin.Citation2,Citation4 In contrast to the previously reported ability of rapamycin administration to generate tolerance,Citation5 due to CD4+ T-cell anergy,Citation6 and/or deviation to regulatory cells,Citation7 several recent studies have reported exacerbated immune responses by using lower doses of rapamycin for shorter administration times.Citation3,Citation4 The likelihood that distinct regimens of rapamycin administration may produce distinct functional outcomes in antigen-specific T cells implies that rapamycin, an FDA approved drug, may be a useful tool if carefully applied to generate memory CD8+ T cells.Citation3 It is noteworthy that the potential clinical benefits of rapamycin must be carefully weighed against its immunosuppressive properties and its renal toxicity, to maximize therapeutic index.
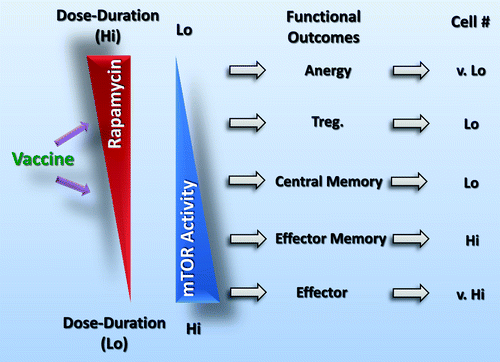
Vaccination with viral vectors has been extensively used to generate high numbers of tumor-specific CD8+ T cells with robust Type 1 effector functions. However, modest clinical benefits for cancer patients have been noted.Citation8 Recent data demonstrating rapamycin treatment induced enhanced memory CD8+ T-cell responses behooves us to carefully test the use of rapamycin for the generation of durable immunity against tumor by vaccine strategies. To this aim, it was imperative to perform studies in murine model systems that would allow for a precise characterization of the impact of rapamycin administration schedule (dose and duration) on antigen-specific CD8+ T cells in vivo. The findings by Li et al.Citation9 reveal () several new insights: (1) transient but complete block of mTOR by a short course of high-dose rapamycin reduces but does not block proliferation of viral vaccine-induced CD8+ T cells; (2) low-dose rapamycin fails to block mTOR activity, CD8+ T-cell proliferation and functional maturation. but augments their survival; (3) short course of high-dose rather than low-dose rapamycin potentiates vaccine-induced memory CD8+ T-cell responses; (4) short-course of high-dose rapamycin generates memory CD8+ T-cell responses that are qualitatively distinct from those elicited by low-dose rapamycin and independent of cell-extrinsic factors such as the presence of interleukin (IL)-7 and IL-15; (5) persistent rapamycin administration at low doses does not enhance, and at high doses this de facto reduces, CD8+ T-cell memory; (6) short course of high-dose rapamycin affords durable protection against tumor better than the persistent administration of either low or high rapamycin doses. The results presented in this paper support the notion that a rational use of rapamycin can augment the efficacy of vaccines against cancer and possibly for various infectious diseases including human immunodeficiency virus (HIV), tuberculosis (TB) and hepatitis C virus (HCV). A recent report by Lamming et al.Citation10 indicates that chronic rapamycin treatment disrupts the association of mTOR with both Raptor (within the multiprotein complex known as mTORC1) and Rictor (within mTORC2) in vivo. This information further validates our findings that the dosage and administration schedule for rapamycin-based regimens is critical in determining CD8+ T cell differentiation for immunity. These observations provide a platform for the use of rapamycin to enhance vaccine-induced clonal expansion of T cells and will be useful in designing preclinical studies that evaluate immune responses and their predictive value for therapeutic outcomes of vaccines against infectious diseases and cancer.
Figure 1. The regimen of rapamycin administration modulates CD8+ T-cell immunity. A short course of high-dose rapamycin transiently blocks mTOR activity in CD8+ T cells, favoring persistence and antigen-recall responses, and facilitates the effector-to-memory transition. This generation of memory CD8+ T-cell responses is independent of various cell-extrinsic factors (e.g., IL-15) and may underlie potent anticancer immune responses.
References
- Zinkernagel RM. Immunology taught by viruses. Science 1996; 271:173 - 8; http://dx.doi.org/10.1126/science.271.5246.173; PMID: 8539616
- Rao RR, Li Q, Odunsi K, Shrikant PA. The mTOR kinase determines effector versus memory CD8+ T cell fate by regulating the expression of transcription factors T-bet and Eomesodermin. Immunity 2010; 32:67 - 78; http://dx.doi.org/10.1016/j.immuni.2009.10.010; PMID: 20060330
- Araki K, Turner AP, Shaffer VO, Gangappa S, Keller SA, Bachmann MF, et al. mTOR regulates memory CD8 T-cell differentiation. Nature 2009; 460:108 - 12; http://dx.doi.org/10.1038/nature08155; PMID: 19543266
- Li Q, Rao RR, Araki K, Pollizzi K, Odunsi K, Powell JD, et al. A central role for mTOR kinase in homeostatic proliferation induced CD8+ T cell memory and tumor immunity. Immunity 2011; 34:541 - 53; http://dx.doi.org/10.1016/j.immuni.2011.04.006; PMID: 21511183
- Colombetti S, Basso V, Mueller DL, Mondino A. Prolonged TCR/CD28 engagement drives IL-2-independent T cell clonal expansion through signaling mediated by the mammalian target of rapamycin. J Immunol 2006; 176:2730 - 8; PMID: 16493028
- Powell JD, Lerner CG, Schwartz RH. Inhibition of cell cycle progression by rapamycin induces T cell clonal anergy even in the presence of costimulation. J Immunol 1999; 162:2775 - 84; PMID: 10072524
- Kang J, Huddleston SJ, Fraser JM, Khoruts A. De novo induction of antigen-specific CD4+CD25+Foxp3+ regulatory T cells in vivo following systemic antigen administration accompanied by blockade of mTOR. J Leukoc Biol 2008; 83:1230 - 9; http://dx.doi.org/10.1189/jlb.1207851; PMID: 18270248
- Odunsi K, Matsuzaki J, Karbach J, Neumann A, Mhawech-Fauceglia P, Miller A, et al. Efficacy of vaccination with recombinant vaccinia and fowlpox vectors expressing NY-ESO-1 antigen in ovarian cancer and melanoma patients. Proc Natl Acad Sci U S A 2012; 109:5797 - 802; http://dx.doi.org/10.1073/pnas.1117208109; PMID: 22454499
- Li Q, Rao R, Vazzana J, Goedegebuure P, Odunsi K, Gillanders W, et al. Regulating mammalian target of rapamycin to tune vaccination-induced CD8(+) T cell responses for tumor immunity. J Immunol 2012; 188:3080 - 7; http://dx.doi.org/10.4049/jimmunol.1103365; PMID: 22379028
- Lamming DW, Ye L, Katajisto P, Goncalves MD, Saitoh M, Stevens DM, et al. Rapamycin-induced insulin resistance is mediated by mTORC2 loss and uncoupled from longevity. Science 2012; 335:1638 - 43; http://dx.doi.org/10.1126/science.1215135; PMID: 22461615