Abstract
Oncogenes have been traditionally viewed as molecular drivers for tumor growth and survival. Recent evidence indicates that oncogenes may facilitate the escape of malignant cells from immune recognition and elimination. In this article, we discuss the implications of the overexpression of epidermal growth factor receptor (EGFR) family members on immune escape of tumors and immunotherapy.
Tumors targeted with cellular immunotherapy can exhibit an “immune escape” phenotype, potentially rendering immunotherapeutic interventions ineffective. As one of the underlying mechanisms, a variety of oncogenes have been shown to interfere with antigen processing and presentation.Citation1 The epidermal growth factor receptor (EGFR) family, consisting of four closely related transmembrane tyrosine kinase receptors (EGFR1–4 also known as HER1–4), is particularly important for the etiology of carcinomas and represents an attractive target for immunotherapy. HER receptors undergo homo- or heterodimerization and autophosphorylation in response to the binding of small peptide ligands, which activate downstream signaling pathways. The HER2/HER3 dimer is the most active HER signaling dimer, and is critical for signaling in HER2-overexpressing tumors.Citation2 We and others have reported that HER2 signaling can lead to the downregulation of MHC Class I molecules and impair MHC Class I-restricted recognition by CTLs.Citation3-Citation5 This is particularly relevant for therapies targeting breast carcinomas, which frequently overexpressed HER2 together with other members of the EGFR family. We recently confirmed the inverse correlation of HER2 and MHC Class I expression by immunohistochemistry (IHC) in a cohort of 70 patients affected by breast carcinomas (unpublished observations). Moreover, we demonstrated that the administration of inhibitors of the Ras/MAPK pathway enhances class MHC I expression in breast cancer, suggesting that this pathway is involved in MHC Class I downregulation by HER-overexpressing tumors.
The HER2-induced loss of MHC Class I expression and the resultant decrease in CTL sensitivity have important implications for cancer immunotherapy. One potential approach to circumvent this issue would be to pre-select breast cancer patients with tumors that express low or intermediate levels of HER2 for CTL-based immunotherapy, excluding patients with high HER2 and low MHC Class Iexpression. At least theoretically, this selection would be of particular importance for patients undergoing vaccination with MHC Class I-restricted CTL epitopes. Benavides et al.Citation6 reported that patients expressing low levels of HER2 (IHC score: 0 or 1+) respond better to vaccination with the HER2 E75 peptide CTL epitope than patients overexpressing (IHC score: 2+ or 3+) HER2, which substantiates the premise for patient selection. Therefore, patients with tumors expressing low levels of HER2, representing > 50% of breast cancer cases, may have greater clinical benefit from MHC Class I-restricted immunotherapy approaches in comparison to the patients whose tumors present high levels of HER2.
MHC Class I inhibits tumor cell lysis by natural killer (NK) cells. Therefore, it may be speculated that MHC Class I downregulation by oncogenes could make transformed cells suitable targets for direct NK cell-mediated tumor rejection or by NK cell-mediated antibody dependent cellular cytotoxicity (ADCC). The balance between activating and inhibiting signals is known to regulate NK cell-mediated cytotoxicity. Thus it is particularly interesting to understand whether oncogenes signaling also affects the ligands for activating NK cell receptors.
In a recent study, we investigated how signaling through HER2/HER3 regulates the MHC Class I chains A and B (MICA and MICB) in human breast cancer cell lines.Citation7 MICA/B are examples of ligands for the activating NK group 2, member D (NKG2D) receptor, which is expressed not only by NK but also by CD8+ and γδ T cells.Citation8 While tumor cell lines and primary tumors express the NKG2D ligand (NKG2DL), MICA/B is also expressed—though at lower levels—by some non-malignant cell types. The extent to which oncogenes can regulate NKG2DL expression is not well understood, but the example of the BCR/ABL oncogene, which can induce MICA in chronic myelogenous leukemia (CML) lends credence to these premises.Citation9 This may relevant for NK cell-mediated tumor surveillance, since tumor cells expressing NKG2DL are often susceptible to killing by NK cells even if they have normal expression of MHC Class I. We tested a number of experimental situations affecting signaling through HER2/HER3 in breast cancer cell lines, including pharmacological and genetic interference, overexpression by transfection, and treatment with the HER3-specific ligand NRG1, and assessed how these manipulations affected MICA/B expression. We concluded that signaling through the HER2/HER3 complex augments the expression of MICA/B, although it appears that HER3 more than HER2 constitutes the rate-limiting factor in this process. Among the multiple pathways activated by HER2/HER3 signaling, the PI3K-AKT pathway was shown to predominantly regulate MICA/B expression. Genotoxic stress, such as ionizing radiation and inhibitors of DNA replication, can induce the upregulation of NKG2DL.Citation10 We believe, however, that a direct effect on HER2/HER3 is responsible for MICA/B induction in breast cancer cell lines, as inferred from the administration of the ataxia-telangiectasia-mutated (ATM) and ATM and Rad-3-related (ATR) protein kinase inhibitor caffeine. The functional consequences of HER2/HER3 signaling manifested as enhanced NKG2D-MICA/B dependent NK cell-mediated cytotoxicity of breast cancer lines.
Combinatorial immunotherapies are becoming increasingly diffuse and appear to yield promising results. The findings discussed above should inspire efforts to develop immunotherapy regimens in which adaptive immunotherapy is combined with strategies based on NK cells for the treatment of carcinomas expressing high levels of HER2 and/or HER3. These combinatorial therapies should be tailored to best fit the individual expression of MHC Class I molecules and NK cell activating receptors on each tumor, a true example of individualized immunotherapy.
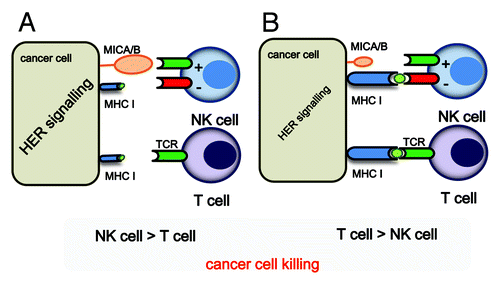
Figure 1. A schematic illustration of how HER2/HER3 signaling might determine whether cancer cells are killed by T cells or NK cells. (A) If HER2/HER3 signaling is strong, MHC Class I expression is weak, while the expression of MICA/B will be enhanced. In these conditions, the NK cell-mediated killing of tumor cells will dominate, while cancer cells will be relatively resistant to CTL-mediated killing. (B) If HER2/HER3 signaling is weak, MHC Class I expression will be unaffected, a conditions in which cancer cells can be killed by CTLs.
References
- Seliger B. Novel insights into the molecular mechanisms of HLA class I abnormalities. Cancer Immunol Immunother 2012; 61:249 - 54; http://dx.doi.org/10.1007/s00262-011-1153-9; PMID: 22120755
- Holbro T, Beerli RR, Maurer F, Koziczak M, Barbas CF 3rd, Hynes NE. The ErbB2/ErbB3 heterodimer functions as an oncogenic unit: ErbB2 requires ErbB3 to drive breast tumor cell proliferation. Proc Natl Acad Sci U S A 2003; 100:8933 - 8; http://dx.doi.org/10.1073/pnas.1537685100; PMID: 12853564
- Vertuani S, Triulzi C, Roos AK, Charo J, Norell H, Lemonnier F, et al. HER-2/neu mediated down-regulation of MHC class I antigen processing prevents CTL-mediated tumor recognition upon DNA vaccination in HLA-A2 transgenic mice. Cancer Immunol Immunother 2009; 58:653 - 64; http://dx.doi.org/10.1007/s00262-008-0587-1; PMID: 18820911
- Herrmann F, Lehr HA, Drexler I, Sutter G, Hengstler J, Wollscheid U, et al. HER-2/neu-mediated regulation of components of the MHC class I antigen-processing pathway. Cancer Res 2004; 64:215 - 20; http://dx.doi.org/10.1158/0008-5472.CAN-2522-2; PMID: 14729627
- Mimura K, Ando T, Poschke I, Mougiakakos D, Johansson CC, Ichikawa J, et al. T cell recognition of HLA-A2 restricted tumor antigens is impaired by the oncogene HER2. Int J Cancer 2011; 128:390 - 401; http://dx.doi.org/10.1002/ijc.25613; PMID: 20715101
- Benavides LC, Gates JD, Carmichael MG, Patil R, Holmes JP, Hueman MT, et al. The impact of HER2/neu expression level on response to the E75 vaccine: from U.S. Military Cancer Institute Clinical Trials Group Study I-01 and I-02. Clin Cancer Res 2009; 15:2895 - 904; http://dx.doi.org/10.1158/1078-0432.CCR-08-1126; PMID: 19351776
- Okita R, Mougiakakos D, Ando T, Mao Y, Sarhan D, Wennerberg E, et al. HER2/HER3 signaling regulates NK cell-mediated cytotoxicity via MHC class I chain-related molecule A and B expression in human breast cancer cell lines. J Immunol 2012; 188:2136 - 45; http://dx.doi.org/10.4049/jimmunol.1102237; PMID: 22301547
- Bauer S, Groh V, Wu J, Steinle A, Phillips JH, Lanier LL, et al. Activation of NK cells and T cells by NKG2D, a receptor for stress-inducible MICA. Science 1999; 285:727 - 9; http://dx.doi.org/10.1126/science.285.5428.727; PMID: 10426993
- Boissel N, Rea D, Tieng V, Dulphy N, Brun M, Cayuela JM, et al. BCR/ABL oncogene directly controls MHC class I chain-related molecule A expression in chronic myelogenous leukemia. J Immunol 2006; 176:5108 - 16; PMID: 16585609
- Gasser S, Orsulic S, Brown EJ, Raulet DH. The DNA damage pathway regulates innate immune system ligands of the NKG2D receptor. Nature 2005; 436:1186 - 90; http://dx.doi.org/10.1038/nature03884; PMID: 15995699