Abstract
In a Phase II clinical study enrolling individuals with melanoma brain metastases, 51 asymptomatic patients (cohort A) and 21 on a stable steroid dose (cohort B) received 4 courses of 10 mg/kg intravenous ipilimumab (induction), then (at 24 weeks) maintenance therapy with the same dose of ipilimumab every 12 weeks. Disease control rate at 12 weeks was 18% (according to the modified WHO criteria) and 26% (according to the immune-related response criteria) in cohort A (median survival = 7 mo) and 5% and 10% in cohort B (median survival = 4 mo). Toxicities were as previously reported for ipilimumab patients without brain metastases.
Keywords: :
Brain metastases are common in melanoma and have been shown to be refractory to nearly all standard therapies until recently. Surgery and stereotactic radiotherapy are the optimal treatments for local disease, but whole brain radiation is of limited value in preventing further brain metastases, which are - in a substantial fraction of patients—the predominant cause of melanoma morbidity and mortality. Systemic therapies available before 2011 were nearly devoid of activity, regardless of their ability to cross the blood brain barrier. While antibodies are not believed to cross an intact blood-brain barrier, activated T cells may be able to penetrate the brain,Citation1,Citation2 providing a rationale for testing immunomodulatory therapies in this setting. Further support for testing this approach came from anecdotal reports of tumor regressions in patients with metastatic melanoma and brain metastasis.
We have reported the results of a prospective clinical trial designed to evaluate the efficacy and safety of ipilimumab in patients with melanoma metastatic to the brain.Citation3 Key eligibility criteria for our Phase II study were as described, with eligible patients enrolled in two cohorts based on whether they received corticosteroids for symptoms or edema. Patients in cohort A were neurologically asymptomatic with no systemic glucocorticosteroid therapy in the 10 d prior to the start of ipilimumab therapy. Patients receiving concurrent systemic corticosteroids for control of neurological signs and symptoms related to brain metastases were enrolled in cohort B.
Study treatment consisted of 10 mg/kg intravenous ipilimumab, every 3 weeks for 4 treatments (induction), followed at 24 weeks by maintenance therapy using the same dose every 12 weeks, for stable or responding patients without severe toxicity. The primary endpoint was disease control rate (DCR), defined as an objective response or stable disease after the 12-week time point, based on modified WHO (mWHO) criteria (bidimensional). We also applied the immune response-related criteria (irRC), in which new lesions are incorporated into the measurement of tumor burden rather than themselves constituting progression.
Seventy-two patients were enrolled between July 31, 2008 and June 3, 2009, 51 patients in cohort A and 21 in cohort B. The median number of doses of induction therapy received by patients was 3 (range 1–4) for subjects in cohort A and 2 (range 1–4) for subjects in cohort B. Patients in cohort A who went on to maintenance therapy received a median of 6 doses (range 1–10), while patients in cohort B who went on to the maintenance phase (n = 2) received a median of 7 doses of ipilimumab.
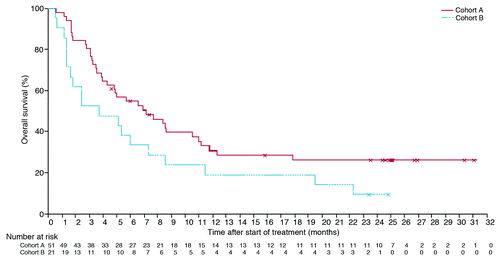
Response and disease control results are summarized in the , while overall survival (OS) is shown in . Median OS was 7 mo (range 0.4–31+) for cohort A and 4 mo (0.5–25+) for cohort B. Survival rates at 6-, 12-, 18-, and 24 mo were 55%, 31%, 26%, and 26% for cohort A and 38%, 19%, 19%, and 10% for cohort B. The most common adverse effects were fatigue, diarrhea, nausea, headache, rash, and pruritus, with immune-related adverse effects clearly attributable to ipilimumab occurring in the reported and expected frequency. Of note is that CNS events were infrequent and attributable to the tumor, with no clearly drug- or immune-related adverse effects occurring in the CNS.
Table 1. Patient response and disease control
Figure 1. Overall survival. Reprinted from Lancet Oncology. Volume 13. Kim Margolin, Marc S Ernstoff, Omid Hamid, Donald Lawrence, David McDermott, Igor Puzanov, Jedd D Wolchok, Joseph I Clark, Mario Sznol, Theodore F Logan, Jon Richards, Tracy Michener, Agnes Balogh, Kevin N Heller, F Stephen Hodi. Ipilimumab in patients with melanoma and brain metastases: an open-label, phase 2 trial. Pages 459–465. Copyright (2012) with permission from Elsevier.
Despite historical assumptions that most therapies would not effectively cross an intact blood brain barrier and the poor prognosis of melanoma patients with brain metastases, who de facto have rarely been allowed to participate in trials testing new therapies, objective responses of melanoma brain metastases to interleukin-2 (IL-2)-based approaches have been reported,Citation4-Citation7 suggesting the possibility of an effective control of CNS metastases by T-cell responses to immunomodulatory therapies. Anecdotal reports of ipilimumab responses in the brain raised the possibility that these assumptions should be questioned and that the role of cellular immunotherapy for melanoma brain metastases should be formally evaluated. Although the primary objective of this study was to estimate DCR, safety was also explored considering that initial tumor growth and/or peritumoral inflammatory changes could cause neurologic complications. Despite early concern that ipilimumab-related inflammation and/or edema in brain lesions would increase morbidity, this did not appear to be the case. In fact, there was at least one occasion in which early progressive disease in the CNS was followed by achievement of partial responses - a pattern that has previously been recognized in extracranial disease and is consistent with the mechanism of action and slow kinetics of tumor responses associated with immunomodulatory therapy. The activity of this regimen without an apparent increase in frequency or emergence of unique CNS toxicities was reassuring evidence that, at least for selected patients, treatment with ipilimumab can be administered with similar efficacy and safety in the brain and extracranial sites. While surgery and/or SRT remains the treatment of choice for most patients at the first occurrence of brain metastasis - in particular for large and/or symptomatic single or oligometastatic disease, ipilimumab is a promising therapy for those patients whose disease has recurred following such frontline therapy and/or who present with multiple, small asymptomatic metastases. The potential impact of systemic corticosteroid therapy at the time of initiation of ipilimumab is unknown, but the apparent low level of benefit for such patients (cohort B) in this study suggests that steroid dependence maybe associated with low benefit from ipilimumab.
References
- Prins RM, Vo DD, Khan-Farooqi H, Yang MY, Soto H, Economou JS, et al. NK and CD4 cells collaborate to protect against melanoma tumor formation in the brain. J Immunol 2006; 177:8448 - 55; PMID: 17142742
- Wilson EH, Weninger W, Hunter CA. Trafficking of immune cells in the central nervous system. J Clin Invest 2010; 120:1368 - 79; http://dx.doi.org/10.1172/JCI41911; PMID: 20440079
- Margolin K, Enrstoff MS, Hamid O, et al. Evaluation of ipilimumab in melanoma patients with brain metastases: a phase II trial. Lancet Oncol http://dx.doi.org/10.1016/S1470-2045(12)70090-6; PMID: 22456429
- Schmittel A, Proebstle T, Engenhart-Cabillic R, Scheibenbogen C, Geueke AM, Thiel E, et al. Brain metastases following interleukin-2 plus interferon-alpha-2a therapy: a follow-up study in 94 stage IV melanoma patients. Eur J Cancer 2003; 39:476 - 80; http://dx.doi.org/10.1016/S0959-8049(02)00731-1; PMID: 12751378
- Powell S, Dudek AZ. Single-institution outcome of high-dose interleukin-2 (HD IL-2) therapy for metastatic melanoma and analysis of favorable response in brain metastases. Anticancer Res 2009; 29:4189 - 93; PMID: 19846971
- Guirguis LM, Yang JC, White DE, Steinberg SM, Liewehr DJ, Rosenberg SA, et al. Safety and efficacy of high-dose interleukin-2 therapy in patients with brain metastases. J Immunother 2002; 25:82 - 7; http://dx.doi.org/10.1097/00002371-200201000-00009; PMID: 11924913
- Dudley ME, Yang JC, Sherry R, Hughes MS, Royal R, Kammula U, et al. Adoptive cell therapy for patients with metastatic melanoma: evaluation of intensive myeloablative chemoradiation preparative regimens. J Clin Oncol 2008; 26:5233 - 9; http://dx.doi.org/10.1200/JCO.2008.16.5449; PMID: 18809613