Abstract
Colorectal cancer metastasis to Ceacam1-/- livers is significantly impaired, compared with wild type livers, due to decreased endothelial cell survival, reduced tumor cell proliferation, diminished immune infiltration and altered chemokine expression. Ceacam1-/- myeloid-derived suppressor cells diminish metastatic burden, as confirmed by bone marrow transplantation and adoptive transfer experiments.
Keywords: :
CEACAM1 in Colorectal Cancer
Carcinoembryonic antigen-related cell adhesion molecule 1 (CEACAM1) is a cell adhesion molecule belonging to the immunoglobulin and CEA gene families. CEACAM1 functions are associated with two particular splicing isoforms carrying either a short (10 aa, CEACAM1-S) or a longer cytoplasmic domain (71–73 aa, CEACAM1-L). On one hand, CEACAM1-L acts as a co-receptor for a number of tyrosine kinase receptors, including the receptors for insulin, EGF, VEGFR and CSF1, as well as the Toll-like receptor 2. All these receptors can phosphorylate CEACAM1-L at its immunoreceptor tyrosine-based inhibitory motifs (ITIMs), leading to the recruitment of the tyrosine phosphatase PTPN6 (SHP-1) and activation of downstream inhibitory signaling pathways in various cells.Citation1 On the other hand, CEACAM1-S plays a role in the activation of apoptosis in breast and colorectal tissues.Citation2 As a co-receptor, CEACAM1 modulates a number of cellular functions such as angiogenesis,Citation3 liver insulin clearance,Citation4 as well as innate and adaptive immune responses,Citation1 including those elicited by microbial and viral infection.Citation1 CEACAM1 is expressed in a number of normal epithelial, endothelial and immune cell compartments, but is downregulated in the early stages of most epithelial cancers. Previous data from our laboratory indicate that the absence of CEACAM1 exacerbates colonic and intestinal tumor burden in azoxymethane-treated and Apc1638N/+ mice, respectively.Citation5 Interestingly, CEACAM1 re-expression often occurs in the advanced stages of multiple malignancies including non-small cell lung cancer, thyroid cancer, gastric carcinoma, pancreatic cancer, malignant melanoma and even metastatic colon cancer.Citation6 Patients whose colon tumors express a predominance of CEACAM1-L relative to CEACAM1-S exhibit accelerated progression to metastasis and shorter survival, compared with patients whose neoplasms predominantly express CEACAM1-S.Citation6 But how CEACAM1 expression in the stromal and immune compartments influences metastatic progression has never been addressed previously.
CEACAM1 in Colorectal Liver Metastasis
Using intrasplenic injection of highly metastatic MC38 colorectal cancer (CRC) cells and intravenous delivery of B16F10 melanoma cells in Ceacam1−/− mice,Citation7 we defined that, irrespective of inoculation route, tumor type and target organ (liver vs. lung), Ceacam1−/− mice developed only a small number of metastatic lesions of reduced size as compared with wild type (WT) animals, underscoring the pro-metastatic role of this protein. Primary tumor cells breach the epithelial tissue barrier (intravasation) and travel via blood vessels to their target destination, which in the case of CRC is often represented by the liver. Intrasplenic injection bypasses the intravasation step to deliver, within a few minutes, tumor cells to the liver sinusoids via the portal vein. Intravital microscopy on live animals demonstrated a reduction in the arrest of CSFE-labeled MC38 cells within Ceacam1−/− hepatic sinusoids 30 min post-injection. Unfavorable microenvironmental conditions resulted in a 3-fold further decrease in tumor cell survival within the Ceacam1−/− hepatic sinusoids over the next 48 h. Moreover, after colonization of Ceacam1−/− livers, tumor cell proliferation was decreased - as compared with the WT setting - by 2-fold. The metastatic nodules developing in Ceacam1−/− mice displayed enhanced vascular density, albeit with less mature vessels. Interestingly, increased tumor angiogenesis observed in Ceacam1−/− mice appeared to result from the granulocyte colony-stimulating factor (G-CSF)-induced expression of prokineticin 2 (Bv8) from infiltrating Gr1+CD11b+ myeloid-derived suppressor cells (MDSCs), rather than from increased VEGF levels.Citation8
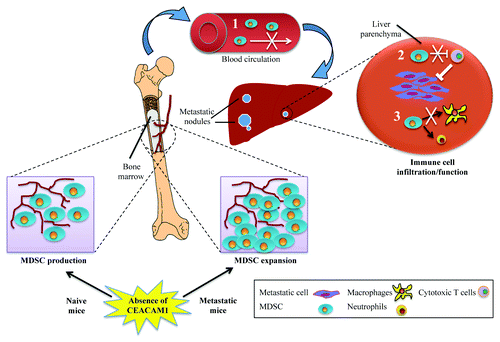
We then examined whether the development of smaller metastatic nodules depended on the dysregulation of bone marrow-derived cell (BMDC) production or on restrained infiltration of immune cells into the liver. Naïve WT and Ceacam1−/− mice produced equivalent amounts of total colony-forming cells, but naïve Ceacam1−/− BMs exhibited significantly reduced levels of total MDSCs (CD11b+Gr1+) as well as of cells belonging to the monocytic (CD11b+Ly6ChiLy6G-) and granulocytic (CD11b+Ly6CloLy6G+) lineages, compared with WT animals. Conversely, metastasis-carrying Ceacam1−/− mice had significantly augmented levels of MDSCs in their BM. When immune cells were profiled in metastatic lesions 14 d after the injection of MC38 cells, we observed a significant suppression in the mobilization and/or infiltration of both myeloid (macrophages, granulocytes, dendritic cells, natural killer cells and MDSCs) and lymphoid (T and B lymphocytes) cells in Ceacam1−/− livers. Profiling of 12 cytokines/chemokines indicated increased levels of interleukin 6 (IL-6), IL-10 and tumor necrosis factor α (TNFα), and decreased amounts of CCL2, CCL3 and CCL5 in Ceacam1−/− vs. WT mice under metastatic conditions.
Transplantation of WT BMDCs into Ceacam1−/− recipients produced a full rescue of metastatic development, whereas the reverse approach (WT recipients of Ceacam1−/− BMs) resulted in a reduced metastatic burden. Other liver stromal cells (such as stellate and Kuppfer cells) appear to be contributing to metastasis development, as the metastatic burden in the liver was always higher than that obtained with Ceacam1−/− BMDCs transplanted into Ceacam1−/− mice. Adoptive transfers of WT MDSCs into Ceacam1−/− mice increased metastasis relative to transfer of Ceacam1−/− MDSCs, confirming that CEACAM1 controls MDSC-dependent processes.
Conclusion
Several mechanisms can be proposed to explain the role of CEACAM1 in tumor metastasis (). These include: (1) a reduced MDSC expansion with enhanced T-cell responses in the Ceacam1−/− liver microenvironment, a finding further supported by the decreased activity of STAT3 previously observed in this context;Citation7 (2) perhaps, a migratory defect of BM MDSCs into the metastatic liver; or (3) a block in the differentiation of monocytes and macrophages within the Ceacam1−/− liver microenvironment. The systemic deletion of CEACAM1 has highlighted a number of important interactions between malignant cell and CEACAM1+ cells participating in metastatic development, such as hepatocytes, endothelial and immune cells. However, many other factors must be considered for a better understanding of the cell biology underlying our observations. First, the cancer cells used in this study (MC38) do not express CEACAM1, whereas many advanced cancers, including colorectal neoplasms, re-express CEACAM1. CEACAM1-L re-expression in advanced colon cancer usually constitutes a worse prognostic marker.Citation6 Second, intrasplenic injections result in a significant influx of highly aggressive metastatic cells into the liver niche, as opposed to a slow and constant delivery from an orthotopic primary tumors. New advances in orthotopic intracecal injections will give us an opportunity to re-examine this issue.Citation9 Finally, discrepancies between our data relating to the role of CEACAM1 and MDSCs in CRC liver metastasisCitation7 and recently published results obtained with xenograft modelsCitation8 will need to be resolved. At least in part, these discrepancies may be due to the distinct types of macrophages that differentiate from MDSCs in primary tumors vs. in metastases.Citation10
Figure 1. Dysregulation of bone marrow myeloid-derived suppressor cells in CEACAM1-deficient mice under metastatic challenge. Upon formation of metastatic nodules in the liver, Ceacam1−/− (but not wild type) bone marrow (BM) myeloid-derived suppressor cells (MDSCs) undergo expansion. However, this is not followed by increased MDSC infiltration into metastatic livers. Given that the adoptive transfer of wild type MDSCs into Ceacam1−/− mice results in increased metastatic burden, several possible mechanisms can be proposed to explain the role of CEACAM1 in tumor metastasis. (1) migratory defect of BM MDSCs; (2) reduced cytotoxic T-cell inhibition by MDSCs; or (3) blockage of monocytic and/or granulocytic differentiation. As a consequence, tumor infiltration/effector functions of MDSCs are diminished in Ceacam1−/− livers, resulting in enhanced immune responses that efficiently eliminate/control metastatic tumor cells.
References
- Gray-Owen SD, Blumberg RS. CEACAM1: contact-dependent control of immunity. Nat Rev Immunol 2006; 6:433 - 46; http://dx.doi.org/10.1038/nri1864; PMID: 16724098
- Kirshner J, Chen CJ, Liu P, Huang J, Shively JE. CEACAM1-4S, a cell-cell adhesion molecule, mediates apoptosis and reverts mammary carcinoma cells to a normal morphogenic phenotype in a 3D culture. Proc Natl Acad Sci U S A 2003; 100:521 - 6; http://dx.doi.org/10.1073/pnas.232711199; PMID: 12522268
- Ergün S, Kilik N, Ziegeler G, Hansen A, Nollau P, Götze J, et al. CEA-related cell adhesion molecule 1: a potent angiogenic factor and a major effector of vascular endothelial growth factor. Mol Cell 2000; 5:311 - 20; PMID: 10882072
- Poy MN, Yang Y, Rezaei K, Fernström MA, Lee AD, Kido Y, et al. CEACAM1 regulates insulin clearance in liver. Nat Genet 2002; 30:270 - 6; http://dx.doi.org/10.1038/ng840; PMID: 11850617
- Leung N, Turbide C, Balachandra B, Marcus V, Beauchemin N. Intestinal tumor progression is promoted by decreased apoptosis and dysregulated Wnt signaling in Ceacam1-/- mice. Oncogene 2008; 27:4943 - 53; http://dx.doi.org/10.1038/onc.2008.136; PMID: 18454175
- Ieda J, Yokoyama S, Tamura K, Takifuji K, Hotta T, Matsuda K, et al. Re-expression of CEACAM1 long cytoplasmic domain isoform is associated with invasion and migration of colorectal cancer. Int J Cancer 2011; 129:1351 - 61; http://dx.doi.org/10.1002/ijc.26072; PMID: 21413011
- Arabzadeh A, Chan C, Nouvion AL, Breton V, Benlolo S, DeMarte L, Turbide C, Brodt P, Ferri L, Beauchemin N. Host-related carcinoembryonic antigen cell adhesion molecule 1 promotes metastasis of colorectal cancer. Oncogene 2012; Epub ahead of print; PMID:22469976; 10.1038/onc.2012.112.
- Lu R, Kujawski M, Pan H, Shively JE. Tumor Angiogenesis Mediated by Myeloid Cells Is Negatively Regulated by CEACAM1. Cancer Res 2012; 72:2239 - 50; http://dx.doi.org/10.1158/0008-5472.CAN-11-3016; PMID: 22406619
- Hackl C, Man S, Francia G, Milsom C, Xu P, Kerbel RS. Metronomic oral topotecan prolongs survival and reduces liver metastasis in improved preclinical orthotopic and adjuvant therapy colon cancer models. Gut 2012; Epub ahead of print; PMID:22543158.
- Hanahan D, Coussens LM. Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell 2012; 21:309 - 22; http://dx.doi.org/10.1016/j.ccr.2012.02.022; PMID: 22439926