Abstract
For a long time, cancer immunotherapy has focused on the induction of tumor-specific T cell-mediated immune responses. Now, a mounting body of evidence indicates that efficient anticancer immune responses also rely on innate immunity. Tietze et al. have recently elucidated an antigen-nonspecific role for memory CD8+ T cells in cytokine-based cancer immunotherapy.
With the recent FDA-approval of Provenge® and Yervoy®, the field of cancer immunology has gone through a renaissance. A number of therapies including antigen-specific (e.g., vaccination using tumor antigens, adoptive transfer of tumor-infiltrating leukocytes)Citation1 and nonspecific (cytokines, immunostimulatory monoclonal antibodies, TLR antibodies)Citation2 have been attempted both preclinically and clinically with varying degrees of success. While the mechanisms underlying the anticancer effects of immunotherapy are not always understood, it was widely speculated that persistent antitumor effects might be achieved through the generation of antigen-specific T cells. In a recent issue of Blood, Tietze et al. presents an alternative hypothesis, suggesting that - upon the nonspecific stimulation of the immune system - antitumor effects can be mediated by bystander activated memory T cells that are not specific to the tumor itself via their T cell receptors (TCRs).Citation3
The authors observed that - following cytokine-based immunotherapies such as the administration of agonistic anti-CD40 mAbs and interleukin 2 (IL-2) - CD8+ memory T cells expressing granzyme B and possessing elevated lytic capability expand. These cells failed to upregulate CD25 (indicative of TCR engagement) yet highly upregulated the natural killer (NK) cell activating receptor NKG2D. Similar phenotypes were observed using transgenic mice bearing ovalbumin (OVA)-specific TCRs, which therefore were not capable of mounting tumor-specific responses following the same immunotherapeutic regimen. Importantly, OVA-specific T cells elicited by this approach were capable of lysing both OVA-expressing and OVA-negative target cells in vitro, as well as delaying the growth of OVA-negative tumors in vivo. The authors showed that the mechanism by which these nonspecific T cells exert antitumor effects depends - at least partially - on NKG2D, as the blockade of this receptor in vivo resulted in partial abrogation of the anticancer response. Finally, human T cells of a similar phenotype were observed in melanoma patients upon imiquimod treatment, suggesting that this process may be remarkably conserved across species.Citation3 This novel, nonspecific role for CD8+ memory T cells in cancer therapy raises many questions.
Presumably, memory T cells have undergone multiple rounds of selection, including (1) central tolerance, (2) peripheral tolerance, and (3) the antigen-driven maturation from a naïve to a memory phenotype. Perhaps, because of this rigorous selection process, these cells may “safely” become activated in a nonspecific (TCR-independent) fashion. Additionally, because these cells are elicited by cytokines yet do not upregulate CD25, they require extremely large amounts of cytokines for survival, perhaps constituting an intrinsic safeguard mechanism. The memory/naïve T cell ratio becomes markedly skewed toward the memory phenotype over the course of a lifespan, due to the facts that the thymus involutes with ageCitation4 and that antigen exposure occurs in a near-to-continuous fashion.Citation5 With such a limited ability to generate novel immune responses as we age, this pathway of activation may represent a means to conserve resources and elicit multiple functions (antigen-specific and nonspecific) from a single cell type. Along similar lines, it was recently reported that memory cells are abundant at different sites throughout the body, including the skin.Citation6 Inflammation brought about at these sites by infection, trauma, etc… might generate cytokine-rich environments capable of temporarily inducing local nonspecific memory T-cell activation. Such cells may contribute to immunosurveillance by detecting NKG2D ligand expression on infected and/or transformed cells.
Tietze et al. also suggests that nonspecific CD8+ memory T cells may be equipped with inherent advantages over their antigen-specific counterparts, based on their unique phenotype and functional characteristics. For instance, while antigen-nonspecific CD8+ memory T cells were found to express NKG2D and low levels of programmed death 1 (PD-1), antigen-specific T cells displayed a NKG2D- and PD-1high phenotype. The description of antigen nonspecific T-cell activation is not novel to cancer immunotherapies, and generally results from potent immunostimulatory interventions. Indeed, similar “bystander” activated cells have been characterized upon viral and bacterial infectionsCitation7 as well as during autoimmunity.Citation8 Tietze et al. adds to this body of literature with their description of the unique phenotype (NKG2Dpos and PD-1low) and function (non-MHC restricted cytoxicity) of nonspecific CD8+ memory T cells. Tumor cells often downregulate both MHC and tumor antigen expression to avoid recognition by CD8+ T cells.Citation9 In this context, nonspecific CD8+ T cells that can become activated upon the engagement of NKG2D present an advantage, as this occurs independently of MHC-antigen recognition by the TCR. Furthermore, the differential expression of immunoregulatory molecules such as PD-1 by nonspecific CD8+ memory T cells suggests that they may not be susceptible to the same inhibitory pathways as antigen-specific T cells, perhaps constituting an advantage within immunosuppressive tumor microenvironments.Citation10
From a therapeutic standpoint, the manuscript by Tietze et al. lays the groundwork for how both antigen-specific and nonspecific approaches can be rationally combined to maximize their therapeutic potential. How might this novel role for memory CD8+ T cells be exploited for optimizing the efficacy of immunotherapy? Induction of nonspecific bystander CD8+ T cells by immunostimulatory therapies might contribute to tumor debulking and hence facilitate the release of tumor antigens. Subsequent vaccination strategies would induce antigen-specific and perhaps prolonged antitumor responses. The activation of the immune system and the release of cytokines at the tumor site by antigen-specific cells may then propagate the survival of nonspecific cells, possibly eliminating the need for further immunostimulatory interventions. This combination could have a 2-fold effect by (i) inducing enhanced, polyclonal specific responses upon the release of otherwise sequestered tumor antigens, and (ii) reducing the side effects that are normally associated with repeated dosing of immunostimulatory therapies (through the generation of antigen-specific cells capable of maintaining nonspecific cells at the tumor and metastatic sites). Importantly, this hypothetical approach may bypass the production of tumor antigen-loss variants that often lead to relapse after immunotherapy. The study by Tietze et al. demonstrates that memory T cells are capable of both extreme specificity as well as nonspecific activities, contingent upon the nature of their stimulation.
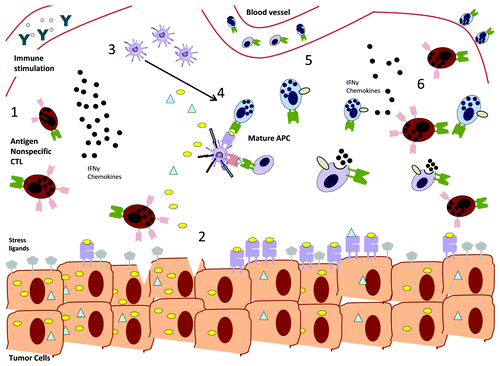
Figure 1. Potential synergy between antigen-specific and nonspecific CD8+ T cell responses. (1) Immunostimulatory therapies induce αβ memory CD8+ T cells to become activated in an antigen-nonspecific fashion, upregulating receptors such as NKG2D. (2) Antigen nonspecific cells can recognize transformed cells via stress ligands, lyse them and hence cause the release of sequestered tumor antigens. (3) Cytokines and chemokines released by nonspecific cells recruit other immune cells including antigen-presenting cells (APCs), which can engulf tumor antigens and hence (4) activate antigen specific CD4+ and CD8+ T cells. At this stage, vaccination strategies, may aggravate tumor-specific immune responses. (5) Antigen-specific and nonspecific cells are able to attack tumor cells by targeting both tumor antigens and stress ligands, making the arisal of escape variants less likely. (6) Cytokine secretion by antigen-specific cells maintains and continuously recruits antigen nonspecific cells to the tumor site.
| Abbreviations: | ||
| FDA | = | Food and Drug Administration |
| mAbs | = | monoclonal antibodies |
| TLR | = | Toll-like receptor |
| IL-2 | = | interleukin 2 |
| TCR | = | T cell receptor |
| NK | = | natural killer |
| OVA | = | ovalbumin |
| PD-1 | = | programmed death 1 |
| PD-1L | = | programmed death 1 ligand |
References
- Gattinoni L, Powell DJ Jr., Rosenberg SA, Restifo NP. Adoptive immunotherapy for cancer: building on success. Nat Rev Immunol 2006; 6:383 - 93; http://dx.doi.org/10.1038/nri1842; PMID: 16622476
- Weiner LM, Surana R, Wang S. Monoclonal antibodies: versatile platforms for cancer immunotherapy. Nat Rev Immunol 2010; 10:317 - 27; http://dx.doi.org/10.1038/nri2744; PMID: 20414205
- Tietze JK, Wilkins DE, Sckisel GD, Bouchlaka MN, Alderson KL, Weiss JM, et al. Delineation of antigen-specific and antigen-nonspecific CD8(+) memory T-cell responses after cytokine-based cancer immunotherapy. Blood 2012; 119:3073 - 83; http://dx.doi.org/10.1182/blood-2011-07-369736; PMID: 22251483
- Naylor K, Li G, Vallejo AN, Lee WW, Koetz K, Bryl E, et al. The influence of age on T cell generation and TCR diversity. J Immunol 2005; 174:7446 - 52; PMID: 15905594
- Schwab R, Szabo P, Manavalan JS, Weksler ME, Posnett DN, Pannetier C, et al. Expanded CD4+ and CD8+ T cell clones in elderly humans. J Immunol 1997; 158:4493 - 9; PMID: 9127016
- Clark RA, Chong B, Mirchandani N, Brinster NK, Yamanaka K, Dowgiert RK, et al. The vast majority of CLA+ T cells are resident in normal skin. J Immunol 2006; 176:4431 - 9; PMID: 16547281
- Ehl S, Hombach J, Aichele P, Hengartner H, Zinkernagel RM. Bystander activation of cytotoxic T cells: studies on the mechanism and evaluation of in vivo significance in a transgenic mouse model. J Exp Med 1997; 185:1241 - 51; http://dx.doi.org/10.1084/jem.185.7.1241; PMID: 9104811
- Meresse B, Chen Z, Ciszewski C, Tretiakova M, Bhagat G, Krausz TN, et al. Coordinated induction by IL15 of a TCR-independent NKG2D signaling pathway converts CTL into lymphokine-activated killer cells in celiac disease. Immunity 2004; 21:357 - 66; http://dx.doi.org/10.1016/j.immuni.2004.06.020; PMID: 15357947
- Dunn GP, Bruce AT, Ikeda H, Old LJ, Schreiber RD. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol 2002; 3:991 - 8; http://dx.doi.org/10.1038/ni1102-991; PMID: 12407406
- Zhang L, Gajewski TF, Kline J. PD-1/PD-L1 interactions inhibit antitumor immune responses in a murine acute myeloid leukemia model. Blood 2009; 114:1545 - 52; http://dx.doi.org/10.1182/blood-2009-03-206672; PMID: 19417208