Abstract
Natural indoleamine-2,3-dioxygenase (IDO)-reactive CD4+ T cells have been shown to release interferonγ (IFNγ), tumor necrosis factor α (TNFα), as well as interleukin 17 (IL-17). In some individuals, these cells also demonstrated the ability to suppress IL-10 production. IDO-specific CD4+ helper T cells among peripheral blood lymphocytes may participate in immunoregulatory networks by delaying the immune suppressive actions of IDO. However, IDO-specific CD4+ T cells may also have a regulatory phenotype, de facto exerting immunosuppressive functions.
Natural Anti-IDO CD4+ T-cell Responses
Indoleamine 2,3-dioxygenase (IDO) underpin a significant counter-regulatory mechanism induced by pro-inflammatory signals such as interferon γ (IFNγ). Recently, we described cytotoxic CD8+ T-cell reactivity toward IDO both in cancer patients as well as in healthy individuals.Citation1 We showed that the presence of such IDO-specific CD8+ T cells boosts T-cell immunity against viral as well as tumor-associated antigens by eliminating IDO+ suppressive cells, suggesting a role of such effector T cells in the regulation of immune responses. This made us scrutinize CD4+ IDO-specific T cells. Indeed, the release of pro-inflammatory cytokines by CD4+ IDO-specific T cells may be important as a counter-response to IDO-induced immunosuppression. Hence, we first sat out to analyze if CD4+ T cells naturally recognize IDO. In this respect, we frequently observed detectable numbers of IDO-specific CD4+ T cells in cancer patients and, to a lesser extent, in healthy individuals.Citation2 Thus, we identified a Class II-restricted IDO-derived CD4+ T-cell epitope in the vicinity of a previously described Class I-restricted IDO-epitope. We found that IDO-specific CD4+ T cells released IFNγ as well as tumor necrosis α (TNFα). The cancer relevance of these CD4+ T cells was further emphasized by the findings that IDO-specific T-cells reacted toward dendritic cells (DCs) pulsed with IDO+ tumor lysates. Interestingly, we detected a correlation between the presence of IDO-specific CD4+ cells and CD8+ T-cell responses against IDO, suggesting that MHC Class I- and Class II-restricted IDO responses co-develop (). In contrast to Th1 cytokines, we could not detect any release of inteleukin 4 (IL-4, a prototypic Th2 cytokine) in response to the IDO-derived CD4 epitope.Citation2
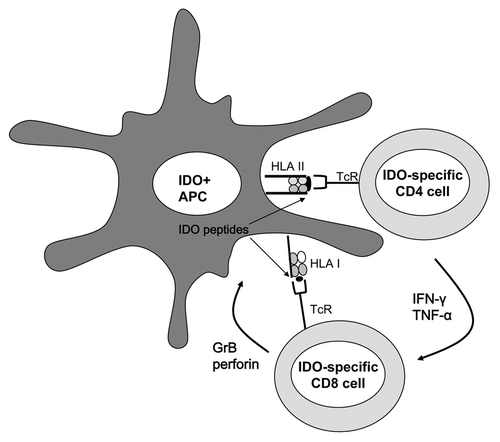
Figure 1. Upregulation of indoleamine 2,3-dioxygenase expression is an early event in antigen-presenting cells, since it is induced by pro-inflammatory signals. Indoleamine 2,3-dioxygenase (IDO) protein is processed and IDO-derived peptides are presented on the cell surface of antigen-presenting cells (APCs) by class II HLA molecules, from where they are recognized by CD4+ T cells. The release of pro-inflammatory cytokines by CD4+ IDO-specific T cells may be important as a counter-response to IDO-induced immunosuppression. These CD4+ T cells may indeed help overcoming the immunosuppressive effects of IDO in the early phases of inflammatory responses. Moreover, IDO-specific CD4+ T cells may promote CD8+ cytotoxic T-cell responses, including anti-CMV and anti-IDO responses. IDO-specific CD8+ T cells may further boost T-cell immunity by eliminating IDO+ suppressive cells, for instance by releasing granzyme B (GrB) and perforins.
Th17 Cells
Since the release of IL-17 is mediated by a very specific subset of CD4+ T-helper cells (Th17 cells), IL-17 has recently been the focus of great interest. Upon stimulation with the IDO-derived CD4 epitope, we frequently detected IDO-specific CD4+ T cells that released IL-17. Th17 cells are thought to be particularly important in maintaining barrier immunity at mucosal surfaces such as the gut and skin.Citation3 Interestingly, IDO is expressed at high levels in the gastrointestinal tract, although its precise role in intestinal immunity is not well understood.Citation4 One could speculate that a fraction of Th17 cells that are highly prevalent at the mucosal tissues of healthy individualsCitation3 is recognizing IDO. Th17 cells might have been proposed to play a protective role against cancer. Tumor-infiltrating Th17 cells express other cytokines in addition to IL-17, which may be functionally relevant. These include IFNγ, TNFα as well as IL-2.Citation5 IDO-specific Th17 cells appeared to exhibit a similar effector T-cell cytokine profile.Citation2 Recently, a FOXP3+ T regulatory (Treg) cell lineage was described that could be redirected to become “Th17-like effector cells.”Citation7 It was suggested that these CD4+ cells correspond to a pool of constitutively primed “first responder” cells capable of rapid suppression as well as of supplying help when redirected by the innate immune system.Citation7 IDO may play a vital role in the conversion of such FOXP3+ Tregs to Th17-like effector cells and hence it could be speculated that IDO-specific T cells may be such constitutively primed “first responder” Th17-like T cells.
Regulatory T Cells
To examine if CD4+ IDO-specific T cells may also function as immune suppressive cells we examined IL-10 release in response to the IDO-derived CD4 epitope. IL-10 is mainly expressed by Tregs.Citation6 In some individuals, we could indeed detect IL-10 responses. Thus, IDO-specific Tregs may enhance IDO-mediated immunosuppression, thus protecting cancer cells from an immune attack. Along similar lines, specific regulatory CD8+ T cells in cancer patients have been described to recognize another immunosuppressive molecule, heme oxygenase 1.Citation8 Interestingly, in some instances, we detected background IL-10 release in vitro, by ELISPOT assays on cells from pre-stimulated subjects. This enabled us to recognize that stimulation with the IDO-derived peptide in two healthy donors triggered an overall suppression of IL-10. In this regard, we have previously observed a decrease in IL-10 when IDO-specific CD8+ T cells were present.Citation1 Thus, the role of IDO-specific CD4+ T cells in immunoregulatory networks may be a complex balance between activation and inhibition, with a predominant role played by the local microenvironment.
Correlation with Cytomegalovirus Immunity
Cytomegalovirus (CMV) is the most immunodominant antigen to be encountered by the human immune system.Citation9 The CD8+ T-cell response to CMV typically consists of a sizeable percentage of the total CD8+ T-cell repertoire in CMV-seropositive individuals. Monocytes are major CMV target cells in vivo, and CMV has been shown to induce IDO expression in these cells. Such an expression of IDO is believed to confer an advantage to CMV-infected monocytes, de facto facilitating their escape from T-cell responses.Citation10 We observed that the presence of IDO-specific CD4+ T-cell responses correlated with the presence of CMV-directed responses.Citation2 In light of this, it could well be possible that the observed IDO-specific T cell responses have developed as a support for constitutive anti-CMV T-cell responses in the individuals.
Conclusions
In conclusion, we have described the presence of naturally occurring IDO-specific CD4+ T-helper cells. Many questions remain unanswered, however, regarding the basic properties of IDO-specific T cells and the potential role of these cells in the regulation of immune responses. We suggest that the activation of such CD4+ cells could help overcoming the immunosuppressive actions of IDO, which otherwise result from the early expression of IDO in maturing antigen-presenting cells. However, since IDO-specific CD4+ T cells may also secrete immunoregulatory cytokines, the balance between immune activation and inhibition may depend largely on the local microenvironment.
References
- Sørensen RB, Hadrup SR, Svane IM, Hjortsø MC, Thor Straten P, Andersen MH. Indoleamine 2,3-dioxygenase specific, cytotoxic T cells as immune regulators. Blood 2011; 117:2200 - 10; PMID: 21079151
- Munir S, Larsen SK, Iversen TZ, Donia M, Klausen TW, Svane IM, et al. Natural CD4(+) T-Cell Responses against Indoleamine 2,3-Dioxygenase. PLoS One 2012; 7:e34568; PMID: 22539948
- O'Quinn DB, Palmer MT, Lee YK, Weaver CT. Emergence of the Th17 pathway and its role in host defense. Adv Immunol 2008; 99:115-63.:115-163.
- Cherayil BJ. Indoleamine 2,3-dioxygenase in intestinal immunity and inflammation. Inflamm Bowel Dis 2009; 15:1391 - 6; PMID: 19322906
- Martin-Orozco N, Muranski P, Chung Y, Yang XO, Yamazaki T, Lu S, et al. T helper 17 cells promote cytotoxic T cell activation in tumor immunity. Immunity 2009; 31:787 - 98; PMID: 19879162
- Sakaguchi S. Naturally arising Foxp3-expressing CD25+CD4+ regulatory T cells in immunological tolerance to self and non-self. Nat Immunol 2005; 6:345 - 52; PMID: 15785760
- Sharma MD, Hou DY, Baban B, Koni PA, He Y, Chandler PR, et al. Reprogrammed foxp3(+) regulatory T cells provide essential help to support cross-presentation and CD8(+) T cell priming in naive mice. Immunity 2010; 33:942 - 54; PMID: 21145762
- Andersen MH, Sørensen RB, Brimnes MK, Svane IM, Becker JC, thor Straten P. Identification of heme oxygenase-1-specific regulatory CD8+ T cells in cancer patients. J Clin Invest 2009; 119:2245 - 56; PMID: 19662679
- Sylwester AW, Mitchell BL, Edgar JB, Taormina C, Pelte C, Ruchti F, et al. Broadly targeted human cytomegalovirus-specific CD4+ and CD8+ T cells dominate the memory compartments of exposed subjects. J Exp Med 2005; 202:673 - 85; PMID: 16147978
- Furset G, Fløisand Y, Sioud M. Impaired expression of indoleamine 2, 3-dioxygenase in monocyte-derived dendritic cells in response to Toll-like receptor-7/8 ligands. Immunology 2008; 123:263 - 71; PMID: 17725606