Abstract
Protective T‑cell immunity against cancer and infections is dependent on the generation of a durable effector and memory T‑cell pool. Studies from cancer and chronic infections reveal that B7-H1 (PD-L1) engagement with its receptor PD-1 promotes apoptosis of effector T cells. It is not clear how B7-H1 regulates T‑cell apoptosis and the subsequent impact of B7-H1 on the generation of memory T cells. In immunized B7-H1-deficient mice, we detected an increased expansion of effector CD8+ T cells and a delayed T‑cell contraction followed by the emergence of a protective CD8+ T‑cell memory capable of completely rejecting tumor metastases in the lung. Intracellular staining revealed that antigen-primed CD8+ T cells in B7-H1-deficient mice express lower levels of the pro-apoptotic molecule Bim. The engagement of activated CD8+ T cells by a plate-bound B7-H1 fusion protein led to the upregulation of Bim and increased cell death. Assays based on blocking antibodies determined that both PD-1 and CD80 are involved in the B7-H1-mediated regulation of Bim in activated CD8+ T cells. Our results suggest that B7-H1 may negatively regulate CD8+ T‑cell memory by enhancing the depletion of effector CD8+ T cells through the upregulation of Bim. Our findings may provide a new strategy for targeting B7-H1 signaling in effector CD8+ T cells to achieve protective antitumor memory responses.
Keywords: :
Introduction
The metastatic spread of tumor cells is the primary cause of cancer related mortalities, indicating a need for therapeutic approaches capable of controlling or preventing metastasis.Citation1 The presence of tumor-infiltrating effector and memory T cells is correlated with decreased metastatic spread, consistent with a role for T cells in preventing metastasis of primary tumors.Citation2-Citation4 As effector and memory T cells preferentially migrate to non-lymphoid organs, where they persist for long periods and undergo rapid re-activation upon antigen stimulation,Citation5 a reasonable approach for therapy would be to generate a durable and effective antitumor memory CD8+ T-cell response within sites of potential or existing metastasis. Although many efforts have focused on inducing effector or memory CD8+ T cells, the eventual emergence or progression of tumor metastases suggests that T-cell immunity to primary and metastatic tumor cells is compromised or ineffective. A wide range of immunosuppressive mechanisms allow for tumor progression and metastatic spread, including the expression of the co-inhibitory molecule B7-H1 (PD-L1, CD274) by primary and metastatic tumor cells as well as by antigen presenting cells.Citation6,Citation7
B7-H1, first identified by our laboratory in 1998,Citation8 is expressed by a variety of tumor cells. B7-H1 is also constitutively expressed by macrophages and dendritic cells, its expression being upregulated upon cell activation.Citation9 Classically, B7-H1 is thought to deliver inhibitory signals to CD8+ T cells by binding to PD-1, resulting in negative regulation of TCR signaling and apoptosis of activated T cells.Citation10-Citation12 Recently, CD80 (B7–1) was identified as an additional binding partner for B7-H1.Citation13 The exact mechanism whereby B7-H1 leads to apoptosis of effector CD8+ T cells is unknown. Despite many studies of the B7-H1/PD-1 pathway in regulating activated T cells interacting with tumor cells during the effector phase of the immune response,Citation13,Citation26,Citation28 the mechanism by which B7-H1 expressed by host cells regulates primary T cell responses remains unresolved. Using B7-H1-deficient mice, we and others have reported that host B7-H1 limits the expansion and survival of effector CD8+ T cells.Citation25,Citation37 However, it is not clear whether increased accumulation of effector CD8+ T cells in B7-H1-deficient mice is due to decreased apoptosis or increased proliferation of effector CD8+ T cells in vivo. The role of B7-H1 in regulation of memory T cell responses also remains unclear.
A primary CD8+ T cell response is characterized by an expansion phase followed by a contraction phase, during which a majority of effector CD8+ T cells are deleted. Importantly, a small population of antigen-specific effector CD8+ T cells survives and develops into the memory population. The contraction phase must be tightly regulated: having too many cells survive contraction would be unfavorable for the host, and could lead to autoimmunity. Conversely, having too few cells survive contraction could compromise the establishment of a protective memory response.Citation14
The regulation of apoptosis in effector CD8+ T cells has been the focus of numerous studies. While the contribution of the death-receptor pathway of apoptosis during contraction remains controversial,Citation15-Citation18 the involvement of the mitochondrial pathway of apoptosis is well established.Citation19-Citation21 Bcl-2 family members regulate the mitochondrial pathway of apoptosis, and some of these proteins have been characterized as the main regulators of apoptosis in activated T cells. Bcl-2, the most important anti-apoptotic molecule in activated T cells, functions by binding to the pro-apoptotic BH3-only molecule Bim.Citation22 At the peak of activation, Bcl-2 levels significantly decline,Citation23 allowing free Bim molecules to activate Bax/Bak-like molecules. Upon activation, Bax/Bak-like molecules form pores in the mitochondrial membrane, leading to the release of cytochrome c, activation of caspases, and initiation of apoptosis.Citation24 Fewer T cells undergo contraction and more memory T cells form in Bim-deficient mice,Citation25,Citation26 indicating that Bim could be a key regulator in limiting effector T cell entry into the memory T cell pool. How Bim levels are regulated in effector T cells following antigen-stimulation is not fully understood.
In this study, we sought to investigate the mechanism by which B7-H1 regulates apoptosis in effector CD8+ T cells and subsequently to address the effect of B7-H1 on memory generation. We used a non-proliferative immunization approach and performed ex vivo assays to directly examine the levels of pro- and anti-apoptotic molecules in antigen-specific CD8+ T cells. We found that more memory CD8+ T cells were generated in B7-H1-deficient mice following immunization as compared with wild type (WT) mice. At the peak of the expansion phase, CD8+ T cells expressed lower levels of Bim in B7-H1-deficient mice than in WT mice. In vitro assays revealed that stimulation by plate-bound B7-H1 led to Bim upregulation in activated CD8+ T cells, and antibodies which inhibit the B7-H1/PD-1 or B7-H1/CD80 interaction blocked this upregulation. Our results suggest that B7-H1 may be a previously unknown regulator of Bim expression, and B7-H1 may negatively regulate CD8+ T cell memory by enhancing the depletion of antigen-primed CD8+ T cells through the upregulation of Bim.
Results
More memory T cells are generated in the absence of B7-H1
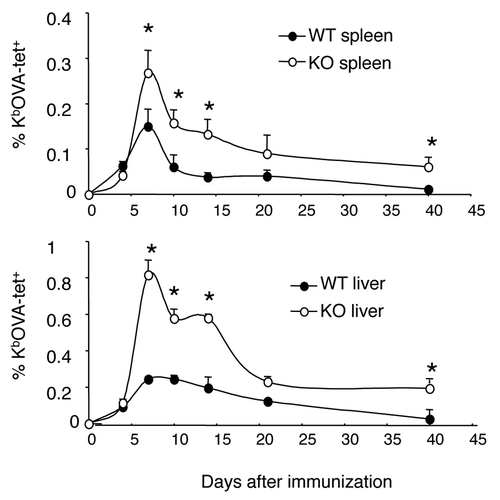
We compared the kinetics of CD8+ T cell responses in the spleen and liver of WT and B7-H1-deficient C57BL/6 mice following immunization with ovalbumin (OVA) protein and polyinosinic:polycytidylic acid (poly (I:C)) as adjuvant. We observed an increased number of CD8+ T cells at the peak of the immune response (day 7-post immunization) in the spleen and liver of B7-H1-deficient mice as compared with WT mice. During the contraction phase (days 7 to 14-post immunization) there was a significant delay in the reduction of antigen-specific CD8+ T cells in the spleen and liver of B7-H1-deficient mice as compared with WT mice. On day 40 following immunization, more antigen-specific memory CD8+ T cells were detected in B7-H1-deficient mice as compared with WT mice. (). These data suggest that host B7-H1 may regulate the extent of expansion and contraction of effector CD8+ T cells, thus influencing the size of the memory CD8+ T cell pool in both lymphoid and non-lymphoid tissues.
Figure 1. Kinetics of CD8+ T cell responses to antigen stimulation. Wild type (WT) and B7-H1-deficient (KO) mice were immunized (i.p.) with OVA plus poly I:C. Kb/OVA tetramer was used to identify antigen-specific CD8+ T cells in spleen and liver at the indicated times after immunization. Data show the percentage of tetramer+ CD8+ T cells (mean ± SD of three mice per time point). One of two independent experiments is shown. * p < 0.05 compared with WT mice.
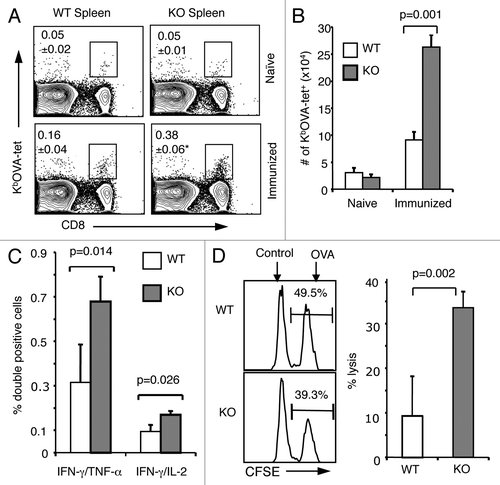
We examined to what extent B7-H1 regulates the generation of memory CD8+ T cells in immunized mice by using KbOVA257–264 tetramer (KbOVA-tet) to detect antigen-primed memory CD8+ T cells in the spleen on day 4 after in vivo re-stimulation (OVA protein, administered on day 40 after primary immunization). Day 4 was selected for analysis because at this time point it is possible to distinguish a recall response from the primary response (which takes 7 d to establish). Thus, naïve mice did not show a significant increase of antigen-specific CD8+ T cells on day 4 after immunization (). The frequency of KbOVA-tet+ CD8+ T cells increased more than 2-fold in immunized B7-H1 deficient mice (0.38%) as compared with WT mice (0.16%; p < 0.05; ). This increase was reflected in the absolute cell numbers (p = 0.001; ). In addition to having increased numbers of memory CD8+ T cells, an increased percentage of memory CD8+ T cells capable of producing multiple cytokines was detected in the spleens of B7-H1 deficient mice (0.73% IFNγ+/TNFα+, 0.17% IFNγ+/IL-2+) as compared with WT mice (0.24% IFNγ+/TNFα+, 0.07% IFNγ+/IL-2+; p < 0.05; ). We also performed an in vivo CTL assay to measure cytolytic activity of the memory CD8+ T cells. On day 4 after in vivo re-stimulation we injected OVA peptide- or control peptide-pulsed target cells (syngeneic splenocytes labeled with either high or low CFSE) into immunized WT and B7-H1 deficient mice. Four hours following cell injection, we analyzed the remaining CFSE positive cells in the spleen. Memory CD8+ T cells in the B7-H1-deficient mice lysed more OVA-peptide pulsed target cells (33.5%) than those in WT mice (9.3%, p < 0.01; ). Collectively, these data suggest that B7-H1 negatively regulates the generation of memory CD8+ T cells in immunized mice.
Figure 2. Enhanced memory CD8+ T-cell population in the absence of B7-H1. Mice were immunized with OVA plus poly I:C and were re-stimulated with OVA on day 40 after immunization. On day 4 after re-stimulation, spleen cells were isolated from naïve or immunized WT and B7-H1-deficient mice for analysis. (A) Percentage of OVA-specific tetramer+ CD8+ T cells, *p < 0.05 compared with WT mice. (B) Graph shows absolute number of OVA-specific tetramer+ CD8+ T cells (mean ± SD, n = 3). (C) Flow cytometry analysis of intracellular production of cytokines in CD8+ T cells from immunized mice (mean ± SD, n = 3). (D) In vivo cytolytic activity in immunized mice. OVA-peptide or control-peptide pulsed target cells (syngeneic splenocytes) were labeled with high or low dose CFSE (5 μM for OVA-peptide pulsed cells; 0.5 μM for control-peptide pulsed cells) and mixed (1:1, 2.5x106 of each) and injected i.v. into WT or B7-H1-deficient mice. Histogram plots show the percentage of remaining target cells in the spleen 4 h after target cell transfer. Bar graph shows percentage of specific lysis in the spleen (mean ± SD, n = 3).
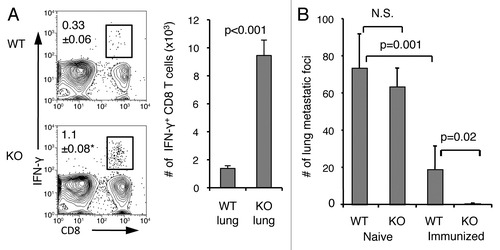
A hallmark of memory CD8+ T cells is their rapid recall response to cognate antigens, so we next examined if the increased memory pool in B7-H1-deficient mice would lead to a more protective recall response. We injected B16-OVA melanoma tumor cells (engineered to express OVA) into immunized WT and B7-H1 deficient mice. Intravenously injected B16-OVA tumor cells form metastases in the lung, and antitumor immunity can be monitored by counting the number of tumor foci. On day 4 following intravenous injection of 5 × 105 B16-OVA tumor cells, the frequency of functional memory CD8+ T cells in the lungs of WT and B7-H1-deficient mice was determined by intracellular staining for IFNγ. We detected ~4–5 fold more IFNγ+ CD8+ T cells in the lungs of B7-H1 deficient mice as compared with WT mice (p < 0.01; ). On day 21-post tumor injection the number of tumor metastases in the lungs of naïve B7-H1 deficient mice was comparable to that of naïve WT mice (p = 0.43; ). Fewer tumor metastases formed in the lungs of immunized WT mice as compared with naïve WT mice (p = 0.001). Significantly, tumor metastases were completely rejected in the lungs of immunized B7-H1 deficient mice (), suggesting that a more efficient CD8+ T cell memory population is established in the absence of B7-H1.
Figure 3. Enhanced memory CD8+ T-cell recall responses and improved antitumor immunity in the lung in the absence of B7-H1. On day 35 after immunization, immunized or naïve WT and B7-H1-deficient mice were injected (i.v.) with 5x105 B16-OVA tumor cells. (A) Percentage and absolute numbers of IFNγ+ CD8+ T cells in the lung of immunized mice (mean ± SD, n = 3) on day 4 after tumor injection. *p < 0.01 compared with WT mice. (B) Metastatic tumor foci in the lung tissue were identified and counted on day 20 after tumor injection (mean ± SD, n = 5). One of two independent experiments is shown. N.S.: not significant.
Bim expression is reduced in antigen-primed CD8+ T cells in the absence of B7-H1
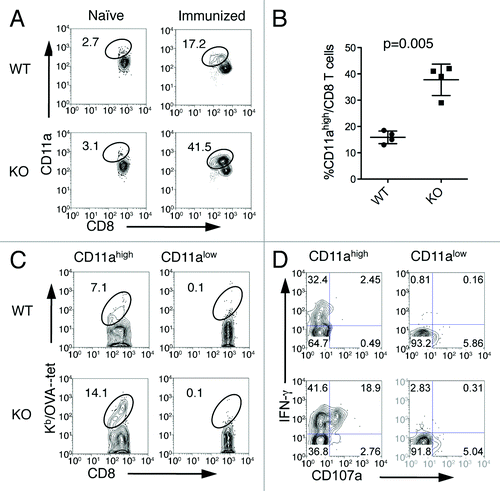
We investigated which mechanisms could be responsible for the increased population of memory CD8+ T cells in B7-H1-deficient mice by examining the proliferation and apoptosis of antigen-primed CD8+ T cells following immunization. We used CD11a as a surrogate activation marker.Citation27 An advantage of this method is that CD11ahigh CD8+ T cells represent antigen-primed CD8+ T cells that are responsive to undefined antigen epitopes not recognized by tetramers. CD11ahigh CD8+ T cells were detected at low levels in the spleens of naïve WT and B7-H1-deficient mice (). On day 7 after immunization, the percentage of CD11ahigh CD8+ T cells increased more than 2-fold in the spleens of B7-H1 deficient mice (41.5%) as compared with immunized WT mice (17.2%; p < 0.01, ), consistent with the results obtained by tetramer staining (). 7–15% of CD11ahigh CD8+ T cells from WT and B7-H1-deficient mice were specific for the known H-2Kb-restricted OVA257–264 epitope based on tetramer staining, and CD11alow CD8+ T cells did not contain tetramer+ cells (), suggesting that all antigen-specific CD8+ T cells are found in the CD11ahigh CD8+ T cell population. In addition, the CD11ahigh CD8+ population from both WT and B7-H1-deficient mice, but not the CD11alow CD8+ T cell population, produced IFNγ and underwent degranulation (indicated by CD107a surface expression) following ex vivo re-stimulation (). As T cell responses against diverse epitopes are coordinately regulated, these data further support the concept that the CD11ahigh CD8+ T cell population represents true OVA-specific CD8+ T cells. Nearly 80–90% of OVA-induced CD11ahigh CD8+ T cells were reactive against undefined antigen epitopes of the OVA protein (). Therefore, the CD11ahigh CD8+ T cell population could be used to represent a majority of the antigen-primed CD8+ T cells during primary T cell responses. In the following studies we used CD11ahigh as a marker to track antigen-specific CD8+ T cells.
Figure 4. CD11ahigh CD8+ T cells represent antigen-primed effector T cells. Spleen cells from naïve or immunized WT and B7-H1-deficient mice were analyzed by co-staining with anti-CD11a and Kb/OVA tetramer or functional markers. (A) Percentage of CD11ahigh CD8+ T cells from WT and B7-H1-deficient immunized mice. (B) Graph shows average percentage of CD11ahigh CD8+ T cells from WT and B7-H1-deficient immunized mice (mean ± SD, n = 4). (C) Percentage of antigen-specific tetramer+ (Kb/OVA-tet) cells in CD11ahigh and CD11alow CD8+ T cell population. (D) CTL functional assay of CD11ahigh and CD11alow CD8+ T cells after a brief re-stimulation in vitro. Degranulation of CTLs was analyzed by CD107a mobilization, followed by intracellular staining for IFN-γ. Numbers indicate percentages of gated areas. One of three independent experiments is shown.
We examined the proliferation of effector CD8+ T cells following immunization by staining cells for Ki67, a nuclear protein associated with cell proliferation.Citation28 The percent of Ki67+ cells increased in CD11ahigh CD8+ T cells from B7-H1-deficient mice (9.32%) as compared with WT mice (7.5%), but this increase was not statistically significant (). We also monitored proliferation by performing a BrdU incorporation assay to measure the ongoing proliferation of CD8+ T cells following immunization. Again, we found that the percentage of BrdU+ CD11ahigh CD8+ T cells was similar between WT (6.05%) and B7-H1 deficient mice (5.59%; ). Ki67+ or BrdU+ cells were mainly detected in the CD11ahigh CD8+ T cells but not in CD11alow CD8+ T cells, suggesting that CD11ahigh CD8+ T cells are proliferating following antigen-stimulation (). Our results suggest that the observed increased population of antigen-primed CD8+ T cells in B7-H1-deficient mice is not due to an increased proliferation of this cell compartement, as compared with WT mice.
Figure 5. Fewer apoptotic antigen-primed CD8+ T cells in B7-H1-deficient mice. On day 7 after immunization, spleen cells were analyzed for proliferation and apoptosis. (A) Ki67 expression and (B) BrdU incorporation were analyzed in CD11ahigh or CD11alow CD8+ T cells. Numbers are percentages of gated area in total CD8+ T cells. (C) TMRElow Annexin V+ apoptotic cells were measured in CD11ahigh and CD11alow CD8+ T cells. (D) Graph shows percentage of apoptotic cells (TMRElow Annexin V+) in CD11ahigh CD8+ T cells (mean ± SD, n = 4). One of three experiments is shown.
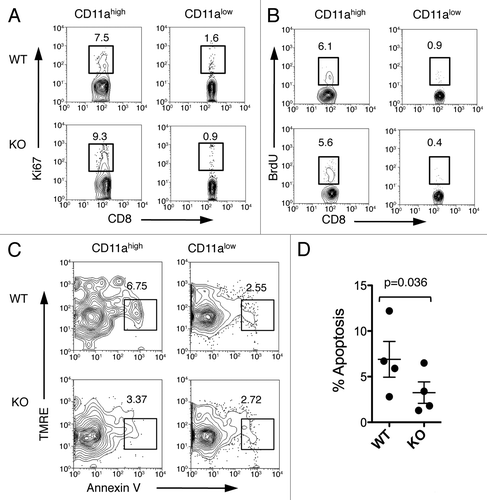
We next asked if decreased apoptosis of antigen-primed CD8+ T cells could contribute to the observed increased population of antigen-primed CD8+ T cells in immunized B7-H1-deficient mice. As discussed earlier, the Fas/Fas ligand death receptor pathway is implicated in regulation of T cell contraction, so we measured the surface expression levels of Fas and Fas ligand on effector CD8+ T cells on day 7 after immunization. Expression of Fas and Fas ligand was detected at similar levels in WT and B7-H1 deficient mice (Fig. S1). These results suggest that the observed increased population of effector CD8+ T cells is not due to a change in Fas-induced apoptosis in B7-H1-deficient mice. We also investigated the mitochondrial pathway for apoptosis by analyzing levels of Annexin V and tetramethylrhodamine ethyl ester (TMRE) staining. TMRE is a fluorescent marker that is incorporated into intact mitochondria, and cells undergoing apoptosis show reduced TMRE staining as compared with live cells.Citation29 Fewer antigen-primed CD11ahigh CD8+ T cells were undergoing apoptosis (TMRElow Annexing V+) in B7-H1-deficient mice (3.4%) as compared with WT mice (6.7%, p < 0.05, ). These results suggest that decreased levels of mitochondrial apoptosis may contribute to the observed increased population of antigen-primed CD8+ T cells in B7-H1-deficient mice.
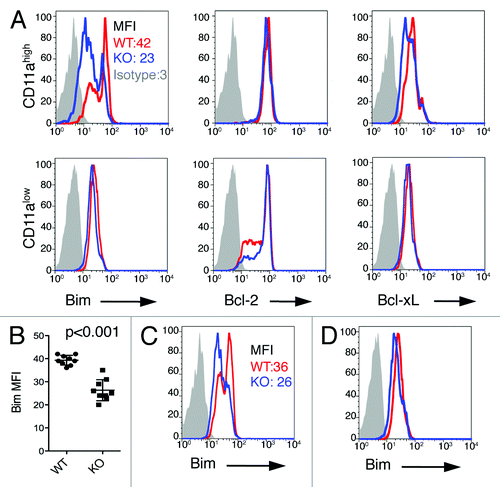
We next looked for alterations in the expression of apoptosis-regulating molecules in effector CD8+ T cells. We measured intracellular levels of the pro-apoptotic molecule Bim, and the anti-apoptotic molecules Bcl-2 and Bcl-xL in CD11ahigh CD8+ T cells freshly isolated from the spleen on day 7 after immunization of naïve mice. We observed lower intracellular expression levels of Bim in CD11ahigh CD8+ T cells from B7-H1-deficient mice than in the same cells obtained from WT mice (p < 0.001, ), while the expression levels of Bcl-2 and Bcl-xL were comparable in WT and B7-H1 deficient mice (). The expression of Bim, Bcl-2 and Bcl-xL were comparable in CD11alow CD8+ T cells from B7-H1 deficient and WT mice (). We also analyzed intracellular expression levels of Bim in CD11ahigh CD8+ T cells isolated from the liver on day 7 after immunization of naïve mice. Again, we observed lower intracellular expression levels of Bim in CD11ahigh CD8+ T cells from B7-H1-deficient mice as compared with WT mice (). Finally, we examined intracellular expression levels of these proteins in CD11ahigh CD8+ T cells isolated from the spleen of naïve mice, and observed no significant differences in B7-H1-deficient vs. WT mice in the expression levels of Bim (), Bcl-2 or Bcl-xL (data not shown). Our data suggests that the downregulation of the pro-apoptotic molecule Bim may contribute to the observed increased population of antigen-primed effector CD8+ T cells in B7-H1-deficient mice.
Figure 6. Lower Bim levels in antigen-primed CD8+ T cells in B7-H1-deficient mice. (A) Flow cytometry assay of the intracellular expression of Bim, Bcl-2 and Bcl-xL in gated CD11ahigh CD8+ T cells in the spleen of WT (red) and B7-H1-deficient (blue) mice on day 7 after immunization. Numbers are mean fluorescence intensity (MFI) of Bim expression. (B) Graph shows average MFI of Bim expressed by CD11ahigh CD8+ T cells (mean ± SD, n = 9). (C) Intracellular expression of Bim in CD11ahigh CD8+ T cells in the liver of immunized mice. Numbers are MFI. (D) Bim expression in total CD8+ T cells in the spleen of naive WT (red) and B7-H1-deficient (blue) mice. One of three experiments is shown.
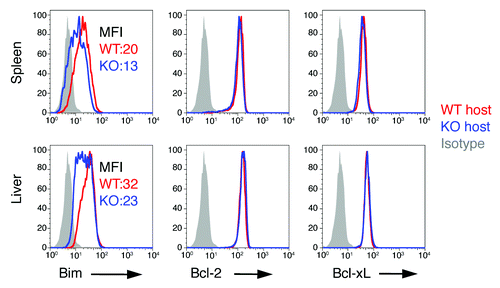
To exclude the possibility that the downregulation of Bim in B7-H1-deficient mice would be due to an intrinsic change in B7-H1-deficient T cells, we performed transfer experiments in which we injected naïve OT-1 CD8+ T cells (Thy1.1+) into WT or B7-H1 deficient mice (Thy1.2+). Following transfer of the OT-1 CD8+ T cells, host mice were immunized with OVA plus poly I:C. On day 7 after immunization we measured the intracellular levels of Bim, Bcl-2, and Bcl-xL in the transferred OT-1 CD8+ T cells freshly isolated from spleen and liver. OT-1 CD8+ T cells transferred into B7-H1-deficient hosts expressed lower levels of Bim in both the spleen and liver as compared with the OT-1 CD8+ T cells transferred into WT hosts (). The expression of Bcl-2 and Bcl-xL in OT-1 CD8+ T cells transferred into WT or B7-H1-deficient mice was comparable (). These data suggest that the downregulation of Bim in B7-H1-deficient mice is not due to an intrinsic change in B7-H1-deficient T cells, but rather to host B7-H1 interacting with one of its binding partners on CD8+ T cells.
Figure 7. Extrinsic role of B7-H1 in regulation of Bim. WT OT-1 CD8+ T cells (Thy1.1+) were transferred in WT (red) or B7-H1-deficient (blue) host mice one day before immunization with OVA plus poly I:C. On day 7 after immunization, the OT-1 CD8+ T cells in the spleen and liver were identified by the Thy1.1 marker and analyzed for intracellular expression of Bim. Numbers are mean fluorescent intensity. One of two experiments is shown.
Next, we used antibodies that block the interaction between B7-H1 and PD-1 or between B7-H1 and CD80 to examine if blocking either of these pathways would impact Bim expression levels. On days 1 and 3 after immunization of WT mice with OVA plus poly I:C, we injected an anti-PD-1 antibody (G4) that only blocks PD-1 binding to B7-H1,Citation30 or an anti-B7-H1 antibody (43H12) that only blocks B7-H1 binding to CD80.Citation31 On day 7 after immunization we compared Bim expression levels in CD11ahigh CD8+ T cells between groups with or without antibody blockade. Antibodies blocking the interaction between B7-H1 and PD-1 or between B7-H1 and CD80 both reduced the expression of Bim in primed CD8+ T cells as compared with control antibodies, whereas the expression of Bcl-2 and Bcl-xL remained unaffected (Fig. S2). Our results suggest that the downregulation of Bim in B7-H1-deficient mice might be due to a lack of interaction between B7-H1 and its binding partners PD-1 and CD80.
The influence of PD-1 signaling on memory generation was addressed previously by Allie et al.Citation32 They demonstrated that after an acute viral infection more central memory T cells accumulate in the lymphoid organs of PD-1-deficient mice as compared with WT mice, indicating that PD-1 signaling negatively regulates memory T-cell generation. We addressed the relevance of CD80 signaling in the regulation of memory generation by transferring equal numbers of CD80-deficient OT-1 and WT OT-1 naïve CD8+ T cells into CD45.1+ mice. One day after T-cell transfer, host mice were immunized with OVA plus poly I:C. On day 21 after immunization the frequency and phenotype of the transferred CD80-deficient and WT OT-1 CD8+ T cells was analyzed. On day 21 after immunization, a 2-fold increased percentage of CD80-deficient OT-1 CD8+ T cells as compared with WT OT-1 CD8+ T cells was detected in the spleen (Fig. S3A), indicating that the transferred CD80-deficient OT-1 CD8+ T cells generated more memory T cells as compared with WT OT-1 CD8+ T cells. Surface staining confirmed that these cells had a central memory phenotype (CD44hi CD62Lhi, Figure S3B). We investigated the recall response of the memory population generated from transferred cells by injecting the hosts with OVA plus poly I:C on day 30 after the initial immunization, and 3 d later the frequency and phenotype of the transferred cells was analyzed. An increased percentage of CD80-deficient OT-1 CD8+ T cells as compared with WT OT-1 CD8+ T cells was detected in the spleen (p = 0.013; Figure S3C,D). Surface staining confirmed that these cells had an effector memory phenotype (CD44hi CD62Llo, Figure S3E). Taken together, these data demonstrate that Cd80−/− OT-1 CD8+ T cells generated more memory T cells as compared their WT counterparts, indicating that CD80 expressed by CD8+ T cells may negatively regulate memory T cell generation.
B7-H1 enhances Bim expression in activated CD8+T cells
We investigated how B7-H1 could regulate Bim levels in activated CD8+ T cells by incubating pre-activated WT CD8+ T cells with plate-bound B7-H1 fusion protein for 48 h in the presence of TCR stimulation (anti-CD3 antibody). We analyzed Bim expression by western blot and found increased expression levels in CD8+ T cells cultured in the presence of B7-H1 fusion protein compared with a control fusion protein (). We also analyzed Bim expression by intracellular flow cytometry and observed that B7-H1 fusion protein dramatically increases the levels of Bim protein in CD8+ T cells compared with a control fusion protein (p < 0.02, ). In the absence of anti-CD3 antibodies, Bim levels did not increase upon incubation with B7-H1 fusion protein (data not shown), suggesting that B7-H1 provides a co-stimulatory signal for Bim upregulation. Accordingly, the absolute number of live cells was also reduced in CD8+ T cells cultured in the presence of B7-H1 fusion protein compared with a control protein (p < 0.01, ). We observed increased levels of cells undergoing apoptosis (TMRElow Annexin V+) in cultures of activated CD8+ T cells exposed to B7-H1 fusion protein and anti-CD3 (12.4%) as compared with cells cultured with a control fusion protein and anti-CD3 (4.1%, ). The induction of apoptosis by B7-H1 fusion protein was lost in CD8+ T cells isolated from Bim-deficient and Bcl-2 transgenic mice (), suggesting that B7-H1-induced T cell apoptosis may be dependent on the Bim-mediated mitochondrial pathway of apoptosis.
Figure 8. B7-H1 co-stimulation induces upregulation of Bim protein levels in activated T cells. Pre-activated CD8+ T cells were incubated with plate-bound B7-H1 or control fusion protein (Fc) for 48 h in the presence of anti-CD3. (A) Bim isoform expression in CD8+ T cells was analyzed by western blot. (B) Histogram shows the expression of total Bim in CD8+ T cells co-stimulated with B7-H1 (blue line) or control protein (red line). Numbers are mean fluorescent intensity (MFI) (C) Graph shows average MFI of Bim expressed by activated CD8+ T cells (mean ± SD, n = 5). (D) Graph shows the percentage of live (trypan blue exclusive) CD8+ T cells in culture (mean ± SD, n = 5). (E) Apoptosis of CD8+ T cells isolated from WT, Bim-deficient and Bcl-2 transgenic (Tg) mice. Numbers show percentage of TMRElow Annexin V+ apoptotic T cells in total CD8+ T cells. One of three experiments is shown. (F) Graph shows average MFI of Bim expressed by CD8+ T cells in culture with anti-B7-H1 Ab (10B5, blocking B7-H1 binding to both PD-1 and CD80; 43H12, blocking B7-H1 binding to CD80 only), anti-PD-1 Ab (G4) or control Ab (10 μg/mL of each) (mean ± SD, n = 3).
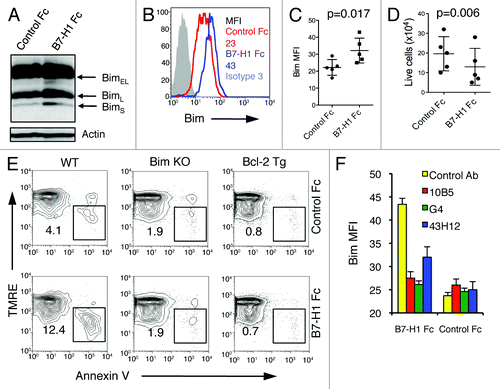
To examine which receptor of B7-H1 is involved in mediating Bim upregulation, we incubated pre-activated WT CD8+ T cells with plate-bound B7-H1 fusion protein pre-blocked with anti-B7-H1 (10B5 or 43H12) or anti-PD1 (G4) antibodies. The 10B5 antibody blocks the interaction of B7-H1 with both PD-1 and CD80. Both 10B5 and G4 antibodies completely blocked Bim upregulation induced by B7-H1 fusion protein, while 43H12 only partially, but significantly, did so (). None of the antibodies used in this experiment had effects on Bim expression levels in cells cultured with control fusion protein, indicating that their effect on Bim expression levels is due to blocking the interaction between B7-H1/PD-1 or B7-H1/CD80, and not due to a non-specific effect. These results suggest that B7-H1 may use PD-1 or CD80 on CD8+ T cells to deliver co-stimulatory signals for the upregulation of Bim.
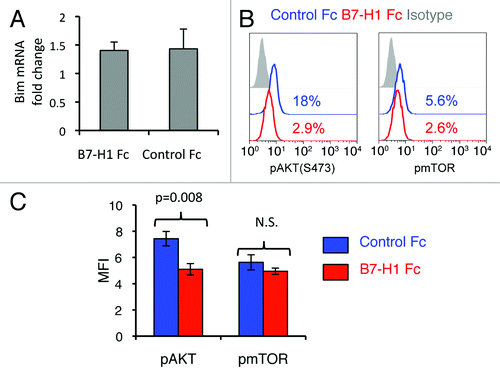
We next investigated the mechanism by which B7-H1 regulates Bim expression levels. We analyzed the mRNA levels of Bcl211, which encodes the Bim protein, by quantitative real-time PCR analysis on mRNA isolated from pre-activated CD8+ T cells exposed to B7-H1 fusion protein or to a control fusion protein and anti-CD3 for 24 h. Incubation of pre-activated CD8+ T cells with B7-H1 fusion protein did not increase the levels of Bcl211 (), indicating that the B7-H1-mediated upregulation of Bim does not result from transcriptional regulation. The degradation of Bim is tightly regulated, one mechanism being the activation of Akt followed by Akt-mediated Bim phosphorylation and degradation.Citation33 The level of Akt activation in CD8+ T cells after B7-H1 engagement was measured by intracellular flow cytometry for phosphorylated-Akt (Ser473). CD8+ T cells cultured with B7-H1 fusion protein exhibited decreased levels of phosphorylated Akt as compared with CD8+ T cells cultured with a control fusion protein (p < 0.01, ). As phosphorylation of Akt at Ser473 is regulated by activation of mTOR,Citation34,Citation35 we next examined whether B7-H1 regulates phosphorylation of mTOR in vitro. Unexpectedly, there was no difference in levels of phospho-mTOR in CD8+ T cells cultured with B7-H1 fusion protein and cells cultured with control fusion protein (). Our results suggest that CD8+ T cell engagement with B7-H1 inhibits the activation of Akt, resulting in decreased degradation of Bim.
Figure 9. B7-H1 co-stimulation inhibits activation of Akt. Pre-activated CD8+ T cells were stimulated with plate-bound B7-H1 or control fusion protein (Fc). After 24 h of stimulation, CD8+ T cells were harvested and used for analysis. (A) Analysis of Bcl2l11 transcript levels by real-time qPCR using the comparative CT method. GAPDH served as the internal control gene. Graph shows fold change (mean ± SD, n = 4). (B) Phosphorylation of Akt and mTOR was analyzed by intracellular staining of CD8+ T cells with anti-phospho-Akt and anti-phospho-mTOR Abs. Numbers show percentage of positive stained cells. (C) Bar graph of average MFI of phospho-Akt and phospho-mTOR expression (mean ± SD, n = 3). N.S.: not significant.
Discussion
In this study, we report that B7-H1 negatively regulates the establishment of CD8+ T cell memory by inducing the upregulation of Bim, resulting in apoptosis of effector CD8+ T cells. Accordingly, B7-H1-deficient mice develop enhanced memory CD8+ T cell populations following immunization, resulting in improved protective immunity. Bim expression levels are reduced in CD8+ T cells when there is a lack of B7-H1 signaling, either in B7-H1-deficient mice, when WT CD8+ T cells are transferred into B7-H1-deficient hosts, or when B7-H1 signaling is inhibited by in vivo administration of blocking antibodies. It appears that both PD-1 and CD80 receptors are involved in the B7-H1-mediated upregulation of Bim. Our results reveal a new mechanism by which B7-H1 regulates CD8+ T cell responses in vivo, and provide a new direction to pursue in dissecting the signaling events downstream of the receptors for B7-H1.
Recent studies in human patients have indicated that tumor-infiltrating CD8+ T cells express PD-1, resulting in impaired function and compromised antitumor immunity.Citation36,Citation37 Analysis of a variety of patient tumor tissues revealed that a majority of tumors express B7-H1, and this tumor-associated B7-H1 increases the apoptosis of infiltrating T cells.Citation11 B7-H1 expression on renal cell carcinoma cells has also been shown to be associated with poor prognosis.Citation38 These findings demonstrate the clinical relevance of B7-H1 signaling in patients with cancer, motivating our studies of how B7-H1 regulates T-cell memory generation and apoptosis. Bim is a key regulator of T cell apoptosis during the contraction phase of CD8+ T-cell responses.Citation39 In a study of patients with chronic HCV infection, Larrubia et al. reported increased levels of Bim expression in CD8+ T cells that upregulated PD-1.Citation40 This and other recent studies also reported that blockade of the B7-H1/PD-1 pathway leads to improved functioning of virus-specific CD8+ T cells.Citation41-Citation43 However, none of these studies addressed the interaction between the B7-H1/PD-1 pathway and Bim expression levels as we have done here.
We observed that B7-H1-deficient mice exhibit delayed contraction following antigen immunization (), and this difference could not be attributed to increased proliferation of antigen-primed CD8+ T cells (). We did observe a lower frequency of TMRElow Annexin V+ CD8+ T cells in B7-H1-deficient mice, which prompted us to examine the mitochondrial pathway of apoptosis (). Both our in vivo and in vitro studies revealed that B7-H1 may upregulate Bim levels in antigen-primed or TCR-activated CD8+ T cells (, and ). The B7-H1-mediated induction of apoptosis in activated CD8+ T cells appears to be dependent on Bim expression, because B7-H1 ligation did not induce apoptosis in activated CD8+ T cells isolated from Bim-deficient or Bcl-2-transgenic mice (). Thus, our studies demonstrate that B7-H1 may induce apoptosis of activated CD8+ T cells via regulation of Bim. In support of this finding, Kryczek et al. reported that Notch/Bcl-2 signaling mediates resistance to apoptosis in human tumor-associated memory TH17 cells.Citation44 Altogether, these studies demonstrate the importance of the Bcl-2 family of pro- and anti-apoptotic molecules in the regulation of antitumor memory T cell responses.
Our group has recently published a study revealing a cell-intrinsic role for B7-H1 expressed by CD8+ T cells in upregulating the anti-apoptotic molecule Bcl-xL.Citation45 Our data presented here clearly shows that regulation of Bim expression levels in CD8+ T cells is an extrinsic function of B7-H1. This was demonstrated by transferring congenically marked naïve WT OT-1 CD8+ T cells into WT or B7-H1-deficient hosts, followed by immunization of the mice and analysis of the resulting effector CD8+ T cells. As shown in , WT CD8+ T cells expressed lower levels of Bim upon transfer into B7-H1-deficient hosts as compared with transfer into WT hosts, due to a lack of extrinsic B7-H1 signaling. B7-H1 regulation of Bim expression is likely mediated through B7-H1 interacting with both of its binding partners expressed by CD8+ T cells, PD-1 and CD80, because in vivo antibody blockade of either B7-H1/PD-1 or B7-H1/CD80 interactions resulted in reduced Bim expression levels in CD8+ T cells (Fig. S2). This function of B7-H1 appears to depend on TCR signaling, because in the absence of anti-CD3 stimulation the B7-H1 protein failed to enhance Bim levels in pre-activated CD8+ T cells (data not shown).
Work investigating signaling events downstream of PD-1 have revealed that PD-1 ligation results in inhibition of phosphoinositide-3-kinase (PI3K) activity, leading to reduced activation of Akt.Citation12 Akt inhibits Bim activity by phosphorylating Bim, leading to its degradation.Citation33 Hence, it is likely that B7-H1 signaling through PD-1 inhibits Akt, resulting in enhanced expression of Bim in activated CD8+ T cells. Accordingly, our data shows that B7-H1 engagement of pre-activated CD8+ T cells leads to decreased phosphorylation of Akt (). Phosphorylation of Akt following PI3K activation is mediated by PDK1 at Thr308 and mTOR at Ser473.Citation34,Citation46,Citation47 We observed down-regulation of phospho-Akt at Ser473 () but not at Thr308 (data not shown), suggesting that B7-H1 may use the mTOR pathway to regulate Akt activation. Recently, Francisco et al. reported that B7-H1 ligation reduces the phosphorylation of mTOR at Ser2448 during the induction or regulatory T cells.Citation48 However, we could not detect changes in the phosphorylation of mTOR Ser2448 in CD8+ T cells cultured with a B7-H1 fusion protein (). This discrepancy might be due to the different T cell subsets studied (CD4+ vs. CD8+) and/or to differences in the duration of the culture (18 h vs. 48 h). Nevertheless, the regulation of Akt activation downstream of B7-H1 signaling in T cells warrants further investigation.
Protective immunity is dependent on the generation of durable memory T-cell responses.Citation49-Citation51 However, the signals required to induce the differentiation of functional long-lived memory T cells are not fully understood. Most studies of T-cell memory generation support a linear model.Citation52 According to this model, upon antigen stimulation naïve T cells undergo a massive level of expansion, during which they differentiate into effector T cells. Following clearance of the initiating antigen stimulus, effector T cells enter a contraction phase, during which a majority of them die. The cells that survive the contraction phase further differentiate to make up the antigen-specific memory T cell population. In support of this model, it has been shown that the transition from naïve to effector to memory T cells is a gradual process,Citation53,Citation54 and that a large portion of memory T cells arise from effector T cell progenitors, as determined by their inherent markers.Citation52,Citation55,Citation56 Because not all effector T cells enter the memory cell pool, factors that control the survival or depletion of effector cells may play a key role in determining the size of the memory cell population.
Recent studies indicate that negative T-cell regulators affect the differentiation of memory T cells. Blockade of CTLA-4 inhibitory signals results in more memory T cells generated following bacterial infection.Citation57 In PD-1-deficient mice, Allie et al. observed that more central memory T cells accumulate in the lymphoid organs after acute viral infections.Citation32 Those studies did not address the molecular mechanisms by which negative regulators influence the process of memory T-cell development. Our studies suggest that B7-H1-mediated upregulation of the pro-apoptotic molecule Bim in effector T cells may be a key factor in limiting effector T-cell entry into the memory pool. In line with previous studies unveiling more memory T cells in Bim deficient mice,Citation25,Citation26 our results show that, in the absence of B7-H1, Bim levels are downregulated in effector CD8+ T cells and apoptosis of effector CD8+ T cells is reduced. Thus, more effector CD8+ T cells are allowed to enter the memory pool. Because Bim upregulation by B7-H1 is dependent on PD-1 (Fig. S2), our data may explain why more memory CD8+ T cells are generated in PD-1-deficient mice after acute infection.Citation32
In addition, our studies show that, in the absence of B7-H1, a robust antitumor immune response can be established that prevents the formation of tumor metastases in the lung (). As tumor metastases frequently occur in peripheral tissues, the generation or maintenance of a functional memory T-cell response in the periphery will provide a first line of protection against tumor metastases. Although we did not directly address to what extent B7-H1 influences the maintenance of memory T cells in peripheral tissues, our data strongly indicate that enhancing primary T-cell responses may promote effector T cells to enter the memory T cell pool. In this regard, B7-H1 may function as a checkpoint in limiting the number of effector T cells that enter the memory pool. Future studies are needed to address which subsets of effector T cells are more sensitive to B7-H1 signals and whether TCR affinity may affect the sensitivity of effector T cells to B7-H1 regulation. This knowledge is important in vaccine design for treatment of cancer and chronic infections for which the B7-H1/PD-1 blockade is being evaluated in phase I clinical trials.Citation58-Citation60
In summary, our findings suggest that B7-H1 signaling through both PD-1 and CD80 on antigen-primed CD8+ T cells negatively regulates the establishment of memory populations by upregulating expression of the pro-apoptotic molecule Bim. Our results add to the understanding of how memory T-cell generation is controlled by inhibitory molecules, and also have important implications for rational vaccine design and the development of immunotherapies targeting tumor metastases.
Materials and Methods
Mice, cell lines, and reagents
Female CD45.2+ C57BL/6 mice were purchased from Taconic Farms (Germantown, NY) and CD45.1+ congenic C57BL/6-Ly5.1 mice were purchased from National Cancer Institute. OT-1 TCR (Thy 1.1+) transgenic mice were provided by T. Tian (Harvard University, Boston, MA). B7-H1-deficient C57BL/6 mice were provided by L. Chen (Yale University, New Haven, CT).Citation61 Bcl211−/− mice and Cd80−/− mice were purchased from Jackson Laboratory (Bar Harbor, ME). Cd80−/− mice were crossbred into WT OT-1 mice and produced Cd80−/− OT-1 mice. Bcl-2 transgenic mice were provided by V. Shapiro (Mayo Clinic, Rochester). Mice were maintained under pathogen-free conditions and used at 8–12 weeks of age. B16-OVA murine melanoma cells were provided by R. Vile (Mayo Clinic, Rochester, MN), and were cultured in RPMI 1640 medium (Cellgro, Hendon, VA) with 10% FBS (Life Technologies, Carlsbad, CA), 1 U/mL penicillin, 1 μg/mL streptomycin, and 20 mM HEPES buffer (all from Mediatech, Manassas, VA). Hamster anti-mouse B7-H1 mAb (10B5) and PD-1 (G4) was obtained from hybridoma cells provided by L. Chen. Hamster anti-mouse B7-H1 mAb (43H12) was provided by K. Tamada (John Hopkins University, Baltimore, MD). Studies were conducted in accordance with the National Institutes of Health guidelines for the proper use of animals in research and with local Institutional Animal Care and Use Committee approval.
Flow cytometry analysis. class I MHC (KbOVA peptide SIINFEKL) tetramer and negative control tetramer were purchased from Beckman Coulter (Brea, CA). Fluorochrome-conjugated Abs against CD8, CD11a, Fas (CD95), Fas ligand, CD90.1 (Thy 1.1), CD90.2 (Thy 1.2), CD107a, IFNγ, IL-2, and TNFα were purchased from BD Biosciences (Mountain View, CA), BioLegend (San Diego, CA), or eBiosciences (San Diego, CA). To detect intracellular cytokine levels cells were incubated with GolgiPlug (BD Biosciences) for 4 h prior to analysis. Cells were stained for surface antigens, and then incubated in Fixation Buffer (BioLegend) for 20 min at room temperature, followed by permeabilization in Permeabilization Wash Buffer (BioLegend). Fixed and permeabilized cells were then stained with Abs for 20 min at room temperature. Abs to Akt, Bcl-xL, Bcl-2, Bim, and mTOR and fluorochrome-conjugated secondary Abs were purchased from Cell Signaling (Danvers, MA). To detect the intracellular levels of Akt, Bcl-xL, Bcl-2, Bim, and mTOR, T cells were first stained for surface antigens, then fixed with 2% paraformaldehyde for 10 min at 37°C, followed by permeabilization with ice-cold methanol for 30 min. After blocking with 15% rat serum for 15 min, cells were stained with Abs for 1 h at room temperature. After staining, cells were washed three times with incubation buffer before analysis. At least 100,000 viable cells were live gated on FACScan or FACSCailbur (BD Biosciences, USA) instrumentation. Flow cytometry analysis was performed using FlowJo software (Tree Star, Ashland, OR).
T cell immunization, activation, apoptosis assay, and proliferation assay
Mice were immunized by i.p. injection of 0.5 mg ovalbumin (OVA, from Sigma-Aldrich, St. Louis, MO) and 50 μg poly (I:C) (Sigma Aldrich). For in vitro T cell activation and apoptosis assay, purified CD8+ T cells were labeled with CFSE (Invitrogen-Molecular Probes, Eugene, OR) and incubated with OVA peptide257–264 (Mayo Clinic Core Facilities) at 0.2 μg/mL for 72 h. Apoptosis of CD8+ T cells was analyzed by staining using Annexin V (BD Biosciences) and TMRE (tetramethylrhodamine ethyl ester, Invitrogen/Molecular Probes T-669, Eugene, OR). Proliferation was also measured by detection of BrdU incorporation and Ki67 staining. Immunized mice were injected i.p. with 0.8 mg/mL BrdU (BD Biosciences) on days 4 through 6 following immunization. On day 7 after immunization BrdU incorporation was determined by intra-nuclear staining with anti-BrdU (B9285, Sigma-Aldrich, St. Louis, MO) and anti-Ki67 (556027, BD Biosciences, San Jose, CA).
In vivo CTL assay
For the in vivo CTL assay, OVA 257–264 peptide-pulsed or control peptide-pulsed spleen cells (as target cells) from syngeneic mice were labeled with a high dose of CFSE (5 μM) or low dose of CFSE (0.5 μM), mixed at 1:1 (2.5 × 106 of each) before injection. Target cells were i.v. injected into immunized mice on day 4 after re-challenge with cognate antigen protein. The CTL activity was determined 4 h after target cell transfer. Specific lysis is calculated using the following formulas: ratio = (%CFSEhigh/%CFSElow), % specific lysis = [1-(ratio primed/ratio unprimed)] x 100%.
Tumor studies
Mice were inoculated i.v. with 5 × 105 B16-OVA tumor cells on day 25 after immunization. On day 21-post tumor injection mice were sacrificed and the lung tissue was perfused with PBS. The number of tumor foci on the lung tissue was counted.
T cell transfer experiments
Purified CD8+ T cells (1 × 106) from Thy1.1+ OT-1 transgenic mice were i.v. injected into Thy 1.2+ WT or B7-H1-deficient recipient mice followed with immunization with OVA plus poly I:C. On day 7 after immunization, transferred CD8+ T cells were identified by their expression of Thy1.1 and used for detection of intracellular expression of Bim, Bcl-2 and Bcl-xL. Equal numbers of Cd80−/− (CD45.2+) and WT OT-1 (Thy1.1+, CD45.2+) CD8+ T cells (106 of each) were i.v. injected into CD45.1+ mice followed with immunization of OVA and poly I:C. The transferred OT-1 CD8+ T cells in the spleen were identified by flow cytometry.
In vitro T cell activation and culturing with fusion proteins
Spleen cells were harvested from naïve mice and pre-activated with ConA (5 μg/mL, L7647, Sigma-Aldrich, St. Louis, MO) for 48 h. Following activation, CD8+ T cells were purified (EasySep CD8+ T cell negative selection kit, Stem Cell Technologies, Vancouver, British Columbia) and incubated with plate-bound anti-CD3 (BD Biosciences) and B7-H1 Fc fusion protein or control Fc protein (R&D Systems, Minneapolis, MN). Cultures were maintained for indicated time periods, and then cells were harvested for analysis.
Western blotting
Cells were lysed with NETN buffer (0.5% NP40, 150 mM NaCl, 50 mM Tris, and 1 mM EDTA). Cell lysates were boiled and run on SDS-PAGE gels (BioRad, Hercules, CA), transferred to nitrocellulose membrane (Millipore, Billerica, MA), and blotted using standard procedures.
Quantitative RT-PCR
Total RNA was isolated from purified CD8+ T cells (RNeasy Kit, Qiagen, Valencia, CA), and reverse transcribed (iScript cDNA synthesis kit, BioRad). Samples were analyzed for Bim transcript levels using Bcl211 primers (Qiagen) and QuantiFast SYBR Green PCR Master Mix (Qiagen) on an iCycler (BioRad). GAPDH levels were used to normalize data by the comparative CT method.
Statistical analysis
A two-sided, unpaired or paired Student T test was used to assess statistical differences in experimental groups. A p value < 0.05 was considered statistically significant.
| Abbreviations: | ||
| BrdU | = | bromodeoxyuridine |
| CFSE | = | carboxyfluorescein succinimidyl ester |
| CTL | = | cytotoxic T lymphocyte |
| KbOVA-tet | = | KbOVA257-264 tetramer |
| OVA | = | ovalbumin |
| Poly I:C | = | polyinosinic:polycytidylic acid |
Additional material
Download Zip (2.6 MB)Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We are thankful to Dr. Lieping Chen for providing B7-H1-deficient mice, Dr. Koji Tamada for anti-B7-H1 (43H12) monoclonal antibody, Dr. Richard Vile for B16-OVA tumor cells, and Dr. Virginia Shapiro for T cells from Bcl-2 transgenic mice. This study was supported by the American Cancer Society's New Investigator Award and Wendy Case Cancer Research Fund, and in part by NIH R01 CA134345.
Supplemental Materials
Supplemental materials can be found at:
www.landesbioscience.com/journals/oncoimmunology/article/20850
References
- Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell 2010; 140:883 - 99; http://dx.doi.org/10.1016/j.cell.2010.01.025; PMID: 20303878
- Pagès F, Berger A, Camus M, Sanchez-Cabo F, Costes A, Molidor R, et al. Effector memory T cells, early metastasis, and survival in colorectal cancer. N Engl J Med 2005; 353:2654 - 66; http://dx.doi.org/10.1056/NEJMoa051424; PMID: 16371631
- Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pagès C, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science 2006; 313:1960 - 4; http://dx.doi.org/10.1126/science.1129139; PMID: 17008531
- Leffers N, Gooden MJM, de Jong RA, Hoogeboom BN, ten Hoor KA, Hollema H, et al. Prognostic significance of tumor-infiltrating T-lymphocytes in primary and metastatic lesions of advanced stage ovarian cancer. Cancer Immunol Immunother 2009; 58:449 - 59; http://dx.doi.org/10.1007/s00262-008-0583-5; PMID: 18791714
- Masopust D, Vezys V, Marzo AL, Lefrançois L. Preferential localization of effector memory cells in nonlymphoid tissue. Science 2001; 291:2413 - 7; http://dx.doi.org/10.1126/science.1058867; PMID: 11264538
- Zhang X, Strome SE. B7-H1-targeted immunotherapy for head and neck cancer. Expert Opin Biol Ther 2004; 4:1577 - 83; http://dx.doi.org/10.1517/14712598.4.10.1577; PMID: 15461569
- Thompson RH, Dong H, Kwon ED. Implications of B7-H1 expression in clear cell carcinoma of the kidney for prognostication and therapy. Clin Cancer Res 2007; 13:709s - 15s; http://dx.doi.org/10.1158/1078-0432.CCR-06-1868; PMID: 17255298
- Dong H, Zhu G, Tamada K, Chen L. B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat Med 1999; 5:1365 - 9; http://dx.doi.org/10.1038/70932; PMID: 10581077
- Pulko V, Liu X, Krco CJ, Harris KJ, Frigola X, Kwon ED, et al. TLR3-stimulated dendritic cells up-regulate B7-H1 expression and influence the magnitude of CD8 T cell responses to tumor vaccination. J Immunol 2009; 183:3634 - 41; http://dx.doi.org/10.4049/jimmunol.0900974; PMID: 19710456
- Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med 2000; 192:1027 - 34; http://dx.doi.org/10.1084/jem.192.7.1027; PMID: 11015443
- Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med 2002; 8:793 - 800; PMID: 12091876
- Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol 2008; 26:677 - 704; http://dx.doi.org/10.1146/annurev.immunol.26.021607.090331; PMID: 18173375
- Butte MJ, Keir ME, Phamduy TB, Sharpe AH, Freeman GJ. Programmed death-1 ligand 1 interacts specifically with the B7-1 costimulatory molecule to inhibit T cell responses. Immunity 2007; 27:111 - 22; http://dx.doi.org/10.1016/j.immuni.2007.05.016; PMID: 17629517
- Kurtulus S, Tripathi P, Opferman JT, Hildeman DA. Contracting the ‘mus cells’--does down-sizing suit us for diving into the memory pool?. Immunol Rev 2010; 236:54 - 67; http://dx.doi.org/10.1111/j.1600-065X.2010.00920.x; PMID: 20636808
- Singer GG, Abbas AK. The fas antigen is involved in peripheral but not thymic deletion of T lymphocytes in T cell receptor transgenic mice. Immunity 1994; 1:365 - 71; http://dx.doi.org/10.1016/1074-7613(94)90067-1; PMID: 7533645
- Mogil RJ, Radvanyi L, Gonzalez-Quintial R, Miller R, Mills G, Theofilopoulos AN, et al. Fas (CD95) participates in peripheral T cell deletion and associated apoptosis in vivo. Int Immunol 1995; 7:1451 - 8; http://dx.doi.org/10.1093/intimm/7.9.1451; PMID: 7495753
- Miethke T, Vabulas R, Bittlingmaier R, Heeg K, Wagner H. Mechanisms of peripheral T cell deletion: anergized T cells are Fas resistant but undergo proliferation-associated apoptosis. Eur J Immunol 1996; 26:1459 - 67; http://dx.doi.org/10.1002/eji.1830260709; PMID: 8766547
- Hildeman DA, Zhu Y, Mitchell TC, Bouillet P, Strasser A, Kappler J, et al. Activated T cell death in vivo mediated by proapoptotic bcl-2 family member bim. Immunity 2002; 16:759 - 67; http://dx.doi.org/10.1016/S1074-7613(02)00322-9; PMID: 12121658
- Pellegrini M, Belz G, Bouillet P, Strasser A. Shutdown of an acute T cell immune response to viral infection is mediated by the proapoptotic Bcl-2 homology 3-only protein Bim. Proc Natl Acad Sci U S A 2003; 100:14175 - 80; http://dx.doi.org/10.1073/pnas.2336198100; PMID: 14623954
- Wojciechowski S, Jordan MB, Zhu Y, White J, Zajac AJ, Hildeman DA. Bim mediates apoptosis of CD127(lo) effector T cells and limits T cell memory. Eur J Immunol 2006; 36:1694 - 706; http://dx.doi.org/10.1002/eji.200635897; PMID: 16761315
- Prlic M, Bevan MJ. Exploring regulatory mechanisms of CD8+ T cell contraction. Proc Natl Acad Sci U S A 2008; 105:16689 - 94; http://dx.doi.org/10.1073/pnas.0808997105; PMID: 18946035
- Zhu Y, Swanson BJ, Wang M, Hildeman DA, Schaefer BC, Liu X, et al. Constitutive association of the proapoptotic protein Bim with Bcl-2-related proteins on mitochondria in T cells. Proc Natl Acad Sci U S A 2004; 101:7681 - 6; http://dx.doi.org/10.1073/pnas.0402293101; PMID: 15136728
- Grayson JM, Zajac AJ, Altman JD, Ahmed R. Cutting edge: increased expression of Bcl-2 in antigen-specific memory CD8+ T cells. J Immunol 2000; 164:3950 - 4; PMID: 10754284
- Korsmeyer SJ, Wei MC, Saito M, Weiler S, Oh KJ, Schlesinger PH. Pro-apoptotic cascade activates BID, which oligomerizes BAK or BAX into pores that result in the release of cytochrome c. Cell Death Differ 2000; 7:1166 - 73; http://dx.doi.org/10.1038/sj.cdd.4400783; PMID: 11175253
- Sabbagh L, Srokowski CC, Pulle G, Snell LM, Sedgmen BJ, Liu Y, et al. A critical role for TNF receptor-associated factor 1 and Bim down-regulation in CD8 memory T cell survival. Proc Natl Acad Sci U S A 2006; 103:18703 - 8; http://dx.doi.org/10.1073/pnas.0602919103; PMID: 17116875
- Wojciechowski S, Tripathi P, Bourdeau T, Acero L, Grimes HL, Katz JD, et al. Bim/Bcl-2 balance is critical for maintaining naive and memory T cell homeostasis. J Exp Med 2007; 204:1665 - 75; PMID: 17591857
- Rai D, Pham NLL, Harty JT, Badovinac VP. Tracking the total CD8 T cell response to infection reveals substantial discordance in magnitude and kinetics between inbred and outbred hosts. J Immunol 2009; 183:7672 - 81; http://dx.doi.org/10.4049/jimmunol.0902874; PMID: 19933864
- Gerdes J, Lemke H, Baisch H, Wacker HH, Schwab U, Stein H. Cell cycle analysis of a cell proliferation-associated human nuclear antigen defined by the monoclonal antibody Ki-67. J Immunol 1984; 133:1710 - 5; PMID: 6206131
- Jayaraman S. Flow cytometric determination of mitochondrial membrane potential changes during apoptosis of T lymphocytic and pancreatic beta cell lines: comparison of tetramethylrhodamineethylester (TMRE), chloromethyl-X-rosamine (H2-CMX-Ros) and MitoTracker Red 580 (MTR580). J Immunol Methods 2005; 306:68 - 79; http://dx.doi.org/10.1016/j.jim.2005.07.024; PMID: 16256133
- Hirano F, Kaneko K, Tamura H, Dong H, Wang S, Ichikawa M, et al. Blockade of B7-H1 and PD-1 by monoclonal antibodies potentiates cancer therapeutic immunity. Cancer Res 2005; 65:1089 - 96; PMID: 15705911
- Park JJ, Omiya R, Matsumura Y, Sakoda Y, Kuramasu A, Augustine MM, et al. B7-H1/CD80 interaction is required for the induction and maintenance of peripheral T-cell tolerance. Blood 2010; 116:1291 - 8; http://dx.doi.org/10.1182/blood-2010-01-265975; PMID: 20472828
- Allie SR, Zhang W, Fuse S, Usherwood EJ. Programmed death 1 regulates development of central memory CD8 T cells after acute viral infection. J Immunol 2011; 186:6280 - 6; http://dx.doi.org/10.4049/jimmunol.1003870; PMID: 21525385
- Qi XJ, Wildey GM, Howe PH. Evidence that Ser87 of BimEL is phosphorylated by Akt and regulates BimEL apoptotic function. J Biol Chem 2006; 281:813 - 23; http://dx.doi.org/10.1074/jbc.M505546200; PMID: 16282323
- Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science 2005; 307:1098 - 101; http://dx.doi.org/10.1126/science.1106148; PMID: 15718470
- Jacinto E, Facchinetti V, Liu D, Soto N, Wei S, Jung SY, et al. SIN1/MIP1 maintains rictor-mTOR complex integrity and regulates Akt phosphorylation and substrate specificity. Cell 2006; 127:125 - 37; http://dx.doi.org/10.1016/j.cell.2006.08.033; PMID: 16962653
- Sfanos KS, Bruno TC, Meeker AK, De Marzo AM, Isaacs WB, Drake CG. Human prostate-infiltrating CD8+ T lymphocytes are oligoclonal and PD-1+. Prostate 2009; 69:1694 - 703; http://dx.doi.org/10.1002/pros.21020; PMID: 19670224
- Ahmadzadeh M, Johnson LA, Heemskerk B, Wunderlich JR, Dudley ME, White DE, et al. Tumor antigen-specific CD8 T cells infiltrating the tumor express high levels of PD-1 and are functionally impaired. Blood 2009; 114:1537 - 44; http://dx.doi.org/10.1182/blood-2008-12-195792; PMID: 19423728
- Thompson RH, Kuntz SM, Leibovich BC, Dong H, Lohse CM, Webster WS, et al. Tumor B7-H1 is associated with poor prognosis in renal cell carcinoma patients with long-term follow-up. Cancer Res 2006; 66:3381 - 5; http://dx.doi.org/10.1158/0008-5472.CAN-05-4303; PMID: 16585157
- Weant AE, Michalek RD, Khan IU, Holbrook BC, Willingham MC, Grayson JM. Apoptosis regulators Bim and Fas function concurrently to control autoimmunity and CD8+ T cell contraction. Immunity 2008; 28:218 - 30; http://dx.doi.org/10.1016/j.immuni.2007.12.014; PMID: 18275832
- Larrubia JR, Benito-Martínez S, Miquel J, Calvino M, Sanz-de-Villalobos E, González-Praetorius A, et al. Bim-mediated apoptosis and PD-1/PD-L1 pathway impair reactivity of PD1(+)/CD127(-) HCV-specific CD8(+) cells targeting the virus in chronic hepatitis C virus infection. Cell Immunol 2011; 269:104 - 14; http://dx.doi.org/10.1016/j.cellimm.2011.03.011; PMID: 21481848
- Trautmann L, Janbazian L, Chomont N, Said EA, Gimmig S, Bessette B, et al. Upregulation of PD-1 expression on HIV-specific CD8+ T cells leads to reversible immune dysfunction. Nat Med 2006; 12:1198 - 202; http://dx.doi.org/10.1038/nm1482; PMID: 16917489
- Penna A, Pilli M, Zerbini A, Orlandini A, Mezzadri S, Sacchelli L, et al. Dysfunction and functional restoration of HCV-specific CD8 responses in chronic hepatitis C virus infection. Hepatology 2007; 45:588 - 601; http://dx.doi.org/10.1002/hep.21541; PMID: 17326153
- Urbani S, Amadei B, Tola D, Pedrazzi G, Sacchelli L, Cavallo MC, et al. Restoration of HCV-specific T cell functions by PD-1/PD-L1 blockade in HCV infection: effect of viremia levels and antiviral treatment. J Hepatol 2008; 48:548 - 58; http://dx.doi.org/10.1016/j.jhep.2007.12.014; PMID: 18280607
- Kryczek I, Zhao E, Liu Y, Wang Y, Vatan L, Szeliga W, et al. Human TH17 Cells Are Long-Lived Effector Memory Cells. Sci Transl Med. 2011; 3(104):104ra100.
- Pulko V, Harris KJ, Liu X, Gibbons RM, Harrington SM, Krco CJ, et al. B7-h1 expressed by activated CD8 T cells is essential for their survival. J Immunol 2011; 187:5606 - 14; http://dx.doi.org/10.4049/jimmunol.1003976; PMID: 22025548
- Alessi DR, Andjelkovic M, Caudwell B, Cron P, Morrice N, Cohen P, et al. Mechanism of activation of protein kinase B by insulin and IGF-1. EMBO J 1996; 15:6541 - 51; PMID: 8978681
- Alessi DR, James SR, Downes CP, Holmes AB, Gaffney PR, Reese CB, et al. Characterization of a 3-phosphoinositide-dependent protein kinase which phosphorylates and activates protein kinase Balpha. Curr Biol 1997; 7:261 - 9; http://dx.doi.org/10.1016/S0960-9822(06)00122-9; PMID: 9094314
- Francisco LM, Salinas VH, Brown KE, Vanguri VK, Freeman GJ, Kuchroo VK, et al. PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. J Exp Med 2009; 206:3015 - 29; http://dx.doi.org/10.1084/jem.20090847; PMID: 20008522
- Harty JT, Badovinac VP. Shaping and reshaping CD8+ T-cell memory. Nat Rev Immunol 2008; 8:107 - 19; http://dx.doi.org/10.1038/nri2251; PMID: 18219309
- Jiang X, Clark RA, Liu L, Wagers AJ, Fuhlbrigge RC, Kupper TS. Skin infection generates non-migratory memory CD8+ T(RM) cells providing global skin immunity. Nature 2012; 483:227 - 31; http://dx.doi.org/10.1038/nature10851; PMID: 22388819
- Nanjappa SG, Heninger E, Wüthrich M, Sullivan T, Klein B. Protective antifungal memory CD8(+) T cells are maintained in the absence of CD4(+) T cell help and cognate antigen in mice. J Clin Invest 2012; 122:987 - 99; http://dx.doi.org/10.1172/JCI58762; PMID: 22354169
- Arens R, Schoenberger SP. Plasticity in programming of effector and memory CD8 T-cell formation. Immunol Rev 2010; 235:190 - 205; PMID: 20536564
- Opferman JT, Ober BT, Ashton-Rickardt PG. Linear differentiation of cytotoxic effectors into memory T lymphocytes. Science 1999; 283:1745 - 8; http://dx.doi.org/10.1126/science.283.5408.1745; PMID: 10073942
- Wherry EJ, Teichgräber V, Becker TC, Masopust D, Kaech SM, Antia R, et al. Lineage relationship and protective immunity of memory CD8 T cell subsets. Nat Immunol 2003; 4:225 - 34; http://dx.doi.org/10.1038/ni889; PMID: 12563257
- Teixeiro E, Daniels MA, Hamilton SE, Schrum AG, Bragado R, Jameson SC, et al. Different T cell receptor signals determine CD8+ memory versus effector development. Science 2009; 323:502 - 5; http://dx.doi.org/10.1126/science.1163612; PMID: 19164748
- Bannard O, Kraman M, Fearon DT. Secondary replicative function of CD8+ T cells that had developed an effector phenotype. Science 2009; 323:505 - 9; http://dx.doi.org/10.1126/science.1166831; PMID: 19164749
- Pedicord VA, Montalvo W, Leiner IM, Allison JP. Single dose of anti-CTLA-4 enhances CD8+ T-cell memory formation, function, and maintenance. Proc Natl Acad Sci U S A 2011; 108:266 - 71; http://dx.doi.org/10.1073/pnas.1016791108; PMID: 21173239
- Berger R, Rotem-Yehudar R, Slama G, Landes S, Kneller A, Leiba M, et al. Phase I safety and pharmacokinetic study of CT-011, a humanized antibody interacting with PD-1, in patients with advanced hematologic malignancies. Clin Cancer Res 2008; 14:3044 - 51; http://dx.doi.org/10.1158/1078-0432.CCR-07-4079; PMID: 18483370
- Brahmer JR, Drake CG, Wollner I, Powderly JD, Picus J, Sharfman WH, et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol 2010; 28:3167 - 75; http://dx.doi.org/10.1200/JCO.2009.26.7609; PMID: 20516446
- Topalian SL, Drake CG, Pardoll DM. Targeting the PD-1/B7-H1(PD-L1) pathway to activate anti-tumor immunity. Curr Opin Immunol 2012; 24:207 - 12; http://dx.doi.org/10.1016/j.coi.2011.12.009; PMID: 22236695
- Dong H, Zhu G, Tamada K, Flies DB, van Deursen JMA, Chen L. B7-H1 determines accumulation and deletion of intrahepatic CD8(+) T lymphocytes. Immunity 2004; 20:327 - 36; http://dx.doi.org/10.1016/S1074-7613(04)00050-0; PMID: 15030776