Abstract
Microarray-based studies by our laboratory confirm that the immune control of tumor progression extends to the “immunoprivileged” central nervous system, identifying prognostic immune gene signatures in primary tumor specimens. Our results provide rationale and mechanistic insights for the development of immunotherapeutic strategies against brain tumors.
The immune system has long been credited with controlling tumor progression, based on a variety of lines of evidence. The most compelling research has demonstrated that the number of tumor-infiltrating immune cells often correlates with improved patient outcome. This has not been so clearly demonstrated in the central nervous system (CNS), considered unique in that immune responses are more tightly controlled to avoid concurrent neurotoxicity. Studies evaluating the role of infiltrating memory T cells (CD8+CD45RO+) in cancer prognosis have recently been reviewed by Fridman et al.,Citation1 listing over 50 independent studies in 14 solid tumor types pointing to a prognostically favorable impact of infiltration. Notably, none of these tumor types were located in the central nervous system. Earlier studies measuring gross lymphocyte infiltration in glioblastoma, the most common brain tumor of adults, have been inconclusive, finding a positive correlation of lymphocyte infiltration with outcome in some studiesCitation2 but not in others.Citation3
Two recent studies by our laboratory have indirectly provided data implicating antitumor immunity in two distinct CNS tumor types—high-grade astrocytoma and ependymoma.Citation4,Citation5 Microarray gene expression analysis of primary surgical samples was utilized as an unbiased approach to identify novel prognostic factors. In ependymoma, a common childhood brain tumor, approximately 50% of patients suffer from tumor recurrence. We identified genes that were significantly higher in tumors that did not subsequently recur, and in those that did recur that were correlated with a longer time to recurrence. Unexpectedly, immune function was found to be the predominant role of genes associated with good prognosis.Citation4 We hypothesized that these prognostic immune function genes were restricted to tumor-infiltrating immune cells, and of those tested—human leukocyte antigen DR (HLA-DR) and allograft-inhibitory factor 1 (AIF1; Iba1)—both were shown by histology to be restricted to the tumor-infiltrating microglia.
These results were recapitulated in a follow-up microarray gene expression analysis of high-grade astrocytoma, a disease most commonly seen in adults but also presenting in children, which conveys a very poor prognosis (median survival 1.5 y). A small proportion (< 10%) of patients survive longer than 5 y, and this rare population was the focus of a similar unbiased study of genes associated with favorable clinical outcome. Again, immune function was the predominant role of genes correlated with long-term survival in high-grade astrocytoma in two independent microarray data sets.Citation5 Infiltration of cytotoxic T cells, helper T cells and microglia was measured by immunohistochemistry and high infiltration was demonstrated to be predictive of longer survival. Total immune cell infiltration was an independent predictor of longer survival even when clinical performance score, traditionally the strongest prognostic factor for high-grade astrocytoma, was included in the analysis ().
Figure 1. Kaplan-Meier survival analysis demonstrating total immune cell infiltration in high-grade astrocytoma as an independent prognostic variable when combined with clinical performance score. High immune cell infiltration was defined as greater than median cytotoxic T cell, helper T cell or > 75th percentile microglia. Karnofsky/Lansky clinical performance score, was used to measure patient well-being. In this case, good performance was defined as greater than median. Cox proportional hazard multivariate analysis demonstrated that both high immune and good performance significantly contributed to longer survival (p values = 0.0077 and 0.0027, respectively).
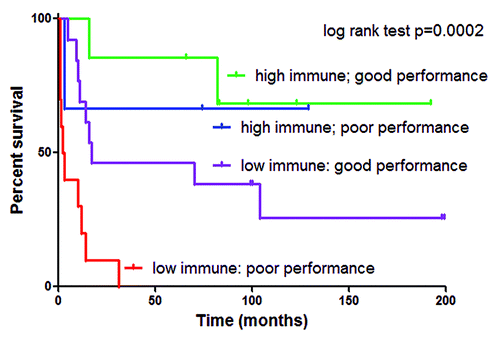
Together, these studies show that on a gross level, an intense immune cell infiltrate is associated with better prognosis in both the brain tumor types that we studied. From this we infer that the host immune system is actively controlling tumor growth in both of these CNS tumor types. Several recent studies have reported that myeloid-derived suppressor cells contribute to tumor growth in the CNS.Citation6,Citation7 Our data provide a cautionary counterpoint, suggesting that therapies that grossly target the myeloid population may eliminate microglia, which is potentially critical for complete tumor eradication.
Although microarray based gene expression analysis is a relatively indirect method of measuring immune cell infiltration it has a number of positive elements. The most significant merit lies in the unbiased screening of the entire transcriptome that is inherent in microarray analyses. This approach provides a relative measure of the contribution of multiple factors to prognosis, immune-related factors attaining a high rank in the case of our two studies. A second attribute is that this approach is not biased by the analysis of only those immune cell phenotypes traditionally measured in infiltration studies. Extrapolation of infiltrating immune cell characteristics defined outside of the CNS may be misleading. For example, regulatory T cells (Tregs) have been ascribed with an immunosuppressive role, contributing to a dismal prognosis in a variety of non-CNS tumors. However, two studies of Tregs in glioblastoma outcome failed to find an association with prognosis.Citation8,Citation9 Unbiased microarray analysis has the power to identify novel immune-related genes that are strongly associated with improved clinical outcome, thus implying a critical role in antitumor immune mechanisms. A small number of immune-related genes were identified as prognostic in all three microarray data sets utilized in our studies, namely coronin, actin binding protein, 1A (CORO1A), dedicator of cytokinesis 2 (DOCK2) and selectin P ligand (SELPLG). Even though each of these genes had previously been attributed with critical roles in immune function, specifically as they regulate cell migration, none of them had been implicated in antitumor immunity.
The identification of putative CNS antitumor immunity has prominent clinical implications, providing a rationale for development of immunotherapy against brain tumors. A detailed characterization of immune effector cells and mechanisms, as implicated by our studies, may help refine existing therapeutic strategies. In general, prognostic immune-related genes in high-grade astrocytoma were dissimilar to those seen in ependymoma, suggesting that different brain tumors may engender different types of immune responses and therefore may require different immunotherapeutic strategies. In both high-grade astrocytoma and ependymoma, mitosis-related genes were strongly associated with a worse prognosis, demonstrating an inverse relationship with prognostic immune-related genes. The cancer immunoediting hypothesis suggests the existence of an equilibrium between rapidly dividing tumor cells and antitumor immunity that, when tipped, can lead to either tumor growth / recurrence or tumor elimination. This provides a plausible biological explanation for the inverse correlation observed in our studies.Citation10 Ideal therapeutic combinations would therefore preserve or promote antitumor immune activity while selectively inhibiting tumor cell proliferation.
References
- Fridman WH, Pagès F, Sautès-Fridman C, Galon J. The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer 2012; 12:298 - 306; http://dx.doi.org/10.1038/nrc3245; PMID: 22419253
- Brooks WH, Markesbery WR, Gupta GD, Roszman TL. Relationship of lymphocyte invasion and survival of brain tumor patients. Ann Neurol 1978; 4:219 - 24; http://dx.doi.org/10.1002/ana.410040305; PMID: 718133
- Rossi ML, Jones NR, Candy E, Nicoll JA, Compton JS, Hughes JT, et al. The mononuclear cell infiltrate compared with survival in high-grade astrocytomas. Acta Neuropathol 1989; 78:189 - 93; http://dx.doi.org/10.1007/BF00688208; PMID: 2750489
- Donson AM, Birks DK, Barton VN, Wei Q, Kleinschmidt-Demasters BK, Handler MH, et al. Immune gene and cell enrichment is associated with a good prognosis in ependymoma. J Immunol 2009; 183:7428 - 40; http://dx.doi.org/10.4049/jimmunol.0902811; PMID: 19917695
- Donson AM, Birks DK, Schittone SA, Kleinschmidt-Demasters BK, Sun DY, Hemenway MF, et al. Increased immune gene expression and immune cell infiltration in high-grade astrocytoma distinguish long-term from short-term survivors. J Immunol 2012; 189:1920 - 7; http://dx.doi.org/10.4049/jimmunol.1103373; PMID: 22802421
- Fujita M, Kohanbash G, Fellows-Mayle W, Hamilton RL, Komohara Y, Decker SA, et al. COX-2 blockade suppresses gliomagenesis by inhibiting myeloid-derived suppressor cells. Cancer Res 2011; 71:2664 - 74; http://dx.doi.org/10.1158/0008-5472.CAN-10-3055; PMID: 21324923
- Raychaudhuri B, Rayman P, Ireland J, Ko J, Rini B, Borden EC, et al. Myeloid-derived suppressor cell accumulation and function in patients with newly diagnosed glioblastoma. Neuro Oncol 2011; 13:591 - 9; http://dx.doi.org/10.1093/neuonc/nor042; PMID: 21636707
- Heimberger AB, Abou-Ghazal M, Reina-Ortiz C, Yang DS, Sun W, Qiao W, et al. Incidence and prognostic impact of FoxP3+ regulatory T cells in human gliomas. Clin Cancer Res 2008; 14:5166 - 72; http://dx.doi.org/10.1158/1078-0432.CCR-08-0320; PMID: 18698034
- Jacobs JF, Idema AJ, Bol KF, Grotenhuis JA, de Vries IJ, Wesseling P, et al. Prognostic significance and mechanism of Treg infiltration in human brain tumors. J Neuroimmunol 2010; 225:195 - 9; http://dx.doi.org/10.1016/j.jneuroim.2010.05.020; PMID: 20537408
- Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science 2011; 331:1565 - 70; http://dx.doi.org/10.1126/science.1203486; PMID: 21436444