Abstract
Tumor-derived transforming growth factor β1 (TGFβ1) generally abrogates the immunogenicity of dendritic cells (DCs) fused to whole cancer cells. We have recently revealed that ethanol-treated neoplastic cells fused to DCs exposed to 2 Toll-like receptor agonists efficiently induce cytotoxic T lymphocytes via TGFβ1 blockade and the production of interleukin-12.
Several strategies have been developed to deliver tumor-associated antigens (TAAs) to dendritic cells (DCs) for the elicitation of efficient antitumor immune responses. One of these strategies consists in the fusion of DCs with whole cancer cells, resulting in the processing of a broad array of TAAs followed by their presentation in complex with MHC Class I and II molecules and in the context of co-stimulatory signals.Citation1,Citation2 DC/cancer cell fusion-based vaccines have successfully been employed to achieve tumor regression in preclinical models, but have not yet demonstrated their potential in clinical trials.Citation3 Thus, improving the therapeutic efficacy of cell fusion-based anticancer vaccine may require an increased immunogenicity not only of DCs but also of malignant cells.
Immunosuppressive molecules such as interleukin-10 (IL-10), vascular endothelial growth factor (VEGF), and transforming growth factor β1 (TGFβ1) in the tumor microenvironment can inhibit the activation of DC/cancer cell fusions, resulting in the accumulation of regulatory T cells (Tregs).Citation4 In particular, TGFβ1 plays a critical immunosuppressive function as it reduces the number and function of circulating DCs, promotes the generation of Tregs, and inhibits cytotoxic T lymphocytes (CTLs).Citation4 Interestingly, DC/cancer cell fusions simultaneously exposed to Toll-like receptor 2 (TLR2) and TLR4 agonists, but not fusions receiving either agonist alone, have been shown to overcome the immunosuppressive activity of TGF-β1.Citation4 However, fusions that were generated with cancer cells producing high levels of TGFβ1 exhibited a reduced immunogenicity in vitro.Citation4
Therefore, blocking TGFβ1 signaling in DC/cancer cell fusions appears as a meaningful approach to improve their immunogenicity, and hence their therapeutic efficacy as anticancer vaccines. Up to now, the TGFβ1 system has been targeted with neutralizing antibodies,Citation5 small molecular inhibitors,Citation6 specific small interfering RNAs (siRNAs),Citation7 or by engineering tumor cells to express a soluble TGF-β receptor.Citation8
We have recently reported that the production of active TGFβ1, IL-10 and VEGR from malignant cells is significantly inhibited by the administration of pharmaceutical grade ethanol, a process that is not paralleled by the downregulation of MHC class I molecules or tumor-associated antigens such as mucin 1 (MUC1).Citation9 Importantly, the immunogenicity of DC/cancer cell fusions was synergistically increased if these were formed by ethanol-treated neoplastic cells and DCs exposed to TLR2 and TLR2 agonists, resulting in the blockade of TGFβ1 signaling as well as in interleukin-12 (IL-12) productionCitation9 ().
Figure 1. Fusions generated with ethanol-treated tumor cells and activated dendritic cells. The treatment of neoplastic cells with ethanol results in the emission/exposure of calreticulin (CRT), heat-shock proteins (HSPs) and high-mobility group box 1 (HMGB1) as well as in the blockade of transforming growth factor β1 (TGFβ1) signaling, but not in decreased expression of MHC class I molecules and tumor-associated antigens such as mucin 1 (MUC1). Ethanol-treated malignant cells fused to dendritic cells (DCs) activated by simultaneous exposure to Toll-like receptor 2 (TLR2) and TLR4 agonists inhibit the production of multiple immunosuppressive factors including TGFβ1 while stimulating the secretion of interleukin-12 and HSPs. Such immunogenic DC/cancer cell fusions activate T cells that produce high levels of interferon γ (IFNγ), resulting in the elicitation of MUC1-specific immune responses, at least in vitro.
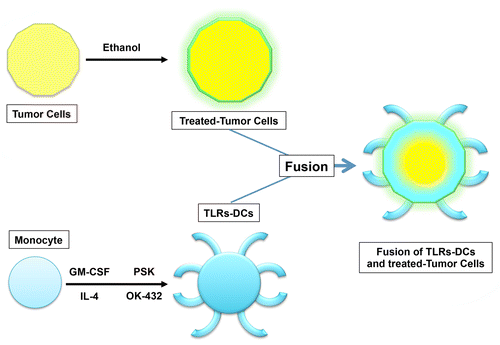
The strategy of using ethanol-treated neoplastic cells for forming DC/cancer cell fusion-based vaccines may have some biological benefits, including 1) an improved sterility and safety; 2) an increased immunogenicity, presumably ensuing the denaturation, modification or aggregation of TAAs by ethanol and hence the exposure of previously concealed, hydrophobic epitopes; 3) a higher immunostimulatory potential, originating from the ability of ethanol to favor the emission of danger signals such as those conveyed by heat-shock proteins (HSPs) and calreticulin (CRT), both serving as “eat-me” signals that allow TAAs to traffic to the antigen-presenting compartment of DC/tumor fusions, as well as by high-mobility group box 1 (HMGB1), which is released from ethanol-treated neoplastic cells to activate DC/cancer cell fusions. This said, it remains unclear which specific agents or cytotoxic chemotherapeutics are best suited to kill malignant cells for the generation of highly immunogenic DC/cancer cell fusions. Understanding how to improve the immunogenicity of both DCs and neoplastic cells may provide a platform for increasing the therapeutic potential of DC/cancer cell fusion-based vaccines. Furthermore, specific chemotherapeutics, irradiation, and immunomodulatory monoclonal antibodies, such inhibitors of the immunological checkpoints orchestrated by programmed cell death protein 1 (PD1) and its ligand (PD-L1),Citation10 may turn out to constitute an efficient means for DC/cancer cell fusion-based vaccines to stimulate therapeutically relevant antineoplastic immune responses.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Gong J, Chen D, Kashiwaba M, Kufe D. Induction of antitumor activity by immunization with fusions of dendritic and carcinoma cells. Nat Med 1997; 3:558 - 61; http://dx.doi.org/10.1038/nm0597-558; PMID: 9142127
- Koido S, Homma S, Okamoto M, Namiki Y, Takakura K, Uchiyama K, et al. Fusions between dendritic cells and whole tumor cells as anticancer vaccines. Oncoimmunology 2013; 2:e24437; http://dx.doi.org/10.4161/onci.24437; PMID: 23762810
- Koido S, Hara E, Homma S, Namiki Y, Ohkusa T, Gong J, et al. Cancer vaccine by fusions of dendritic and cancer cells. Clin Dev Immunol 2009; 2009:657369; http://dx.doi.org/10.1155/2009/657369; PMID: 20182533
- Koido S, Homma S, Okamoto M, Namiki Y, Takakura K, Takahara A, et al. Combined TLR2/4-activated dendritic/tumor cell fusions induce augmented cytotoxic T lymphocytes. PLoS One 2013; 8:e59280; http://dx.doi.org/10.1371/journal.pone.0059280; PMID: 23555011
- Terabe M, Ambrosino E, Takaku S, O’Konek JJ, Venzon D, Lonning S, et al. Synergistic enhancement of CD8+ T cell-mediated tumor vaccine efficacy by an anti-transforming growth factor-beta monoclonal antibody. Clin Cancer Res 2009; 15:6560 - 9; http://dx.doi.org/10.1158/1078-0432.CCR-09-1066; PMID: 19861451
- Ueda R, Fujita M, Zhu X, Sasaki K, Kastenhuber ER, Kohanbash G, et al. Systemic inhibition of transforming growth factor-beta in glioma-bearing mice improves the therapeutic efficacy of glioma-associated antigen peptide vaccines. Clin Cancer Res 2009; 15:6551 - 9; http://dx.doi.org/10.1158/1078-0432.CCR-09-1067; PMID: 19861464
- Conroy H, Galvin KC, Higgins SC, Mills KHG. Gene silencing of TGF-β1 enhances antitumor immunity induced with a dendritic cell vaccine by reducing tumor-associated regulatory T cells. Cancer Immunol Immunother 2012; 61:425 - 31; http://dx.doi.org/10.1007/s00262-011-1188-y; PMID: 22193988
- Zhang M, Berndt BE, Chen JJ, Kao JY. Expression of a soluble TGF-beta receptor by tumor cells enhances dendritic cell/tumor fusion vaccine efficacy. J Immunol 2008; 181:3690 - 7; PMID: 18714045
- Koido S, Homma S, Okamoto M, Namiki Y, Takakura K, Takahara A, et al. Augmentation of Antitumor Immunity by Fusions of Ethanol-Treated Tumor Cells and Dendritic Cells Stimulated via Dual TLRs through TGF-β1 Blockade and IL-12p70 Production. PLoS One 2013; 8:e63498; http://dx.doi.org/10.1371/journal.pone.0063498; PMID: 23717436
- Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 2012; 12:252 - 64; http://dx.doi.org/10.1038/nrc3239; PMID: 22437870