Abstract
Inflammation, which is now recognized as an hallmark of cancer, is intimately linked to the reactivity of stromal fibroblasts. Accumulating evidence indicate that cancer-associated fibroblasts not only drive the epithelial-mesenchymal transition and metabolically sustain the growth of cancer cells, but also engage in a reciprocal relationship with M2 macrophages that dramatically boost malignancy.
Tumors are complex tissues in which different cellular compartments beyond the malignant one coexist, including fibroblasts, endothelial cells, and multiple immune cells such as tumor-infiltrating macrophages. These accessory cells engage with cancer cells in a complex network of interactions that change and evolve alongside malignant progression. Besides influencing their malignant counterparts, stromal cells are also able to modulate the behavior of each other, ultimately promoting tumor progression.Citation1
Cancer-associated fibroblasts (CAFs) play an intriguing role in such context, as they are able to 1) deeply affect the behavior of cancer cells, by exerting trophic functions and favoring motility;Citation2,Citation3 2) shape the reactivity of other stromal cells, by promoting their recruitment and modulating their physical and functional interaction within neoplastic lesions;Citation4,Citation5 3) modify structural features of the tumor microenvironment, by promoting local acidification and secreting proteins that remodel the extracellular matrix;Citation2,Citation6 and 4) escort cancer cells in the bloodstream, granting their survival throughout the migration to metastatic sites.Citation7 Indeed, CAFs have been reported to promote the epithelial-mesenchymal transition (EMT) by activating an epigenetic transcriptional program that stimulates cancer cells to disengage from adhesive cell-to-cell interactions and achieve a mesenchymal motility, culminating in their escape from primary neoplastic lesions and metastasis.Citation1,Citation3 Such a transcriptional program is characterized by gene signatures that are associated with resistance to anoikis, granting for the survival of cancer cells that lack proper adhesion to the extracellular matrix while they circulate in the bloodstream/lymph and colonize new organs.Citation1,Citation4 Fascinatingly, the molecular pathways that promote the reactivity of CAFs and the EMT in cancer cells are very similar, as they engage redox signaling circuitries involving cyclooxygenase 2 (COX2), hypoxia-inducible factor 1 (HIF1), and NF-κB.Citation8,Citation9 In addition, CAFs not only stimulate cancer cells to express stem cell markers like CD133 or CD44, enhancing their anchorage-independent growth potential and tumor-repopulating ability,Citation3 but also influence their metabolic profile. Indeed, the interaction between CAFs and cancer cells shift the latter toward a respiratory metabolism, a process known as reverse Warburg effect. In this context, malignant cells utilize the lactate produced by CAFs to produce ATP. Thus, CAFs play a major trophic role for cancer cells, maintaining them in an optimal bioenergetic status that sustains anabolic metabolism and hence tumor growth.Citation2
Inflammation is nowadays acknowledged as an hallmark of cancer, and several tumor-associated cells appear to operate in this sense to favor tumor progression, including CAFs and macrophages. Recent immunological studies have identified 2 divergent functional states of macrophages: “classically” activated (M1) and “alternatively” activated (M2) macrophages. M1 macrophages produce high amounts of inflammatory cytokines and reactive oxygen species, thus orchestrating an immune response that exert antineoplastic effects. Conversely, M2 macrophages mainly promote tissue repair and angiogenesis, thus favor malignant progression.Citation10 At least in some setting, including aggressive prostate carcinomas, CAFs actively promote the recruitment of monocytes to the tumor site and their differentiation toward M2 macrophages, mostly as they secrete stromal-derived factor 1 (SDF1).Citation5 Noteworthy, the interaction between M2 macrophages and CAFs is reciprocal, as the former are able to affect the reactivity of the latter and promote their transition toward myofibroblasts.
CAFs and M2 macrophages also interact to affect the functional profile of other stromal cells. Indeed, CAFs and M2 macrophages collaborate in activation of endothelial cells to drive de novo angiogenesis, fostering the escape of cancer cells from the primary tumor and ultimately facilitating the metastatic spread.Citation5 The 2 cell populations also cooperate to recruit bone marrow-derived endothelial cell precursors and hence favor local neoangiogenesis. Once endothelial cell precursors have been recruited, they are in turn able to influence the behavior of cancer cells undergoing the EMT, in particular as they favor transendothelial migration and intravasation through an auxiliary motility shift toward amoeboid movements.Citation4 This additional differentiation, called mesenchymal-amoeboid transition, provides an extra advantage to metastatic cells in terms of invasiveness.
Remarkably, cancer cells are able to complete their diabolic escalation by affecting the reactivity of CAFs as well as by promoting the M2 polarization, mostly through the secretion of pro-inflammatory factors such as interleukin 6 (IL-6) and monocyte chemotactic protein 1 (monocyte chemoattractant protein 1)Citation5 (). In support of the translational relevance of these observations, the analysis of prostate carcinoma patients at different stages of the disease revealed a raise in the reactivity of stromal fibroblasts as well as in the M2/M1 macrophage ratio that clearly correlated with clinicopathological parameters, including overall survival and biochemical recurrence-free survival.Citation5
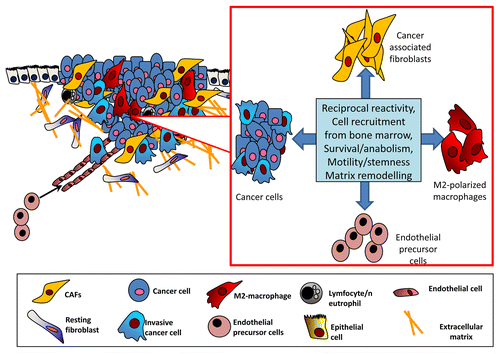
Figure 1. Cross-talk among different components of the tumor stroma. Cancer cells engage in a complex and reciprocal relationship with cancer-associated fibroblasts (CAFs), M2 macrophages, and bone marrow-derived endothelial cell precursors. CAFs not only stimulate malignant cells to undergo the epithelial-mesenchymal transition and to acquire other stem cell-like traits, but also recruit endothelial cell precursors and monocytes to the tumor site, thus stimulating angiogenesis as well as the polarization of monocytes toward the M2 phenotype. In turn, M2 macrophages enhance the reactivity of CAFs, thereby affecting the whole stromal context in which cancer cells progress toward malignancy.
As metabolic adaptation is a key component of macrophage plasticity and M1/M2 polarization,Citation10 it is likely that tumor-infiltrating M2 macrophages resemble CAFs in their ability to affect the bioenergetic metabolism of cancer cells. Nothing more than an attractive hypothesis at the moment, this idea is supported by the ability of macrophage by-products to impact the metabolism of other cells, as previously demonstrated in the setting of obesity-associated pathologies.
Thus, a complex and intimately interrelated ensemble of stromal cells appears to actively participate in tumor progression as it allow malignant cells to expand locally in spite of adverse microenvironmental conditions, invade surrounding tissues, trespass endothelial barriers, survive in the bloodstream, and generate metastases at distant sites, thanks to acquisition of stem cell-like properties. The reaction of the scientific community to this diabolic task-force, synergistically promoting malignancy, should be to design original strategies that simultaneously target inflammatory cells and stromal fibroblasts, hence exerting superior therapeutic effect. In particular, strategies to reeducate M2 macrophages and hence reestablish their antitumor activity, which are currently undergoing preclinical evaluation, could benefit from the concurrent administration of anti-inflammatory molecules, aimed at limiting the reactivity of CAFs as well as at preventing the EMT in cancer cells.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Cirri P, Chiarugi P. Cancer-associated-fibroblasts and tumour cells: a diabolic liaison driving cancer progression. Cancer Metastasis Rev 2012; 31:195 - 208; http://dx.doi.org/10.1007/s10555-011-9340-x; PMID: 22101652
- Fiaschi T, Marini A, Giannoni E, Taddei ML, Gandellini P, De Donatis A, et al. Reciprocal metabolic reprogramming through lactate shuttle coordinately influences tumor-stroma interplay. Cancer Res 2012; 72:5130 - 40; http://dx.doi.org/10.1158/0008-5472.CAN-12-1949; PMID: 22850421
- Giannoni E, Bianchini F, Masieri L, Serni S, Torre E, Calorini L, et al. Reciprocal activation of prostate cancer cells and cancer-associated fibroblasts stimulates epithelial-mesenchymal transition and cancer stemness. Cancer Res 2010; 70:6945 - 56; http://dx.doi.org/10.1158/0008-5472.CAN-10-0785; PMID: 20699369
- Giannoni E, Taddei ML, Parri M, Bianchini F, Santosuosso M, Grifantini R, et al. EphA2-mediated mesenchymal-amoeboid transition induced by endothelial progenitor cells enhances metastatic spread due to cancer-associated fibroblasts. J Mol Med (Berl) 2013; 91:103 - 15; http://dx.doi.org/10.1007/s00109-012-0941-9; PMID: 22903544
- Comito G, Giannoni E, Segura CP, Barcellos-de-Souza P, Raspollini MR, Baroni G, et al. Cancer-associated fibroblasts and M2-polarized macrophages synergize during prostate carcinoma progression. Oncogene 2013; In press http://dx.doi.org/10.1038/onc.2013.191; PMID: 23728338
- Fiaschi T, Giannoni E, Taddei ML, Cirri P, Marini A, Pintus G, et al. Carbonic anhydrase IX from cancer-associated fibroblasts drives epithelial-mesenchymal transition in prostate carcinoma cells. Cell Cycle 2013; 12:1791 - 801; http://dx.doi.org/10.4161/cc.24902; PMID: 23656776
- Duda DG, Duyverman AM, Kohno M, Snuderl M, Steller EJ, Fukumura D, et al. Malignant cells facilitate lung metastasis by bringing their own soil. Proc Natl Acad Sci U S A 2010; 107:21677 - 82; http://dx.doi.org/10.1073/pnas.1016234107; PMID: 21098274
- Toullec A, Gerald D, Despouy G, Bourachot B, Cardon M, Lefort S, et al. Oxidative stress promotes myofibroblast differentiation and tumour spreading. EMBO Mol Med 2010; 2:211 - 30; http://dx.doi.org/10.1002/emmm.201000073; PMID: 20535745
- Giannoni E, Bianchini F, Calorini L, Chiarugi P. Cancer associated fibroblasts exploit reactive oxygen species through a proinflammatory signature leading to epithelial mesenchymal transition and stemness. Antioxid Redox Signal 2011; 14:2361 - 71; http://dx.doi.org/10.1089/ars.2010.3727; PMID: 21235356
- Sica A, Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J Clin Invest 2012; 122:787 - 95; http://dx.doi.org/10.1172/JCI59643; PMID: 22378047