Abstract
The MSP/RON signaling pathway favors the conversion of micrometastatic lesions to overt metastases by suppressing antitumor immune responses. The loss of RON functions in the host potentiates tumor-specific CD8+ T-cell responses, hence inhibiting the outgrowth of metastatic cancer cells. Thus, RON inhibitors may potentially prevent the outgrowth of micrometastases in cancer patients.
The metastatic spread of primary lesions to distant organs is the main cause of death from cancer.Citation1 Although the conversion of dormant micrometastatic cancer cells into overt metastases is a critical and therapeutically relevant point in disease progression, the molecular and cellular mechanisms that underpin this conversion remain unclear. Immunosurveillance appears to play a key role in this process.Citation2 Thus, T lymphocytes appear to prevent the escape of micrometastatic cancer cells from dormancy in many tumor models. However, how micrometastatic cells suppress the immune system to enable metastatic outgrowth is largely unknown. Therefore, identifying and targeting pathways that are employed by neoplastic cells to subvert antitumor immune responses during metastatic outgrowth is particularly important, as the prevention of early metastatic dissemination may not be clinically feasible.
Macrophage stimulating 1 (hepatocyte growth factor-like) (MS1), also known as macrophage-stimulating protein (MSP), is a glycoprotein released from the liver into the blood as an inactive precursor, and is activated locally on macrophages upon cleavage by the macrophage protease suppression of tumorigenicity 14 (colon carcinoma) (ST14, best known as matriptase).Citation3 Upon cleavage, activated MSP can bind to its receptor, macrophage stimulating 1 receptor (c-met-related tyrosine kinase) (MST1R, also known as RON), which is expressed by epithelial cells, osteoclasts, and macrophages. The MSP/RON signaling axis generally promotes cell proliferation, survival, and chemotaxis. Although mice lacking Msp or Ron exhibit no obvious developmental defects, they are susceptible to toxic shock upon infection or injury due to the inability of their macrophages to downregulate pro-inflammatory cytokines while upregulating anti-inflammatory ones.Citation4,Citation5 Thus, the MSP/RON signaling axis is critical for the regulation of immune responses to external insults.
RON has also been reported to play a role in a wide range of human cancers.Citation3 ST14 and RON are overexpressed in around 45% and 50% of human breast carcinomas, respectively.Citation6 Moreover, the co-overexpression of MSP, ST14, and RON constitutes an independent prognostic factor for metastasis and death among breast carcinoma patients. We have previously reported that the overexpression of MSP in MMTV-PyMT mice (which spontaneously develop breast carcinoma upon the mammary gland-specific expression of the polyomavirus middle T antigen, PyMT) promotes the metastatic spread of neoplastic lesions to a wide range of organs, including lungs, lymphatic vessels, bones, and the spleen.Citation6 However, it was unclear whether the MSP/RON signaling axis would contribute to metastasis through cell-intrinsic mechanisms or by modulating inflammation.
To dissect the cell-intrinsic vs. cell-extrinsic role of MSP/RON in metastasis, we performed tissue complementation experiments in the MMTV-PyMT mouse model.Citation7 In particular, we overexpressed MSP in RON-expressing MMTV-PyMT cancer cells, and transplanted them orthotopically into wild-type (WT) or Ron−/− hosts. Although tumor growth was similar in WT and Ron−/− animals, the loss of host RON abrogated pulmonary metastasis. We determined that the absence of metastasis was actually due to the inability of micrometastatic cancer cells to generate macrometastases. In turn, such a defect in metastatic outgrowth exhibited by Ron−/− mice was due to enhanced antitumor CD8+ T-cell responses. CD8+ T cells from Ron−/− hosts secreted higher levels of tumor necrosis factor α (TNFα) and exerted more robust cytolytic activity in vitro than their WT counterparts. Importantly, adoptively transferred Ron−/− CD8+ T cells efficiently blocked the metastatic outgrowth of micrometastatic cancer cells, while WT CD8+ T cells did not. Conversely, both the genetic and the pharmacological ablation of CD8+ T cells was sufficient to restore the capacity of breast carcinoma cells to form macrometastases in Ron−/− mice. The inhibition of RON with BMS-777607 (also known as ASLAN002), a selective inhibitor of its tyrosine kinase activity, reduced metastatic outgrowth, both in prophylactic and adjuvant settings. Significantly, the antimetastatic activity of BMS-777607 relied on CD8+ T cells, as this molecule completely lost its efficacy in the context of CD8+ T-cell depletion. Altogether, our findings reveal a novel pathway that malignant cells harness to suppress immune responses during metastatic outgrowthCitation7 ().
Figure 1. RON signaling suppresses antitumor CD8+ T-cell responses. (A) The binding of cancer cell-derived macrophage-stimulating protein (MSP) to RON stimulated myeloid cells to produce decreased levels of interleukin (IL)-12, interferon γ (IFNγ) and tumor necrosis factor α (TNFα) as well as increased amounts of IL-10. This cytokine profile suppresses antitumor CD8+ T cell responses and enables micrometastatic cancer cells to generate macrometastases. (B) The loss of RON signaling in the host, be it caused by genetic or pharmacological interventions, switches cytokine secretion by myeloid cells to a profile characterized by high levels of IL-12, IFNγ, and TNFα as well as by reduced amount of IL-10. This relieves immunosuppression, potentiating an antitumor CD8+ T-cell response that kills micrometastatic tumor cells.
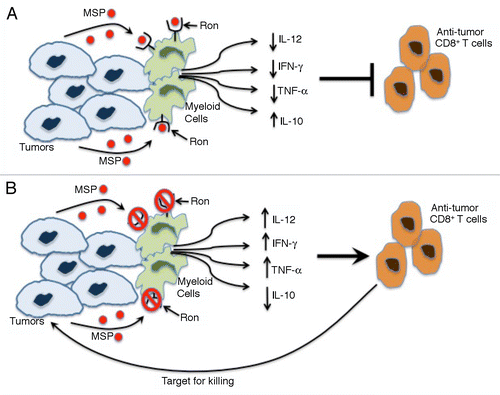
Immunosubversion is a critical step in tumor progression.Citation8 The microenvironment of primary neoplastic lesions is particularly immunosuppressive, featuring increased levels of cytokines and mediators that inhibit CD8+ T-cell responses, such as arginase and interleukin (IL)-10.Citation8 However, tumor cells that have invaded a new microenvironment may be more vulnerable to immunosurveillance. Based on our recent findings, we propose that some cancer cells may upregulate MSP as an additional mechanism to evade antitumor immune responses. Moreover, our data demonstrate that RON is important for immunosuppression even when tumors do not overexpress MSP, perhaps reflecting the activation of circulating MSP by macrophage- and/or tumor-derived serine proteases.
The detailed molecular mechanisms whereby the MSP/RON signaling axis suppresses antitumor immunity are still unknown. Based on previously findingsCitation9 and our recent results, RON appears to favor the polarization of macrophages toward the immunosuppressive M2 phenotype. Alternatively, the pharmacological or genetic inhibition of RON may result in the widespread establishment of a pro-inflammatory cytokine milieu that contains high levels of IL-12 and TNFα and hence boosts CD8+ T-cell responses. Additional genetic models of impaired RON signaling are required to dissect the precise molecular mechanisms that underlie our observations.
Although RON inhibitors are currently explored as targeted cytotoxic agents,Citation10 our findings suggest that these chemicals may also exert therapeutically relevant immunostimulatory effects. Thus, the clinical success of RON inhibitors may require a careful design of clinical trials, the selection of specific patient cohort and the development/exploitation of immunological biomarkers. In conclusion, we propose that the inhibition of the MSP/RON signaling axis may be an exciting addition to the growing armamentarium of cancer immunotherapies.
| Abbreviations: | ||
| IFN | = | interferon |
| IL | = | interleukin |
| MSP | = | macrophage-stimulating protein |
| PyMT | = | polyomavirus middle T antigen |
| SCID | = | Severe combined immunodeficiency |
| TK | = | tyrosine kinase |
| TNFα | = | tumor necrosis factor α |
| WT | = | wild type |
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011; 144:646 - 74; http://dx.doi.org/10.1016/j.cell.2011.02.013; PMID: 21376230
- Vesely MD, Kershaw MH, Schreiber RD, Smyth MJ. Natural innate and adaptive immunity to cancer. Annu Rev Immunol 2011; 29:235 - 71; http://dx.doi.org/10.1146/annurev-immunol-031210-101324; PMID: 21219185
- Kretschmann KL, Eyob H, Buys SS, Welm AL. The macrophage stimulating protein/Ron pathway as a potential therapeutic target to impede multiple mechanisms involved in breast cancer progression. Curr Drug Targets 2010; 11:1157 - 68; http://dx.doi.org/10.2174/138945010792006825; PMID: 20545605
- Waltz SE, Eaton L, Toney-Earley K, Hess KA, Peace BE, Ihlendorf JR, et al. Ron-mediated cytoplasmic signaling is dispensable for viability but is required to limit inflammatory responses. J Clin Invest 2001; 108:567 - 76; PMID: 11518730
- Morrison AC, Wilson CB, Ray M, Correll PH. Macrophage-stimulating protein, the ligand for the stem cell-derived tyrosine kinase/RON receptor tyrosine kinase, inhibits IL-12 production by primary peritoneal macrophages stimulated with IFN-gamma and lipopolysaccharide. J Immunol 2004; 172:1825 - 32; PMID: 14734766
- Welm AL, Sneddon JB, Taylor C, Nuyten DSA, van de Vijver MJ, Hasegawa BH, et al. The macrophage-stimulating protein pathway promotes metastasis in a mouse model for breast cancer and predicts poor prognosis in humans. Proc Natl Acad Sci U S A 2007; 104:7570 - 5; http://dx.doi.org/10.1073/pnas.0702095104; PMID: 17456594
- Eyob H, Ekiz HA, Derose YS, Waltz SE, Williams MA, Welm AL. Inhibition of Ron kinase blocks conversion of micrometastases to overt metastases by boosting antitumor immunity. Cancer Discov 2013; http://dx.doi.org/10.1158/2159-8290.CD-12-0480; PMID: 23612011
- Zitvogel L, Tesniere A, Kroemer G. Cancer despite immunosurveillance: immunoselection and immunosubversion. Nat Rev Immunol 2006; 6:715 - 27; http://dx.doi.org/10.1038/nri1936; PMID: 16977338
- Morrison AC, Correll PH. Activation of the stem cell-derived tyrosine kinase/RON receptor tyrosine kinase by macrophage-stimulating protein results in the induction of arginase activity in murine peritoneal macrophages. J Immunol 2002; 168:853 - 60; PMID: 11777982
- Wang M-H, Padhye SS, Guin S, Ma Q, Zhou YQ. Potential therapeutics specific to c-MET/RON receptor tyrosine kinases for molecular targeting in cancer therapy. Acta Pharmacol Sin 2010; 31:1181 - 8; http://dx.doi.org/10.1038/aps.2010.106; PMID: 20694025