Abstract
In addition to cytotoxic effects, anticancer agents can exert multiple immunomodulatory functions. We have recently described the molecular mechanisms whereby bleomycin can 1) promote endoplasmic reticulum stress, causing the immunogenic death of cancer cells and hence strengthening antitumor CD8+ T cell responses; and 2) induce the secretion of transforming growth factor β (TGFβ), which stimulates regulatory T cells. This suggests that bleomycin may be favorably combined with TGFβ-targeting strategies.
There is now evidence that anticancer chemotherapy can promote various multiple immunomodulatory effects. Multiple reports have indeed underlined how some drugs can stimulate tumor-specific cytotoxic T lymphocytes, either by inhibiting immunosuppressive cells such as regulatory T cells (Tregs),Citation1 either by inducing cancer cell death in an immunogenic fashionCitation2–Citation4 or by facilitating the recognition of neoplastic cells by the immune system.Citation5. However, in some cases, anticancer chemotherapy can have opposite effects on the immune system. For instance, 5-fluorouracil can kill tumor-supporting myeloid-derived suppressor cells (MDSCs), hence exerting antineoplastic effects, while promoting the accumulation of TH17 CD4+ T cells, which generally favors tumor relapse.Citation6,Citation7
Different anticancer agents, including radiotherapy, anthracyclines and oxaliplatin, have been reported to induce immunogenic cell death (ICD), a particular type of cellular demise characterized by specific pathognomonic hallmarks, i.e., the establishment of endoplasmic reticulum (ER) stress, the exposure of ER proteins such as calreticulin and ERp57 on the cell surface, the activation of autophagy and the secretion of ATP. Citation2–Citation4,Citation8
We recently decided to study the immunomodulatory potential of bleomycin (BLM).Citation9 BLM is indeed known to stimulate the production of reactive oxygen species (ROS), which may facilitate ICD via the activation of the ER stress response. Moreover, BLM causes DNA breaks, in thus far resembling other ICD-inducing agents like radiotherapy. Finally, at least in a fraction of patients, BLM-based therapeutic regimens have been associated with cure (long-term patient survival), which may be indicative of the establishment of an efficient immunosurveillance against persisting neoplastic lesions. So, we hypothesized that the administration of BLM could be immunogenic.
We found that BLM can induce ROS-dependent ER stress, calreticulin and ERp57 exposure on the cell surface, autophagy and ATP release, in vitro. Moreover, we found that BLM-treated cancer cells administered to mice can stimulate a TH1-biased immune response that resembles that elicited by malignant cells succumbing to the classical ICD inducer doxorubicin. Conversely, BLM treatment caused no meaningful alteration in cells of the innate immune system, such as natural killer or dendritic cells. Finally, we were able to demonstrate that the in vivo antineoplastic response to BLM relies on the presence of calreticulin in cancer cells and CD8+ T lymphocytes in the host.Citation9 Taken together, these results indicate that part of the antitumor effects of BLM relies on this capacity to trigger ICD.
The systematic immunomonitoring of BLM-treated mice showed that BLM causes the accumulation of regulatory T cells (Treg). In particular, we found that BLM-treated cancer cells secrete transforming growth factor β(TGFβ), which stimulates Treg proliferation in vitro and in vivo. In line with this notion, CD4+ T-cell or Treg depletion, as well as the inhibition of TGFβ, strongly enhanced the antitumor effects of BLM [9].
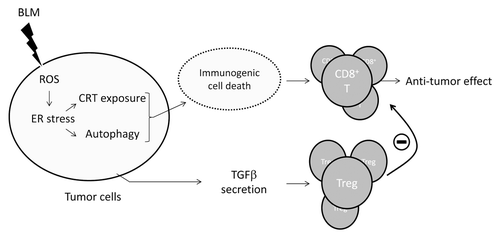
We therefore propose a model in which BLM causes robust antitumor responses while promoting immunosuppression (). Thus, in addition to exerting direct cytotoxic effects on malignant cells, BLM activate immunomodulatory circuitries that may increase or decrease its efficacy.
Figure 1. Immunomodulatory activities of bleomycin. Bleomycin (BLM) stimulated the production of reactive oxygen species (ROS), thus favoring the establishment of endoplasmic reticulum (ER) stress, the exposure of calreticulin (CRT) and ERp57 on the cell surface, the activation of autophagy and ATP secretion. Cancer cells succumbing in this fashion are immunostimulatory, and hence favor the elicitation of a tumor-specific immune response mediated by interferon γ-producing CD8+ T lymphocytes, de facto boosting the antineoplastic activity of BLM. However, BLM also favors the secretion of transforming growth factor β (TGFβ), which promotes the accumulation of regulatory T cells (Treg). By inhibiting CD8+ T lymphocytes, Treg can abolish the immunostimulatory activity of BLM-induced immunogenic cell death, thus limiting therapeutic responses.
Of note, our strategy of studying the immunomodulatory potential of one specific drug by systematically investigating how it influences each immune cell population is complementary to high-throughput screening approaches for the identification of novel ICD inducers. Recently, Guido Kroemer’s laboratory developed fluorescence microscopy-based assays allowing for the automated detection of calreticulin exposure and other ICD hallmarks.Citation10 By screening drug libraries with this system, cardiotonic glycosides were found to stimulate multiple signs of ICD and to synergize with non-ICD inducers in the elicitation of antineoplastic responses in vivo. However, this technique is not adapted to test the bystander effects that several chemotherapeutics exert on the immune system.
The use of BLM is hindered by severe adverse effects. In particular, repetitive injections of BLM have been associated with pulmonary fibrosis, an irreversible alteration linked to tissue inflammation as well as to the production of TGFβ by the bronchial epithelium. Thus, TGFβ stands out as a target for the treatment of pulmonary fibrosis. Our observations reveal that TGFβ also reduces the antineoplastic potential of BLM. Thus, targeting TGFβ or TGFβ-elicited signaling pathways may represent an efficient mean to enhance the efficacy of BLM as well as to prevent (or at least inhibit) its most dangerous side effect.
The opposite effects of BLM on antitumor immunity warrant further consideration. Current research focuses on the development of drugs that neutralize Treg or inhibit the TGFβ receptor, both such therapeutic approaches being under clinical investigation. By combining either of these strategies with BLM, we could kill two birds with one stone, as Treg and TGFβ are not only responsible for BLM-induced pulmonary fibrosis but also liable for its limited efficacy. In conclusion, targeting Treg, TGFβ or TGFβ-elicited signaling pathways may constitute an efficient approach to enhancing the antineoplastic potential of BLM while keeping under control its most dangerous side effect.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Ghiringhelli F, Larmonier N, Schmitt E, Parcellier A, Cathelin D, Garrido C, et al. CD4+CD25+ regulatory T cells suppress tumor immunity but are sensitive to cyclophosphamide which allows immunotherapy of established tumors to be curative. Eur J Immunol 2004; 34:336 - 44; http://dx.doi.org/10.1002/eji.200324181; PMID: 14768038
- Apetoh L, Ghiringhelli F, Tesniere A, Obeid M, Ortiz C, Criollo A, et al. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med 2007; 13:1050 - 9; http://dx.doi.org/10.1038/nm1622; PMID: 17704786
- Ghiringhelli F, Apetoh L, Tesniere A, Aymeric L, Ma Y, Ortiz C, et al. Activation of the NLRP3 inflammasome in dendritic cells induces IL-1beta-dependent adaptive immunity against tumors. Nat Med 2009; 15:1170 - 8; http://dx.doi.org/10.1038/nm.2028; PMID: 19767732
- Obeid M, Tesniere A, Ghiringhelli F, Fimia GM, Apetoh L, Perfettini JL, et al. Calreticulin exposure dictates the immunogenicity of cancer cell death. Nat Med 2007; 13:54 - 61; http://dx.doi.org/10.1038/nm1523; PMID: 17187072
- Hervieu A, Rébé C, Végran F, Chalmin F, Bruchard M, Vabres P, et al. Dacarbazine-mediated upregulation of NKG2D ligands on tumor cells activates NK and CD8 T cells and restrains melanoma growth. J Invest Dermatol 2013; 133:499 - 508; http://dx.doi.org/10.1038/jid.2012.273; PMID: 22951720
- Bruchard M, Mignot G, Derangère V, Chalmin F, Chevriaux A, Végran F, et al. Chemotherapy-triggered cathepsin B release in myeloid-derived suppressor cells activates the Nlrp3 inflammasome and promotes tumor growth. Nat Med 2013; 19:57 - 64; http://dx.doi.org/10.1038/nm.2999; PMID: 23202296
- Vincent J, Mignot G, Chalmin F, Ladoire S, Bruchard M, Chevriaux A, et al. 5-Fluorouracil selectively kills tumor-associated myeloid-derived suppressor cells resulting in enhanced T cell-dependent antitumor immunity. Cancer Res 2010; 70:3052 - 61; http://dx.doi.org/10.1158/0008-5472.CAN-09-3690; PMID: 20388795
- Michaud M, Martins I, Sukkurwala AQ, Adjemian S, Ma Y, Pellegatti P, et al. Autophagy-dependent anticancer immune responses induced by chemotherapeutic agents in mice. Science 2011; 334:1573 - 7; http://dx.doi.org/10.1126/science.1208347; PMID: 22174255
- Bugaut H, Bruchard M, Berger H, Derangère V, Odoul L, Euvrard R, et al. Bleomycin exerts ambivalent antitumor immune effect by triggering both immunogenic cell death and proliferation of regulatory T cells. PLoS One 2013; 8:e65181; http://dx.doi.org/10.1371/journal.pone.0065181; PMID: 23762310
- Menger L, Vacchelli E, Adjemian S, Martins I, Ma Y, et al. (2012) Cardiac glycosides exert anticancer effects by inducing immunogenic cell death. Sci Transl Med 4: 143ra199.