Abstract
The mevalonate pathway is an attractive target for cancer therapy not only to override multidrug resistance but also to promote the immunogenic demise of malignant cells. Recent data indicate that aminobisphosphonates are superior to statins for the pharmacological manipulation of the mevalonate pathway, since they exert therapeutically relevant effects on both cancer cells and the immune system.
The final products of the mevalonate pathway are cholesterol and various isoprenoids including isopentenyl pyrophosphate (IPP), farnesyl pyrophosphate (FPP) and geranylgeranyl pyrophosphate (GGPP). Isoprenoids are critical for the post-translational modification of proteins that are essential for both cell proliferation and differentiation, such as the small GTP-binding proteins RAS and RHOA.
Accumulating evidence indicates that the mevalonate pathway also contributes to multidrug resistance (MDR), a major challenge to durable tumor eradication by chemotherapy. The plasma membrane transporter P-glycoprotein (Pgp) plays a key role in MDR by promoting the efflux of various drugs, including doxorubicin. The activity of Pgp in tumor cells is supported by the mevalonate pathway by several mechanisms including: (1) elevated levels of cholesterol in the plasma membrane;Citation1 (2) a decreased synthesis of the endogenous Pgp-inhibitor nitric oxide, which results from the GGPP-induced activation or RHOA;Citation2 and (3) an enhanced hypoxia-inducible factor 1α (HIF-1α)-dependent transactivation of the Pgp-coding gene, reflecting the activation of the RHOA/RHOA kinase and RAS/ERK signaling pathways.Citation3
Interestingly, the overexpression of Pgp also impairs the biological functions of calreticulin (CRT).Citation4 CRT translocation on the cell surface is an hallmark of immunogenic cell death (ICD), a peculiar type of cell death triggered by specific chemotherapeutic agents, including doxorubicin, that elicits an antitumor immune response. CRT exposure is sensed by dendritic cells (DCs) as an “eat me” signal, promoting the phagocytosis of dying cancer cells and the activation of a cytotoxic T-lymphocyte tumor-specific immune response.Citation5
These data suggest that malignant cells that are resistant to the direct cytotoxic effects of chemotherapy (as a results of MDR) also have a tendency to escape the ICD-dependent induction of antitumor immune responses. We have recently tested this hypothesis and demonstrated that an accelerated mevalonate pathway is a common denominator bridging MDR and ICD resistance in cancer cells.Citation3 Thus, the inhibition of the mevalonate pathway is an attractive strategy to simultaneously override MDR and reinstate the sensitivity of neoplastic cells to ICD.
The mevalonate pathway can be inhibited with statins and aminobisphosphonates (NBPs). The former are specific inhibitors of 3-hydroxy-3-methylglutaryl-CoA reductase (HMGCoAR), the rate limiting enzyme of this metabolic circuitry, while NBPs selectively interfere with the enzymatic activity of FPP synthase (FPPS). NBPs are commonly used to inhibit the activity of osteoclasts in patients affected by bone metastases or other causes of bone fragility (e.g., osteoporosis), zoledronic acid (ZA) being the most potent NBP currently available for clinical use. Indeed, the mevalonate pathway is not unique to tumor cells, and other cells with an accelerated mevalonate pathway activity, such as monocytes and DCs, are efficiently targeted by ZA and statins, such as simvastatin.
Several scenarios can be envisioned as the result of the inhibition of the mevalonate pathway in tumor cells and DCs in the context of doxorubicin-based chemotherapy (). Both ZA and simvastatin cause the deprivation of intracellular FPP and GGPP, hence inhibiting RAS and RHOA signaling, and limit the production of cholesterol, but only ZA (as it targets FPPS downstream to HMGCoAR) increases intracellular IPP levels and stimulates its release in the extracellular space.Citation6 Interestingly, IPP is very similar to natural ligands of the Vγ9Vδ2 T-cell receptor (TCR), which is expressed by a unique subset of unconventional T cells deeply involved in innate immune responses against microbes as well as stressed and transformed cells.Citation7 Several groups have shown that the ZA-induced accumulation of IPP within malignant cells or antigen-presenting cells (APC), such as monocytes and DCs, can be exploited to intentionally activate Vγ9Vδ2 T cells and trigger antitumor immune responses.Citation8
Figure 1. Consequences of mevalonate pathway inhibition in malignant cells and dendritic cells with zoledronic acid or simvastatin. (A) In tumor cells, the zoledronic acid (ZA)-mediated inhibition of farnesyl pyrophosphate (FPP) synthase (FPPS) results in decreased hypoxia-inducible factor 1α (HIF-1α) activity and limited P-glycoprotein (Pgp) expression, thus favoring the intracellular accumulation of doxorubicin (Doxo) and calreticulin (CRT) exposure. In this setting, dying cancer cells are engulfed by dendritic cells (DCs), representing the afferent arm of immunogenic cell death (ICD), and become able to prime antitumor cytotoxic T-cell responses, the efferent arm of ICD. ZA also induces the intracellular accumulation and release of isopentenyl pyrophosphate (IPP), leading to an increased functional activation of Vγ9Vδ2 T cells. In DCs, the ZA-dependent deprivation of isoprenoids stimulated the caspase-1-dependent production of interleukin (IL)-1β and IL-18, in turn promoting the activation of natural killer (NK) cells. ZA-treated DCs also release IPP, further activating Vγ9Vδ2 T cells, which are potent adjuvants for MHC-restricted αβ CD8+ T as well as NK cells. Thus, the afferent and efferent arms of ICD are boosted by the activation of Vγ9Vδ2 T cells. (B) The administration of simvastatin (Sim) to malignant cells inhibits cholesterol synthesis and hence limits the activity of the Pgp, thus favoring (to some extent) the intracellular accumulation of Doxo. However, Sim fails to decrease Pgp expression levels, to stimulate CRT exposure and to promote ICD. Moreover, the Sim-dependent inhibition of 3-hydroxy-3-methylglutaryl-CoA (HMGCoA) reductase (HMGCoAR) is not associate with the accumulation/release of IPP, implying that Sim is incapable of functionally recruiting Vγ9Vδ2 T cells. CoA, coenzyme A; GGPP, geranylgeranyl pyrophosphate.
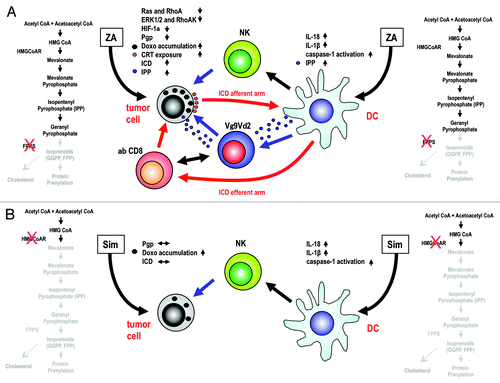
In multidrug resistant cancer cells (, left), ZA interrupts RAS- and RHOA-dependent signaling pathways and abrogates the HIF-1α-driven expression of Pgp, hence promoting the intracellular accumulation of doxorubicin, facilitating doxorubicin-dependent CRT exposure and de facto restoring the sensitivity of neoplastic cells to ICD. This is well documented by the ability of DCs to engulf multidrug resistant cancer cells exposed to ZA and doxorubicin (afferent ICD arm), and prime antitumor cytotoxic CD8+ T lymphocytes (efferent ICD arm).Citation3 Moreover, the ZA-induced release of IPP facilitates the activation of Vγ9Vδ2 T cells, which hence become able to recognize multidrug resistant cancer cells.Citation6
In DCs (, right), the ZA-induced deprivation of isoprenoids promotes the caspase-1-dependent proteolytic maturation and secretion of interleukin (IL)-1β and IL-18, which in turn drive the activation of natural killer (NK) cells.Citation9 ZA-treated DCs also accumulate and release elevated amounts of IPP, favoring the activation of Vγ9Vδ2 T cells.Citation6 Active Vγ9Vδ2 T cells are not detrimental for NK cells, but rather potentiate their immunological functions.Citation10 Lastly, Vγ9Vδ2 T cells activated by ZA-treated DCs and malignant cells can act as cellular adjuvants to boost both the afferent and efferent arm of ICD. The antitumor immune response of MHC-restricted CD8+ αβ T cells is amplified by the concurrent activation of Vγ9Vδ2 T cells as induced by autologous DCs simultaneously exposed to ZA and tumor-associated antigens.Citation6 In conclusion, the ZA-mediated inhibition of the mevalonate pathway in tumor cells and DCs can override MDR and promote the recognition of cancer cells by innate and adaptive immune effectors.
A similarly favorable scenario is not induced by simvastatin (), which is actually unable to downregulate the expression of Pgp.Citation1 Thus, the accumulation of doxorubicin in simvastatin-treated cancer cells is suboptimal, failing to induce CRT exposure and ICD. Moreover, simvastatin does not promote the accumulation of IPP in malignant cells or DCs, and therefore is unable to recruit immune effector cells other than NK cells. These observations and the significant discrepancy between the doses of statins used in vitro and in vivo may explain why these agents have failed to exert bona fide chemosensitizing effects in clinical trials.
In conclusion, accumulating evidence points to ZA, rather than to statins, as the most promising means to inhibit the mevalonate pathway in cancer cells, hence overriding MDR, restoring ICD sensitivity and stimulating robust anticancer responses.
Acknowledgments
This work was supported by AIRC (MFAG 11475 to CR and IG 13119 to MM), PRIN 2010–2011 to MM and FIRB 2012 to CR.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Kopecka J, Campia I, Olivero P, Pescarmona G, Ghigo D, Bosia A, et al. A LDL-masked liposomal-doxorubicin reverses drug resistance in human cancer cells. J Control Release 2011; 149:196 - 205; http://dx.doi.org/10.1016/j.jconrel.2010.10.003; PMID: 20946921
- Riganti C, Orecchia S, Pescarmona G, Betta PG, Ghigo D, Bosia A. Statins revert doxorubicin resistance via nitric oxide in malignant mesothelioma. Int J Cancer 2006; 119:17 - 27; http://dx.doi.org/10.1002/ijc.21832; PMID: 16450390
- Riganti C, Castella B, Kopecka J, Campia I, Coscia M, Pescarmona G, et al. Zoledronic acid restores doxorubicin chemosensitivity and immunogenic cell death in multidrug-resistant human cancer cells. PLoS One 2013; 8:e60975; http://dx.doi.org/10.1371/journal.pone.0060975; PMID: 23593363
- Kopecka J, Campia I, Brusa D, Doublier S, Matera L, Ghigo D, et al. Nitric oxide and P-glycoprotein modulate the phagocytosis of colon cancer cells. J Cell Mol Med 2011; 15:1492 - 504; http://dx.doi.org/10.1111/j.1582-4934.2010.01137.x; PMID: 20716130
- Zitvogel L, Kepp O, Kroemer G. Immune parameters affecting the efficacy of chemotherapeutic regimens. Nat Rev Clin Oncol 2011; 8:151 - 60; http://dx.doi.org/10.1038/nrclinonc.2010.223; PMID: 21364688
- Castella B, Riganti C, Fiore F, Pantaleoni F, Canepari ME, Peola S, et al. Immune modulation by zoledronic acid in human myeloma: an advantageous cross-talk between Vγ9Vδ2 T cells, αβ CD8+ T cells, regulatory T cells, and dendritic cells. J Immunol 2011; 187:1578 - 90; http://dx.doi.org/10.4049/jimmunol.1002514; PMID: 21753152
- Bonneville M, O’Brien RL, Born WK. Gammadelta T cell effector functions: a blend of innate programming and acquired plasticity. Nat Rev Immunol 2010; 10:467 - 78; http://dx.doi.org/10.1038/nri2781; PMID: 20539306
- Castella B, Vitale C, Coscia M, Massaia M. Vγ9Vδ2 T cell-based immunotherapy in hematological malignancies: from bench to bedside. Cell Mol Life Sci 2011; 68:2419 - 32; http://dx.doi.org/10.1007/s00018-011-0704-8; PMID: 21584812
- Gruenbacher G, Gander H, Nussbaumer O, Nussbaumer W, Rahm A, Thurnher M. IL-2 costimulation enables statin-mediated activation of human NK cells, preferentially through a mechanism involving CD56+ dendritic cells. Cancer Res 2010; 70:9611 - 20; http://dx.doi.org/10.1158/0008-5472.CAN-10-1968; PMID: 20947520
- Maniar A, Zhang X, Lin W, Gastman BR, Pauza CD, Strome SE, et al. Human gammadelta T lymphocytes induce robust NK cell-mediated antitumor cytotoxicity through CD137 engagement. Blood 2010; 116:1726 - 33; http://dx.doi.org/10.1182/blood-2009-07-234211; PMID: 20519625