Abstract
Tumor infiltration by effector cells is essential for the efficacy of T cell-based immunotherapeutic approaches against brain malignancies. We found that tumor-associated antigen (TAA)-specific CD8+ T cells are optimally recruited to neoplastic lesions when co-administered with TH1 polarized CD4+ T cells that are also TAA-specific. However, in vitro TH1 polarization is not required for the long-term therapeutic efficacy of the combined transfer of CD4+ and CD8+ T cells.
Anticancer immunotherapy has entered an era in which concepts no longer require validation, but in which an ever increasing immunological knowledge provides an embarrassingly large panel of choices in term of immune cells and mediators to test. Even focusing on T cell-mediated immunotherapy comes with difficult choices, concerning not only which specific T-cell lineage to promote (for instance, CD8+ vs. CD4+ TH1, TH2 or TH17 cells), but which cellular requirements to impose on different stages of the immune response. Indeed, the roles of T cells of a given lineage with varying differentiation statuses can change during the induction phase (for instance, at vaccination), at the effector stage, during chronic stimulation, and along with the establishment of long-term immunological memory.Citation1 Multiple issues related to tumor type and anatomical location further add to this complexity. Solid neoplasms are particularly resistant to T cell-based immunotherapy because the tumor stroma can resist penetration by T lymphocytes. Moreover, tumor-infiltrating T cells generally encounter an hostile and robustly immunosuppressive microenvironment. Along similar lines, the relatively low accessibility of the brain to immune cells may negatively impact the efficacy of cell-based immunotherapeutic strategies for the treatment of both primary and metastatic brain malignancies. Nonetheless, when a robust infiltration of neoplastic lesions by T cells can be achieved (be it spontaneous or induced by immunotherapy), this can favorably correlate with clinical outcome.Citation2 Therefore, T cell-based immunotherapy might stand out as an attractive modality for the treatment of brain tumors, especially if the potentially synergistic interactions between different T-cell subsets could be fully exploited.
The most direct approach to study the role of different immune cell subsets in anticancer therapy is upon adoptive cell transfer (ACT),Citation1 as this avoids the inevitable bias originating from adjuvants and/or other vaccine components. Moreover, in view of clinical applications, it may be advantageous to expand T cells under controlled culture conditions, and notably in the absence of tumor- or chemotherapy-derived deleterious and/or immunosuppressive factors. The opportunity to expand T cells in vitro also imposes a choice on culture conditions. Indeed, culture conditions can be modified to elicit specific phenotypic and functional traits that can be exploited for therapeutic purposes. Historically, ACT-based anticancer therapy has been developed around CD8+ T cells, as they can differentiate to become potent cytotoxic T lymphocytes that specifically lyse malignant cells expressing their cognate antigen. Together with the notion that many cancer cells constitutively express MHC class I, but not class II, molecules (at least in vitro), this focused the discovery of tumor-specific or tumor-associated antigens (TSAs and TAAs) on molecules that can be recognized by CD8+ T cells. Major advances regarding in vivo presented epitopes have been made in the context of glioblastoma.Citation3 Of course, immunologists have long recognized the critical “helper” role of CD4+ T cells, particularly at the priming step of CD8+ T-cell immune responses, when they functionally license dendritic cells and produce high levels of interleukin-2 (IL-2).Citation4 To exploit these functional properties of CD4+ T cells, “universal” (but not tumor-associated) CD4 epitopes such as the pan-DR helper T-cell epitope (PADRE) or peptides from the tetanus toxoid have been incorporated in cancer vaccines.Citation5 Following the administration of CD8+ T cells activated in vitro, the help of CD4+ T cells is no longer required at the induction stage, but rather to support persistence and effector functions. To this aim, CD4+ T cells must presumably co-localize with CD8+ T cells at effector sites. Thus, a profound understanding of the trafficking and functional interactions of CD4+ and CD8+ T cells at tumor sites is essential for the optimization of ACT protocols.
We have recently reported an optimal strategy to exploit CD4+ T cells for ACT in the context of brain tumors.Citation6 In line with the notion that the homing properties of CD4+ T cells are influenced by their functional polarization, in our hands TH1 polarized CD4+ T cells infiltrated an intracranial tumor far more efficiently than their TH2 counterparts (). This correlated with elevated expression levels of α4 integrin and chemokine (C-X-C motif) receptor 3 (CXCR3), two hallmarks of TH1 polarization.Citation7 The objective was to enhance the recruitment of CD8+ T cells to the brain and to augment their ability to secrete interferon γ (IFNγ) and tumor necrosis factor α (TNFα), and this could only be achieved when TAA-specific CD4+ TH1 cells were co-administered (). These results extend to the central nervous system earlier findings exploring the importance of CD4+ T-cell help in the immune response against extracranial tumors.Citation8,Citation9 As for many other malignancies, only a few TSAs/TAAs recognized by CD4+ T cells have been characterized to date for human brain tumors. Our findings should therefore encourage a wave of antigen discovery aimed at identifying targets for synergistic CD4+ and CD8+ T cell-based anticancer immunotherapy. “Universal,” tumor-associated helper peptides from telomerase are interesting candidates to test in this sense.Citation10
Figure 1. Synergic effects of tumor-associated antigen-specific CD4+ TH cells and CD8+ T cells against brain cancer. (A) In vitro polarized TH1 cells preferentially infiltrate an intracranial tumor. (B) TH1 cells specific for a tumor-associated antigen enhanced the infiltration of neoplastic lesions by CD8+ T cells and the ability of the latter to secrete cytokines. (C) Both TH1 and TH2 T cells have therapeutic effects when co-administered with CD8+ T cells, potentially owing to in vivo repolarization. APC, antigen-presenting cell; IFNγ, interferon γ; IL, interleukin.
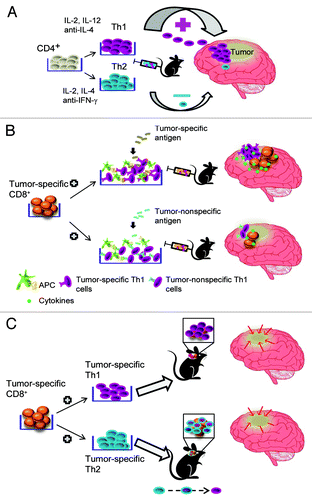
In conclusion, the adoptive transfer of T cells for the immunotherapy of brain tumors does not require the clinically inconvenient approach of local delivery, since systemically delivered TAA-specific TH1 CD4+ and CD8+ T cells exert synergistic anticancer effects. But is TH1 polarization a required complexity? In some circumstances probably not, because we demonstrated that in long-term models of immunotherapy against brain cancer, even TH2 polarized CD4+ cells can synergize with CD8+ effector cells (). This may be because of the inherent plasticity of in vitro polarized TH1 or TH2 CD4+ T cells, which may repolarize in vivo, in appropriate microenvironments.
| Abbreviations: | ||
| ACT | = | adoptive cell transfer |
| TAA | = | tumor-associated antigen |
| TSA | = | tumor -specific antigen |
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Restifo NP, Dudley ME, Rosenberg SA. Adoptive immunotherapy for cancer: harnessing the T cell response. Nat Rev Immunol 2012; 12:269 - 81; http://dx.doi.org/10.1038/nri3191
- Prins RM, Soto H, Konkankit V, Odesa SK, Eskin A, Yong WH, et al. Gene expression profile correlates with T-cell infiltration and relative survival in glioblastoma patients vaccinated with dendritic cell immunotherapy. Clin Cancer Res 2011; 17:1603 - 15; http://dx.doi.org/10.1158/1078-0432.CCR-10-2563
- Dutoit V, Herold-Mende C, Hilf N, Schoor O, Beckhove P, Bucher J, et al. Exploiting the glioblastoma peptidome to discover novel tumour-associated antigens for immunotherapy. Brain 2012; 135:1042 - 54; http://dx.doi.org/10.1093/brain/aws042
- Lanzavecchia A. Immunology. Licence to kill. Nature 1998; 393:413 - 4; http://dx.doi.org/10.1038/30845
- Slingluff CL Jr.. The Present and Future of Peptide Vaccines for Cancer: Single or Multiple, Long or Short, Alone or in Combination?. Cancer J 2011; 17:343 - 50; http://dx.doi.org/10.1097/PPO.0b013e318233e5b2
- Hoepner S, Loh JM, Riccadonna C, Derouazi M, Maroun CY, Dietrich PY, et al. Synergy between CD8 T Cells and Th1 or Th2 Polarised CD4 T Cells for Adoptive Immunotherapy of Brain Tumours. PLoS ONE 2013; 8:e63933; http://dx.doi.org/10.1371/journal.pone.0063933
- Okada H. Brain tumor immunotherapy with type-1 polarizing strategies. Ann N Y Acad Sci 2009; 1174:18 - 23; http://dx.doi.org/10.1111/j.1749-6632.2009.04932.x
- Wang LX, Shu S, Disis ML, Plautz GE. Adoptive transfer of tumor-primed, in vitro-activated, CD4+ T effector cells (TEs) combined with CD8+ TEs provides intratumoral TE proliferation and synergistic antitumor response. Blood 2007; 109:4865 - 76; http://dx.doi.org/10.1182/blood-2006-09-045245
- Bos R, Sherman LA. CD4+ T-cell help in the tumor milieu is required for recruitment and cytolytic function of CD8+ T lymphocytes. Cancer Res 2010; 70:8368 - 77; http://dx.doi.org/10.1158/0008-5472.CAN-10-1322
- Dosset M, Vauchy C, Beziaud L, Adotevi O, Godet Y. Universal tumor-reactive helper peptides from telomerase as new tools for anticancer vaccination. OncoImmunology 2013; 2:e23430; http://dx.doi.org/10.4161/onci.23430