Abstract
Although in the last few years γδ T lymphocytes have been the subject of growing interest as potential anticancer immunotherapeutics, how the proliferative and effector responses of these cells are regulated remains unclear. We have recently reported that the co-receptor B and T lymphocyte associated (BTLA) inhibits the proliferation of human Vγ9Vδ2 T cells, potentially underpinning a mechanism of immune escape by lymphoma cells.
Keywords: :
Vγ9Vδ2 T cells operate both as effector cells and as initiators of immune responses at the interface between innate and adaptive immunity, mediating a robust and MHC-unrestricted cytotoxic activity, displaying an elevated potential for cytokine release and recognizing a broad spectrum of cancer cells. The function of these cells must therefore be finely regulated. Multiple co-receptors have been shown to positively or negatively regulate the activation, expansion and survival of γδ T cells, including several molecules of the CD28 family, which robustly influence T-cell receptor (TCR)-dependent T-cell activation. Some of these co-receptors, such as programmed cell death 1 (PDCD1, best known as PD-1), are also able to modulate the proliferative response of Vγ9Vδ2 T cells.Citation1 B and T lymphocyte associated (BTLA), a recently described member of the CD28 family structurally related to cytotoxic T lymphocyte-associated protein 4 (CTLA4) and PD-1, is expressed by most lymphocytes.Citation2 BTLA binds herpes virus entry mediator A (HVEM), a member of the tumor necrosis factor (TNF) TNF receptor superfamily found on T, B, natural killer (NK), dendritic and myeloid cells.Citation3 We have recently reported that BTLA regulates Vγ9Vδ2 T-cell proliferation and differentiation. In addition, the BTLA-HVEM signaling pathway negatively affects Vγ9Vδ2 T-cell proliferation upon interaction with lymphoma B cells.Citation4
The phenotypic analysis of various human circulating Vγ9Vδ2 T-cell subsets revealed that high expression levels of BTLA inversely correlate with Vγ9Vδ2 T-cell differentiation. In contrast, PD-1 is preferentially expressed on differentiated effector Vγ9Vδ2 T cells. Interestingly, whereas we found PD-1 to be upregulated upon TCR engagement, the expression of BTLA was drastically reduced. These data reveal a differential regulation of BTLA and PD-1 in response to TCR-elicited signals, pointing to distinct functional profiles. Supporting this hypothesis, it has previously been suggested that PD-1 would contribute to the contraction of cellular immune responses,Citation5 while BTLA would preferentially take part in their initiation, similar to inducible T-cell co-stimulator (ICOS).Citation6 As we observed a concurrent downregulation of BTLA and TCR upon the engagement of the latter, we suspected a physical interaction between these two molecules. Indeed, BTLA is recruited to the proximity of the TCR at the surface of activated Vγ9Vδ2 T cells and at the immunological synapse forming between γδ T cells and HVEM+ target cells. The proximal signal transduction cascade elicited by the Vγ9Vδ2 TCR is enhanced upon BTLA blockade, revealing BTLA as a repressor of γδ TCR signaling. These observations may not only improve our understanding of the kinetics of γδ T-cell responses during autoimmune conditions, but also provide a rationale for the development of specific therapeutic approaches.
Upon TCR activation and the elicitation of effector functions, Vγ9Vδ2 T cells, like γδ cells, undergo a rapid and robust proliferative response. Surprisingly, the blockade of BTLA affected neither the degranulation nor the secretion production of pro-inflammatory cytokines including interferon γ (IFNγ) and TNFα by Vγ9Vδ2 T cells. Rather, BTLA appeared to negatively control Vγ9Vδ2 T-cell proliferation by mediating a partial arrest in the S phase of the cell cycle, in thus far resembling CTLA4.Citation7
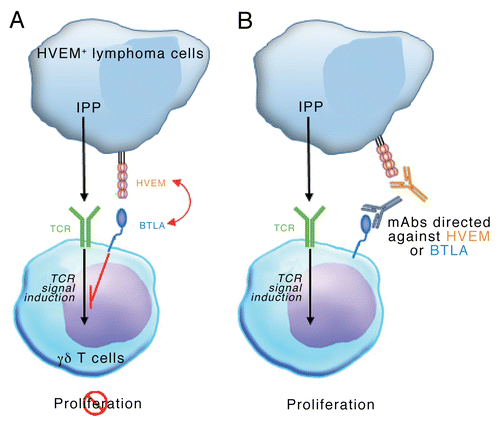
Vγ9Vδ2 T cells mediate robust antineoplastic effects, mostly in a cell-to-cell contact-dependent manner,Citation8 and the control of these cells by stimulatory and inhibitory signals may play a prominent role in preventing tumor-specific immune responses. We therefore asked whether HVEM-expressing cancer cells could affect Vγ9Vδ2 T-cell proliferation? In lymphoma tissue samples, Vγ9Vδ2 T cells are scarce and hence an extensive proliferative response may be required for an efficient control of tumor progression. The phenotypic analysis of tumor-infiltrating immune effectors (NK cells, γδ T cells and Vγ9Vδ2 T cells) and neoplastic cells revealed that the latter express high levels of HVEM, while BTLA expression is restricted to the T-cell compartment. As both CD160 and tumor necrosis factor (ligand) superfamily, member 14 (TNFSF14 best known as LIGHT), two alternative HVEM binding partners, were not expressed, BTLA was the only receptor for HVEM in this setting. Accordingly, blocking the BTLA-HVEM interaction drastically enhanced the proliferation of Vγ9Vδ2 T cells co-cultured with allogeneic and autologous HVEM+ lymphoma cells. These data suggest that lymphoma cells may exert a control on the intratumoral expansion of Vγ9Vδ2 T cells in a BTLA- and HVEM-dependent manner (). This conclusion is in line with recent data showing that loss-of-function mutations TNFRSF14 (the HVEM-coding gene) correlate with improved prognosis in follicular lymphoma patients.Citation9 However, the actual prognostic value of TNFRSF14 mutations in lymphoma patients remains controversial.Citation10
Figure 1. Lymphoma cells may regulate intranodal Vγ9Vδ2 T-cell expansion via a BTLA- and HVEM-dependent signaling pathway. (A) Isopentenyl pyrophosphate (IPP) produced by cancer cells can be recognized by the Vγ9Vδ2 T-cell receptor (TCR), hence stimulating Vγ9Vδ2 T-cell proliferation. Lymphoma cells expressing the herpesvirus entry mediator A (HVEM) inhibit the trasnduction of Vγ9Vδ2 TCR-elicited signals by interacting with B and T lymphocyte associated (BTLA), hence impeding Vγ9Vδ2 T-cell proliferation. (B) Monoclonal antibodies directed against BTLA or HVEM allow Vγ9Vδ2 T cells to proliferate in spite of the presence of HVEM+ lymphoma cells.
Taken together, our findings delineate a novel pathway whereby malignant cells can escape Vγ9Vδ2 T-cell immune responses and provide a solid background for additional studies on the role of Vγ9Vδ2 T cells in lymphoma immunosurveillance.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Iwasaki M, Tanaka Y, Kobayashi H, Murata-Hirai K, Miyabe H, Sugie T, Toi M, Minato N. Expression and function of PD-1 in human γδ T cells that recognize phosphoantigens. Eur J Immunol 2010; Forthcoming PMID: 21225952
- Watanabe N, Gavrieli M, Sedy JR, Yang J, Fallarino F, Loftin SK, Hurchla MA, Zimmerman N, Sim J, Zang X, et al. BTLA is a lymphocyte inhibitory receptor with similarities to CTLA-4 and PD-1. Nat Immunol 2003; 4:670 - 9; http://dx.doi.org/10.1038/ni944; PMID: 12796776
- Sedy JR, Gavrieli M, Potter KG, Hurchla MA, Lindsley RC, Hildner K, Scheu S, Pfeffer K, Ware CF, Murphy TL, et al. B and T lymphocyte attenuator regulates T cell activation through interaction with herpesvirus entry mediator. Nat Immunol 2005; 6:90 - 8; http://dx.doi.org/10.1038/ni1144; PMID: 15568026
- Gertner-Dardenne J, Fauriat C, Orlanducci F, Thibult ML, Pastor S, Fitzgibbon J, et al. The co-receptor BTLA negatively regulates human Vγ9Vδ2 T cell proliferation: a potential way of immune escape for lymphoma cells. Blood 2013; Forthcoming http://dx.doi.org/10.1182/blood-2012-11-464685; PMID: 23692853
- Sakai S, Kawamura I, Okazaki T, Tsuchiya K, Uchiyama R, Mitsuyama M. PD-1-PD-L1 pathway impairs T(h)1 immune response in the late stage of infection with Mycobacterium bovis bacillus Calmette-Guérin. Int Immunol 2010; 22:915 - 25; http://dx.doi.org/10.1093/intimm/dxq446; PMID: 21047981
- van Berkel ME, Schrijver EH, Hofhuis FM, Sharpe AH, Coyle AJ, Broeren CP, Tesselaar K, Oosterwegel MA. ICOS contributes to T cell expansion in CTLA-4 deficient mice. J Immunol 2005; 175:182 - 8; PMID: 15972646
- Brunner MC, Chambers CA, Chan FK, Hanke J, Winoto A, Allison JP. CTLA-4-Mediated inhibition of early events of T cell proliferation. J Immunol 1999; 162:5813 - 20; PMID: 10229815
- Gertner-Dardenne J, Bonnafous C, Bezombes C, Capietto AH, Scaglione V, Ingoure S, Cendron D, Gross E, Lepage JF, Quillet-Mary A. Bromohydrin pyrophosphate enhances antibody-dependent cell-mediated cytotoxicity induced by therapeutic antibodies. Blood 2009; 113:4875 - 84; http://dx.doi.org/10.1182/blood-2008-08-172296; PMID: 19278954
- Launay E, Pangault C, Bertrand P, Jardin F, Lamy T, Tilly H, Tarte K, Bastard C, Fest T. High rate of TNFRSF14 gene alterations related to 1p36 region in de novo follicular lymphoma and impact on prognosis. Leukemia 2012; 26:559 - 62; http://dx.doi.org/10.1038/leu.2011.266; PMID: 21941365
- Cheung KJ, Johnson NA, Affleck JG, Severson T, Steidl C, Ben-Neriah S, Schein J, Morin RD, Moore R, Shah SP. Acquired TNFRSF14 mutations in follicular lymphoma are associated with worse prognosis. Cancer Res 2010; 70:9166 - 74; http://dx.doi.org/10.1158/0008-5472.CAN-10-2460; PMID: 20884631