Abstract
The secretion of interleukin-10 by both malignant and immune cells promotes the progression of lung tumors, hence negatively impacting on patient prognosis. As interleukin-10 mediates oncogenic effects through the PI3K/AKT signaling pathway, PI3K/AKT inhibitors might sensitize cancer cells to chemotherapy, thus favoring tumor regression and improving disease outcome.
Keywords: :
Lung cancer is the leading cause of cancer-related deaths in the world, and the overall 5-y survival rate of patients affected by this malignancy is only 14% to 17%. Cigarette smoking is widely considered as the major cause of lung cancer, since more than 85% of Caucasian lung cancer patients are smokers.Citation1 However, approximately 50% of lung cancer patients in Taiwan never smoked. Interestingly, infection by human papillomavirus (HPV) type 16 or 18 has been detected in lung cancer biopsies and is suspected to play a role in lung oncogenesis, at least in Taiwan.Citation2 Such a high HPV infection rate reflects the possibility that immunological defects might play an important role in the development of lung cancer, especially among Taiwanese women who never smoked.
Interleukin-10 (IL-10) is predominately secreted by immune cells including macrophages, T lymphocytes, and natural killer (killer) NK cells, and constitutes a major determinant of viral clearance vs. persistent infection.Citation3,Citation4 The precise effects of IL-10 on tumor progression remain matter of debate, as IL-10 has been shown to play both oncosuppressive and oncogenic roles in virus-associated human cancers.Citation4,Citation5 In fact, our preliminary data indicated that lung tumors bearing high HPV16/18 genome copy numbers also exhibit a robust expression of IL-10 (at the mRNA level). A large body of studies demonstrates that IL-10 secreted from immune cells is associated with the progression of neoplasms including large B-cell lymphoma, T-cell non-Hodgkin lymphoma, myeloma as well as breast, lung, gastric, colorectal and prostate carcinoma.Citation3 Serum IL-10 levels were found to be significantly higher in HPV+ patients as well as in individuals bearing cervical intraepithelial neoplasia (CIN) than in HPV- healthy subjects. Therefore, IL-10 may play a role in the development of HPV-associated cervical neoplasms by favoring HPV persistence.
Elevated IL-10 levels were frequently observed in the booth the peripheral blood and tumor tissue of advanced stage cancer patients.Citation6 Additionally, robust IL-10 expression was associated with poor disease outcome in HPV-infected cervical and oropharyngeal cancer patients.Citation7 Therefore, we expected that circulating IL-10 might not only originate from immune cells but also from HPV+ malignant cells. In line with this hypothesis, the circulating levels of IL-10 were found to be higher in lung cancer patients than in healthy individuals.Citation6 Moreover, patients with metastatic disease had significantly higher serum IL-10 levels than patients with early stage lesions.Citation6 Of note, a significant elevation in the circulating levels of IL-10 was observed in patients who failed to respond to chemotherapy (exhibiting progressive disease) as compared with patients undergoing complete responses, partial responses, or disease stabilization.Citation6
We have previously demonstrated that the IL10 haplotype, impacting IL-10 expression levels, is associated with disease outcome among lung cancer patients.Citation8 Such haplotypes corresponded to polymorphisms of the IL10 promoter region, notably -1082A > G, -819C > T, and -592C > A. Thus, neoplastic lesions from patients with the IL10 non-ATA haplotype (-1082G, -819C and -592C) expressed higher IL10 mRNA levels were infiltrated by lower amounts of lymphocytes than tumors from individuals with the IL10 ATA haplotype (-1082A, -819T, and -592A).Citation8 As expected, the IL10 non-ATA haplotype was associated with poor survival and increased relapse rate as compared with the IL10 ATA haplotype.Citation8 In addition, we were able to demonstrate that malignant cells suppress the antitumor effects of T cells via IL-10 (at least in vitro), a phenomenon that can be reversed by the neutralization of IL-10 with specific antibodies. These results support the hypothesis that IL-10 expression by tumor cells may promote the progression of lung carcinoma.Citation8
These findings prompted us to investigate the role of IL-10 in HPV-associated lung cancer. Although the contribution of IL-10 to the evasion of immune responses by malignant cells has been extensively studied, the role of IL-10 in tumorigenesis itself remains enigmatic. IL-10 is well known to favor immune escape by inhibiting the antitumor activity of tumor-infiltrating macrophages as well as the cytotoxicity of tumor-specific T cells, and by blocks the presentation of tumor-associated antigens by antigen-presenting cells.Citation3,Citation4 In addition, IL-10 is considered as an autocrine growth factor not only for immune cells, but also for malignant cells of various types, including melanoma, gastric carcinoma, and thyroid cancer cells.Citation9 Apparently at odds with these observations, other studies have shown that IL-10 potently inhibits the growth and metastatic dissemination of colorectal carcinoma, breast cancer, and melanoma.Citation5 Moreover, the administration of IL-10 elicits tumor-specific immune responses in murine models. Collectively, these results indicate that IL-10 may play a dual role in tumor progression, prompting us to explore whether IL-10 expressed by tumor cells might sustain the progression of lung neoplasms ().
Figure 1. Role of interleukin-10 in the progression of human papillomavirus-associated lung cancer. The secretion of interleukin-10 (IL-10) by immune and malignant cells, as induced by the E6 protein of human papillomavirus (HPV) type 16 or 18, might contribute to tumor progression by upregulating cancerous inhibitor of protein phosphatase 2A (CIP2A) and MYC. HPV-infected lung cancer cells that express E6 manifest indeed the activating phosphorylation of cAMP responsive element binding protein 1 (CREB1) and CCAAT/enhancer binding protein β (C/EBPβ), which stimulate the production of IL-10 at the transcriptional level. IL-10 secreted by malignant cells stimulates an autocrine loop relying on the IL-10 receptor (IL-10R). In addition, by binding to IL-10R expressed by immune cells, IL-10 may imbalance TH1 vs. TH2 tumor-specific immune responses. Cumulatively, these effects favor tumor progression.
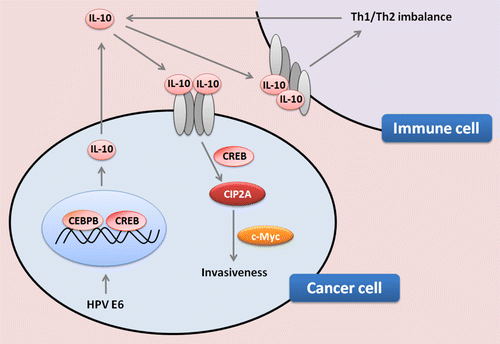
Our mechanistic studies indicate that IL-10 is upregulated by the HPV E6 oncoprotein and acts as an autocrine factor that not only promotes the proliferation of malignant cells, but also anchorage-independent growth and invasiveness.Citation10 Furthermore, we found that (in the context of HPV infections) the transcription of IL10 is predominantly regulated by the E6-dependent phosphorylation of cAMP responsive element binding protein 1 (CREB1) and CCAAT/enhancer binding protein β (C/EBPβ) through the phosphoinositide-3-kinase (PI3K) signaling pathway. The HPV-mediated activation of IL-10 and turned out to induce the expression of cancerous inhibitor of protein phosphatase 2A (CIP2A) and MYC, again via a PI3K-dependent signal transduction cascade.Citation10 This migration- and invasion-promoting activity of IL-10 could be inhibited by the depletion of the IL-10 receptor (IL-10R), suggesting that IL-10 favors the progression of lung carcinoma via an autocrine loop.Citation10 Of note, IL-10 expression levels, as monitored at the mRNA levels in lung cancer biopsies, correlated with those of CIP2A. Both IL10 and CIP2A mRNA levels may therefore predict the prognosis of lung cancer patients, in particular individuals bearing E6+ lesions.Citation10
In summary, the secretion of IL-10 by both malignant and immune cells promotes the progression of HPV-associated lung carcinoma, hence worsening patient prognosis. Our findings indicate that PI3K inhibitors might sensitize lung cancer cells to the cytotoxic effect of chemotherapy, hence favoring tumor regression and providing actual clinical benefits to patients affected by this deadly disease.
Disclosure of Potential Conflicts of Interest
The authors declare no conflicts of interest.
References
- Cokkinides V, Bandi P, McMahon C, Jemal A, Glynn T, Ward E. Tobacco control in the United States--recent progress and opportunities. CA Cancer J Clin 2009; 59:352 - 65; http://dx.doi.org/10.3322/caac.20037; PMID: 19897839
- Cheng YW, Chiou HL, Sheu GT, Hsieh LL, Chen JT, Chen CY, et al. The association of human papillomavirus 16/18 infection with lung cancer among nonsmoking Taiwanese women. Cancer Res 2001; 61:2799 - 803; PMID: 11306446
- Moore KW, de Waal Malefyt R, Coffman RL, O'Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol 2001; 19:683 - 765; http://dx.doi.org/10.1146/annurev.immunol.19.1.683; PMID: 11244051
- Brooks DG, Trifilo MJ, Edelmann KH, Teyton L, McGavern DB, Oldstone MB. Interleukin-10 determines viral clearance or persistence in vivo. Nat Med 2006; 12:1301 - 9; http://dx.doi.org/10.1038/nm1492; PMID: 17041596
- Zheng LM, Ojcius DM, Garaud F, Roth C, Maxwell E, Li Z, et al. Interleukin-10 inhibits tumor metastasis through an NK cell-dependent mechanism. J Exp Med 1996; 184:579 - 84; http://dx.doi.org/10.1084/jem.184.2.579; PMID: 8760811
- De Vita F, Orditura M, Galizia G, Romano C, Roscigno A, Lieto E, et al. Serum interleukin-10 levels as a prognostic factor in advanced non-small cell lung cancer patients. Chest 2000; 117:365 - 73; http://dx.doi.org/10.1378/chest.117.2.365; PMID: 10669676
- Chuang CY, Sung WW, Wang L, Lin WL, Yeh KT, Su MC, et al. Differential impact of IL-10 expression on survival and relapse between HPV16-positive and -negative oral squamous cell carcinomas. PLoS One 2012; 7:e47541; http://dx.doi.org/10.1371/journal.pone.0047541; PMID: 23118878
- Wang YC, Sung WW, Wu TC, Wang L, Chien WP, Cheng YW, et al. Interleukin-10 haplotype may predict survival and relapse in resected non-small cell lung cancer. PLoS One 2012; 7:e39525; http://dx.doi.org/10.1371/journal.pone.0039525; PMID: 22848356
- Todaro M, Zerilli M, Ricci-Vitiani L, Bini M, Perez Alea M, Maria Florena A, et al. Autocrine production of interleukin-4 and interleukin-10 is required for survival and growth of thyroid cancer cells. Cancer Res 2006; 66:1491 - 9; http://dx.doi.org/10.1158/0008-5472.CAN-05-2514; PMID: 16452205
- Sung WW, Wang YC, Lin PL, Cheng YW, Chen CY, Wu TC, et al. IL-10 Promotes tumor aggressiveness via upregulation of CIP2A transcription in lung adenocarcinoma. Clin Cancer Res 2013; Forthcoming http://dx.doi.org/10.1158/1078-0432.CCR-12-3439; PMID: 23743567