Abstract
The use of interleukin (IL)-15 or the IL-15 superagonist RLI as immunological adjuvants presents many advantages over that of IL-2, including a reduced toxicity and an improved efficacy. We have generated an immunocytokine that specifically targets RLI to a tumor-associated antigen, namely, disialoganglioside GD2. This agent displayed robust antitumor activity in 2 syngeneic murine models of malignant disease.
Interleukin (IL)-15 is a cytokine of the four α-helix bundle family structurally related to IL-2.Citation1 IL-15 acts upon binding to a receptor that shares with the IL-2 receptor the β and γ chains, operating as signal transducing components. In addition, the IL-2 and IL-15 receptors each use a private α chain (IL-2Rα or IL-15Rα), which confers cytokine specificity by preferentially enhancing ligand binding affinity. Although displaying similar effects in vitro, IL-2 and IL-15 exert distinct and often competing effects in the course of adaptive immune responses. Indeed, unlike IL-2, IL-15 does not promote activation-induced cell death (AICD) among CD8+ effector cells, and does not seem to exert an important influence on immunosuppressive regulatory T cells (Tregs). However, IL-15 is crucial not only for the development of natural killer (NK) cells and the survival of memory T lymphocytes, but also for initiating T-cell activation. IL-15 therefore plays a major role in anticancer immunosurveillance. In line with this notion, IL-15 has been ranked first among the agents with an elevated potential for the treatment of multiple neoplasms,Citation2 and is currently being evaluated in several Phase I clinical trials enrolling patients with advanced solid tumors such as renal cell carcinoma and melanoma.Citation3
Several preclinical studies have revealed a specific mode of action for IL-15 in vivo, which has been named trans-presentation. In the course of trans-presentation, IL-15Rα expressed at the surface of IL-15-secreting cells (including dendritic cells, macrophages and epithelial cells), presents IL-15 in trans to IL-15-sensitive cells (such as NK cells or memory CD8+ T lymphocytes) that bear IL-15Rβ/γ dimers. A soluble form of IL-15Rα has also been described to result from the proteolytic cleavage of membrane-anchored IL-15Rα by metalloproteases.Citation4 Multiple studies have shown that the soluble IL-15/IL-15Rα complex exerts more consistent immunostimulatory effects (in the context of trans-activation) than soluble IL-15. Based on these premises, we have previously engineered a fusion protein called RLI, linking the sushi domain of human IL-15Rα to human IL-15. As a single molecule, RLI exerted improved biological activities in vitroCitation5 and in vivo, both as a promoter of the development of lymphoid cells and as an adjuvant to immune responses against murine and human cancers.Citation6
To further capitalize on the antitumor activity of RLI, we sought to develop RLI-based immunocytokines (ICKs) by fusing RLI to antibodies targeting tumor-associated antigens. The rationale of ICKs is to specifically direct to the tumor site both the effector activities of tumor-specific antibodies and the cytokine-dependent immunostimulatory signal that is required for the generation of cytotoxic cellular immunity (). An additional advantage of this approach is that reduced concentrations of cytokines are needed to achieve a biological effect in the tumor environment, resulting in minimal systemic toxicity.Citation7 Among the most advanced ICKs, IL-2-based fusion proteins have shown promising results in Phase II clinical trials, yet were associated with adverse effects resembling those observed with recombinant IL-2.Citation7 Based on preclinical studies, IL-15 is considered to have an improved safety profile and immunostimulatory activity over IL-2. In this context, we have developed the first RLI-based ICK targeting the GD2 disialoganglioside (),Citation8 a validated tumor-associated antigen ranked 12th among all promising targets for the prevention or treatment of cancer.Citation9 GD2 is a sialic acid-bearing glycosphingolipid expressed on several tumors of neuroectodermal origin, including melanoma, glioma, neuroblastoma, and small cell lung carcinoma, but only to minimal levels by the peripheral nervous system and the cerebellum.Citation10
Figure 1. Development of a RLI-based immunocytokine targeting the tumor-associated antigen GD2. The C-terminus of the heavy chain of an anti-GD2 antibody was fused to the N-terminus of RLI. The purified anti-GD2-RLI immunocytokine not only efficiently bound GD2 and the interleukin (IL)-15 receptor β/γ dimer, but also conserved both the cytotoxic effector functions of the antibody and the biological activity of the cytokine. Such an RLI-based immunocytokine targeting GD2 has a higher therapeutic activity in vivo than RLI and anti-GD2, whether they be employed as standalone interventions or combined. ADCC, antibody-dependent cell-mediated cytotoxicity; CDC, complement-dependent cytotoxicity.
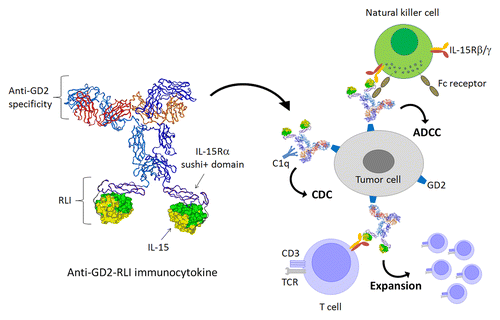
By flow cytometry, we demonstrated the specific binding of our ICK to GD2+ EL4 cells as well as to human lymphoma Kit225 cells, which endogenously express IL-15Rα, IL-15Rβ and IL-15Rγ. Importantly, the anti-GD2 antibody used for our ICK preserved cytotoxic effector functions, i.e., antibody-dependent cell-mediated cytotoxicity (ADCC) and complement-dependent cytotoxicity (CDC), in vitro, even upon fusion with RLI. On a molar basis, the anti-GD2-RLI ICK was found to be more efficient than RLI at inducing cell proliferation through IL-15Rβ/γ, a result that may suggest the bivalency of the RLI moiety in the context of the ICK. Pharmacokinetic experiments performed in mice excluded significant alterations in the integrity of the anti-GD2-RLI fusion protein upon intraperitoneal administration and indicated that the fusion markedly enhanced the bioavailability of RLI (half-life of 17 vs. 3 h). An essential role of the anti-GD2 component to target RLI to the tumor site, where the RLI-based immunostimulatory functions and antibody cytotoxic functions can synergize, has been evidenced in 2 models of syngeneic cancers developing in immunocompetent mice, namely, subcutaneous EL4 lymphomas and metastatic NXS2 neuroblastomas. In the primary tumor model based on EL4 cells, the anti-GD2-RLI ICK robustly inhibited tumor development and increased the mean survival of diseased mice, whereas RLI, the co-administration of anti-GD2 antibodies and RLI, as well as an irrelevant ICK (anti-CD20-RLI), all employed at equimolar doses, had no significant effects. In the metastatic model based on NXS2 cells, the anti-GD2-RLI fusion protein was the only agent able to completely prevent the dissemination of the disease to the liver. The therapeutic benefits of targeting RLI to the tumor site by the means of an anti-GD2 antibody are presumably related to the improved pharmacokinetic properties and immunostimulatory effects of the ICK, both components of which appear to retain complete biological activity. Such a synergistic interaction should allow for the reduction of therapeutic doses, hence limiting adverse effects. Taken together with the improved safety profile of IL-15 (as compared with IL-2), our findings indicate that RLI-based ICKs may represent a promising approach to anticancer immunotherapy.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Citation: Vincent M, Quéméner A, Jacques Y. Antitumor activity of an immunocytokine composed of an anti-GD2 antibody and the IL-15 superagonist RLI. OncoImmunology 2013; 2:e26441; 10.4161/onci.26441
References
- Grabstein KH, Eisenman J, Shanebeck K, Rauch C, Srinivasan S, Fung V, Beers C, Richardson J, Schoenborn MA, Ahdieh M, et al. Cloning of a T cell growth factor that interacts with the beta chain of the interleukin-2 receptor. Science 1994; 264:965 - 8; http://dx.doi.org/10.1126/science.8178155; PMID: 8178155
- Cheever MA. Twelve immunotherapy drugs that could cure cancers. Immunol Rev 2008; 222:357 - 68; http://dx.doi.org/10.1111/j.1600-065X.2008.00604.x; PMID: 18364014
- Steel JC, Waldmann TA, Morris JC. Interleukin-15 biology and its therapeutic implications in cancer. Trends Pharmacol Sci 2012; 33:35 - 41; http://dx.doi.org/10.1016/j.tips.2011.09.004; PMID: 22032984
- Mortier E, Bernard J, Plet A, Jacques Y. Natural, proteolytic release of a soluble form of human IL-15 receptor alpha-chain that behaves as a specific, high affinity IL-15 antagonist. J Immunol 2004; 173:1681 - 8; PMID: 15265897
- Mortier E, Quéméner A, Vusio P, Lorenzen I, Boublik Y, Grötzinger J, Plet A, Jacques Y. Soluble interleukin-15 receptor alpha (IL-15R alpha)-sushi as a selective and potent agonist of IL-15 action through IL-15R beta/gamma. Hyperagonist IL-15 x IL-15R alpha fusion proteins. J Biol Chem 2006; 281:1612 - 9; http://dx.doi.org/10.1074/jbc.M508624200; PMID: 16284400
- Bessard A, Solé V, Bouchaud G, Quéméner A, Jacques Y. High antitumor activity of RLI, an interleukin-15 (IL-15)-IL-15 receptor alpha fusion protein, in metastatic melanoma and colorectal cancer. Mol Cancer Ther 2009; 8:2736 - 45; http://dx.doi.org/10.1158/1535-7163.MCT-09-0275; PMID: 19723883
- Pasche N, Neri D. Immunocytokines: a novel class of potent armed antibodies. Drug Discov Today 2012; 17:583 - 90; http://dx.doi.org/10.1016/j.drudis.2012.01.007; PMID: 22289353
- Vincent M, Bessard A, Cochonneau D, Teppaz G, Solé V, Maillasson M, Birklé S, Garrigue-Antar L, Quéméner A, Jacques Y. Tumor targeting of the IL-15 superagonist RLI by an anti-GD2 antibody strongly enhances its antitumor potency. Int J Cancer J Int Cancer 2013
- Cheever MA, Allison JP, Ferris AS, Finn OJ, Hastings BM, Hecht TT, Mellman I, Prindiville SA, Viner JL, Weiner LM, et al. The prioritization of cancer antigens: a national cancer institute pilot project for the acceleration of translational research. Clin Cancer Res 2009; 15:5323 - 37; http://dx.doi.org/10.1158/1078-0432.CCR-09-0737; PMID: 19723653
- Birklé S, Zeng G, Gao L, Yu RK, Aubry J. Role of tumor-associated gangliosides in cancer progression. Biochimie 2003; 85:455 - 63; http://dx.doi.org/10.1016/S0300-9084(03)00006-3; PMID: 12770784