Abstract
ALT-803, an interleukin-15-based superagonist, induces memory CD8+ T cells to proliferate, upregulate receptors involved in innate immunity, secrete interferon γ and acquire the ability to kill malignant cells in the absence of antigenic stimulation. Thus, ALT-803 can promote the expansion and activation of memory CD8+ T cells while converting them into innate immune effector cells that exhibit robust antineoplastic activity.
Harnessing cytotoxic CD8+ T cells against neoplastic lesions has been a major goal of anticancer immunotherapy.Citation1 The clonal expansion and activation of these cells are triggered by antigen-specific interactions between their T-cell receptors (TCRs) and the cognate tumor-associated antigen (TAA) displayed in complex with MHC molecules on the surface of malignant or antigen-presenting cells.Citation2 Surprisingly, a recent report has shown that cytokine-based systemic immunotherapy can trigger the antitumor functions of memory CD8+ T cells in the absence of specific antigenic stimulation.Citation3 In particular, following the co-administration of interleukin (IL)-2 and a CD40-targeting agonist antibody to mice, memory CD8+ T cells underwent a rapid expansion, upregulated killer cell lectin-like receptor subfamily K, member 1 (KLRK1, best known as NKG2D) and granzyme B, and acquired broad lytic functions. These cells failed to upregulate programmed cell death 1 (PDCD1, best known PD-1) and CD25, suggesting that their activation was independent of TCR signaling. Furthermore, the authors demonstrated that antigen specificity is not mandatory for the expansion and antitumor activity of memory CD8+ T cells as elicited by systemic immunotherapy in TCR-transgenic mice. Immunotherapy-activated ovalbumin (OVA)-specific memory CD8+ T cells were indeed capable of lysing OVA+ as well as OVA- tumors in vitro and also mediated significant antineoplastic effects in vivo. Taken together, these findings indicate that memory CD8+ T cells activated by IL-2 and CD40 signaling can acquire an unusual innate-like phenotype and become capable of mounting antigen-independent cytotoxic responses against tumor cells. Human T cells with a similar phenotype were observed in melanoma patients upon localized imiquimod-based immunotherapy,Citation3 suggesting that such immune responses may be conserved across species.
Recent studies have demonstrated that bacterial, viral and parasitic infections can also trigger memory CD8+ T cells to proliferate and become potent effector cells in the absence of specific antigenic stimulation via a process of natural inflammation known as “bystander” activation.Citation4-Citation6 In a Listeria monocytogenes (Lm) immunization mouse model, Soudja et al. showed that Lm-specific memory CD8+ T cells can acquire strong effector functions and expression of activation markers without the requirement for antigen recognition.Citation4 Such activation and differentiation of memory CD8+ T cells into potent effector cells, which contribute to anti-bacterial immunity, was shown to be orchestrated by IL-15 and IL-18, which are secreted by inflammatory monocytes upon exposure to various classes of microbial pathogens. Along similar lines, Chu et al. subsequently showed that bystander-activated memory CD8+ T cells can control the early pathogen load by killing target cells through an NKG2D-dependent mechanism, importantly mediating anti-influenza responses prior to the initiation of adaptive immunity.Citation5 In a mouse influenza model, Tietze et al. found that adoptively transferred OVA-specific memory CD8+ T cells proliferated in the lungs and displayed increased levels of NKG2D, but not CD25, in response to influenza infection.Citation6 In this setting, the intranasal blockade of NKG2D resulted in a significant increase in viral replication in the early phase of infection. These studies demonstrate that microbial pathogens induce a rapid, antigen-independent expansion of memory CD8+ T cells at the site of inflammation, resulting in the elicitation of NKG2D-dependent innate immune responses against infectious agents.
In studies described above, either multiple immunostimulatory proteins or inflammatory mediators were required to expand and activate memory CD8+ T cells in the absence of specific antigenic stimulation. Conversely, we have recently shown that the systemic administration of an IL-15 superagonist complex, ALT-803 (), is sufficient to trigger memory CD8+ T-cell responses that mediate robust antitumor effects in several mouse models of myeloma.Citation7 ALT-803 contains a mutant form of IL-15 (IL-15N72D) that exhibits a 4–5-fold increase in biological activity as compared with wild-type IL-15 due to an improved affinity for the IL-2 receptor β chain.Citation8 In ALT-803, IL-15N72D is bound to a dimeric IL-15 receptor α chain-IgG Fc fusion to form a complex with optimized in vivo activity, being at least 25-fold more potent than soluble IL-15.Citation8,Citation9 In addition, ALT-803 has a significantly longer serum half-life in vivo than wild-type IL-15 (25 h vs. < 40 min). Hence, a single intravenous injection of ALT-803 is capable of inducing mouse CD8+ T-cell and natural killer (NK)-cell proliferation at a dose at which an equimolar dose of IL-15 has no effects.Citation9
Figure 1. ALT-803 promotes innate-like CD8+ T-cell effector activity and protective antitumor immunity in myeloma-bearing mice. ALT-803 is a supramolecular complex that exhibits superagonist activity and is comprised of a mutant form of interleukin-15 (IL-15N72D) associated with a dimeric IL-15 receptor α chain sushi domain (IL-15RαSu)–IgG1 Fc fusion. The N72D substitution confers to IL-15 increased affinity for the IL-2 receptor β chain (IL-2Rβ) and enhanced biological activity. In addition, association of IL-15N72D with IL-15RαSu further improves the biological activity of IL-15 in vivo, resulting in the potent activation of IL-2Rβ/γ-bearing natural killer (NK) cells and T lymphocytes. (A) In myeloma-bearing mice, ALT-803 promoted the rapid expansion of memory CD8+ T cells but not naïve CD8+ T lymphocytes. (B) Such memory CD8+ T cells secreted high levels of interferon γ (IFNγ) and upregulated killer cell lectin-like receptor subfamily K, member 1 (KLRK1, best known as NKG2D) but not of programmed cell death 1 (PDCD1, best known as PD-1) and CD25, on their surfaces. (C) ALT-803-activated cells also mediated nonspecific cytotoxicity against myeloma cells and other tumor cells, via a mechanism that was partially dependent on IFNγ. By activating such a response, ALT-803 was capable of eliminating well-established myelomas from the bone marrow and significantly prolonging the survival of tumor-bearing mice. (D) Short-term ALT-803 treatment also provided tumor-bearing mice with protective immunity against a subsequent inoculation of myeloma cells. This protective response appeared to rely on CD8+ T lymphocytes. Presumably, ALT-803 treatment stimulated naïve and/or memory CD8+ T cells specific for tumor-associated antigens (TAAs) to acquire effector functions against a subsequent tumor challenge.
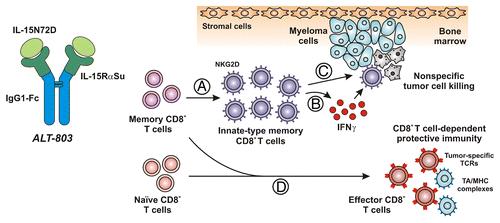
The administration of ALT-803 promoted the rapid expansion of memory CD8+ T cells, but not naïve CD8+ T lymphocytes, in mice ().Citation7 Similar to what was reported in the context of the IL-2- and CD40-based immunotherapy,Citation3,Citation6 ALT-803-activated memory CD8+ T cells upregulated NKG2D, but not PD-1 or CD25, on their cell surface and secreted high amounts of interferon γ (IFNγ) without requiring antigen-specific stimulation in vivo. These lymphocytes also exhibited nonspecific cytotoxicity against cancer cells of several types, including myeloma cells, in vitro. Consistent with the results obtained with IL-2 and CD40-targeting agonist antibodies,Citation3,Citation10 we found that these nonspecific memory CD8+ T-cell responses could not be induced by IL-15 alone, indicating that the long-lived, potent immunostimulatory properties of ALT-803 might alleviate the requirement for CD40 (or other co-stimulatory) signaling.
The treatment of mice bearing 5T33 or MOPC-315 myelomas with ALT-803, but not IL-15, rapidly eliminated malignant cells from the bone marrow and prolonged survival, often curing mice, in a CD8+ T-cell dependent manner.Citation7 NK cells were not required for such anti-myeloma activity. Conversely, the ALT-803-mediated elevation of CD8+ T cells in the bone marrow correlated with therapeutic responses, supporting the hypothesis that ALT-803 induces innate-like memory CD8+ T cells that efficiently kill myeloma cells. Furthermore, as it also activates NK cells in vitro and in vivo,Citation9 ALT-803 might have the potential to elicit broad innate immune responses against neoplastic and infected cells.
Finally, we observed that the curative, short-term administration of ALT-803 to tumor-bearing mice provided them with a CD8+ T cell-dependent protection against a subsequent rechallenge with myeloma performed months later.Citation7 These findings suggest that ALT-803 also elicits efficient adaptive immune responses, resulting in the generation of long-term T cell-based antitumor immunity. Thus, ALT-803 stands out as a potent immunostimulant that is capable of simultaneously activating the innate and adaptive arms of the immune system to elicit both rapid and long-lasting protective responses against infectious or neoplastic challenges to the host.
| Abbreviations: | ||
| IFN | = | interferon |
| IL | = | interleukin |
| IL-2Rβ | = | IL-2 receptor β |
| Lm | = | Listeria monocytogenes |
| NK | = | natural killer |
| OVA | = | ovalbumin |
| TAA | = | tumor-associated antigen |
| TCR | = | T-cell receptor |
Disclosure of Potential Conflicts of Interest
The authors are employees and shareholders of Altor BioScience Corp. Financial support: National Institutes of Health (CA156740) (H. C. Wong).
Citation: Wong HC, Jeng EK, Rhode PR. The IL-15-based superagonist ALT-803 promotes the antigen-independent conversion of memory CD8+ T cells into innate-like effector cells with antitumor activity. OncoImmunology 2013; 2:e26442; 10.4161/onci.26442
References
- Sharma P, Wagner K, Wolchok JD, Allison JP. Novel cancer immunotherapy agents with survival benefit: recent successes and next steps. Nat Rev Cancer 2011; 11:805 - 12; http://dx.doi.org/10.1038/nrc3153; PMID: 22020206
- Hivroz C, Chemin K, Tourret M, Bohineust A. Crosstalk between T lymphocytes and dendritic cells. Crit Rev Immunol 2012; 32:139 - 55; http://dx.doi.org/10.1615/CritRevImmunol.v32.i2.30; PMID: 23216612
- Tietze JK, Wilkins DE, Sckisel GD, Bouchlaka MN, Alderson KL, Weiss JM, Ames E, Bruhn KW, Craft N, Wiltrout RH, et al. Delineation of antigen-specific and antigen-nonspecific CD8(+) memory T-cell responses after cytokine-based cancer immunotherapy. Blood 2012; 119:3073 - 83; http://dx.doi.org/10.1182/blood-2011-07-369736; PMID: 22251483
- Soudja SM, Ruiz AL, Marie JC, Lauvau G. Inflammatory monocytes activate memory CD8(+) T and innate NK lymphocytes independent of cognate antigen during microbial pathogen invasion. Immunity 2012; 37:549 - 62; http://dx.doi.org/10.1016/j.immuni.2012.05.029; PMID: 22940097
- Chu T, Tyznik AJ, Roepke S, Berkley AM, Woodward-Davis A, Pattacini L, Bevan MJ, Zehn D, Prlic M. Bystander-activated memory CD8 T cells control early pathogen load in an innate-like, NKG2D-dependent manner. Cell Rep 2013; 3:701 - 8; http://dx.doi.org/10.1016/j.celrep.2013.02.020; PMID: 23523350
- Tietze JK, Sckisel GD, Zamora AE, Hsiao HH, Priest SO, Wilkins DE, Lanier LL, Blazar BR, Baumgarth N, Murphy WJ. Influenza infection results in local expansion of memory CD8(+) T cells with antigen-nonspecific phenotype and function. [Epub ahead of print] Clin Exp Immunol 2013; Forthcoming http://dx.doi.org/10.1111/cei.12186; PMID: 23937663
- Xu W, Jones M, Liu B, Zhu X, Johnson CB, Edwards AC, Kong L, Jeng EK, Han K, Marcus WD, et al. Efficacy and mechanism-of-action of a novel superagonist interleukin-15: interleukin-15 receptor αSu/Fc fusion complex in syngeneic murine models of multiple myeloma. Cancer Res 2013; 73:3075 - 86; http://dx.doi.org/10.1158/0008-5472.CAN-12-2357; PMID: 23644531
- Zhu X, Marcus WD, Xu W, Lee HI, Han K, Egan JO, Yovandich JL, Rhode PR, Wong HC. Novel human interleukin-15 agonists. J Immunol 2009; 183:3598 - 607; http://dx.doi.org/10.4049/jimmunol.0901244; PMID: 19710453
- Han KP, Zhu X, Liu B, Jeng E, Kong L, Yovandich JL, Vyas VV, Marcus WD, Chavaillaz PA, Romero CA, et al. IL-15:IL-15 receptor alpha superagonist complex: high-level co-expression in recombinant mammalian cells, purification and characterization. Cytokine 2011; 56:804 - 10; http://dx.doi.org/10.1016/j.cyto.2011.09.028; PMID: 22019703
- Murphy WJ, Welniak L, Back T, Hixon J, Subleski J, Seki N, Wigginton JM, Wilson SE, Blazar BR, Malyguine AM, et al. Synergistic anti-tumor responses after administration of agonistic antibodies to CD40 and IL-2: coordination of dendritic and CD8+ cell responses. J Immunol 2003; 170:2727 - 33; PMID: 12594303