Abstract
The elicitation of efficient antitumor immune responses requires the optimal activation of tumor-associated dendritic cells (DCs). Our comparison of the effect of various immunostimulatory treatments on DCs revealed that the best predictor of the success of immunotherapy is not the activation of existing DC populations, but the appearance of a population of monocyte-derived DC in tumor-draining lymph nodes.
Effective anticancer immunotherapies deliver tumor antigens in an immunogenic form, hence supporting the induction of antitumor immune responses of the relevant specificity. Selection of the appropriate tumor antigens is critical for therapeutic success, but it is not straightforward. Owing to the heterogeneity of neoplastic lesions the number of shared tumor antigens is generally limited, and tumor antigens arising from functionally relevant mutations are usually patient-specific. Available evidence also suggests that vaccines that simultaneously target a broad range of tumor antigens are more likely to be successful than monospecific vaccines,Citation1 possibly reflecting a reduced risk that neoplastic cells may escape immune elimination by downregulating antigen expression. For these reasons, autologous tumors may represent an ideal source of antigens for vaccination. For the generation of anticancer vaccines, however, the tumor material must be collected through surgery and is generally available in finite amounts. An alternative, and possibly more attractive, strategy is to activate anticancer immune responses in situ by manipulating the function of tumor antigen-loaded dendritic cells (DCs) that are present within neoplastic lesions or tumor-draining lymph nodes (dLNs), to render them capable of priming effective T-cell responses.
Inducing tumor-specific immune responses by local interventions is a concept that was pioneered by Coley in the 19th century and more recently applied with the intratumoral administration of bacillus Calmette–Guérin or local application of other Toll-like receptor (TLR) ligands (reviewed in refs. Citation2,Citation3). While several studies have documented the activation of robust innate and adaptive immune responses upon such interventions, few have focused on the earliest events of the process and characterized how these agents influence DCs. The reliance on pathogen-associated molecular patterns would suggest that all these treatments can induce some extent of DC activation. However, it is unknown whether such an activation involves specific DC subsets and which ones. As a consequence, the agents that are best suited to trigger antitumor immune responses upon local administration remain to be defined.
Our study compared the effects of various agents that induce the activation of DCs in vitro, upon repeated in vivo administration in the vicinity of established tumors. While several of these regimens elicited an activated DC phenotype in vivo, corresponding to an increased expression of MHC and co-stimulatory molecules on intranodal DCs, only a few of them were able to impact on tumor progression by supporting the induction of adaptive immune responses. A more detailed analysis of DC populations revealed that only the treatments exerting antineoplastic activity were able to induce the rapid appearance of a population of Ly6B+Ly6C+CD64+CD11c+MHCII+ cells (),Citation4,Citation5 a phenotype that is characteristic of inflammatory monocyte-derived DCs (moDCs). This population was located in dLNs,Citation4,Citation5 suggesting that moDCs may play a critical role in the activation of antitumor immune responses. The regimens mediating antineoplastic effects were also the most potent at inducing an early increase in the levels of specific cytokines such as interferon γ (IFNγ) and interleukin (IL)-12, suggesting that, as proposed by others, these mediators may play a critical part in the generation of moDCs.Citation6 In contrast, typical pro-inflammatory cytokines such as tumor necrosis factor α (TNFα) and IL-6 may not be required for the induction of moDCs.
Figure 1. Role of monocyte-derived dendritic cells in anticancer immune responses. The activation of antitumor immune responses is associated with the appearance of monocyte-derived dendritic cells (moDCs) in tumor-draining lymph nodes (dLNs). Conversely, “steady-state” DCs are generally unable to sustain antitumor immunity. MoDCs are defined by the co-expression of DC (e.g., CD11c and high levels of MHC class II molecules) and monocyte (e.g., CD64, Ly6C and Ly6B) markers, by their DC-like morphology, and by their elevated immunostimulatory activity in vitro.
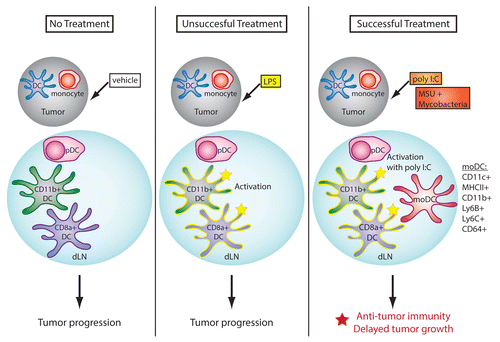
Our study did not directly test whether moDCs are required for the success of immunotherapeutic regimens, or whether their appearance is an inconsequential side effect of the beneficial inflammation induced by the local administration of these treatments. Ongoing work in our laboratory is aimed at addressing this question. Morphologically, moDCs resemble conventional DCs.Citation4 They are also at least as effective as mature DCs at inducing the proliferation of naïve CD8+ T cells in vitro (our unpublished observations). Together with other preliminary data from our laboratory, this suggests that moDCs may indeed play a central role in the antitumor immune response. Further experiments are in progress to evaluate the relative contributions of moDCs and conventional DCs in the activation of antitumor immunity.
Which might be the precise function of moDCs in antitumor immune responses? Published data indicate that moDCs can be highly immunostimulatory and appear to be superior to conventional DCs with respect to the cross-presentation of cell-associated antigens.Citation7 In some situations, moDCs have also been shown to be the principal producers of IL-12, which is critical for the induction of TH1 responses.Citation6 Whether moDCs require each of these functions to mediate antitumor effects remains unknown. In addition, the role of moDCs must be considered in view of their rapid appearance in dLNs, which may be indicative of either direct differentiation from blood monocytes or migration of reprogrammed monocytes from neoplastic lesions. The latter scenario would be compatible with a role in transporting and presenting tumor antigen in the dLN, whereas in the former case the role of moDCs would be restricted to the secretion of cytokines for T-cell differentiation, or perhaps the engulfment of tumor antigens transported by other migratory cell populations.
The observation that steady-state DCs may be unable to drive a productive antitumor immune response, even when activated by exposure to TLR ligands such as lipopolysaccharide (LPS), is also intriguing. In the steady-state, tumor antigens are constitutively presented by DCs within dLNs.Citation8 Why would these DCs be incapable of initiating a productive immune response? Compared with moDCs, steady-state DCs might be skewed to an immunosuppressive phenotype as a result of prolonged exposure to inhibitory factors and/or cytokines from either the neoplastic lesion itself or other tumor-infiltrating immune cells. The immunosuppressive phenotype induced by these signals may be irreversible, and the recruitment of a new DC population may therefore be necessary to bypass this constraint. Defining the role of moDCs in antitumor immune responses may provide a fresh understanding of how the immunological unresponsiveness to malignancy might be unlocked.
| Abbreviations: | ||
| DC | = | dendritic cell |
| dLN | = | draining lymph node |
| LN | = | lymph node |
| LPS | = | lipopolysaccharide |
| moDC | = | monocyte-derived dendritic cell |
| TLR | = | Toll-like receptor |
Disclosure of Potential Conflicts of Interest
The authors declare no competing financial interests.
Citation: Kuhn S, Ronchese F. Monocyte-derived dendritic cells: Emerging players in the antitumor immune response. OncoImmunology 2013; 2:e26443; 10.4161/onci.26443
References
- Neller MA, López JA, Schmidt CW. Antigens for cancer immunotherapy. Semin Immunol 2008; 20:286 - 95; http://dx.doi.org/10.1016/j.smim.2008.09.006; PMID: 18951039
- Fahrer AM. A proposal for a simple and inexpensive therapeutic cancer vaccine. Immunol Cell Biol 2012; 90:310 - 3; http://dx.doi.org/10.1038/icb.2011.42; PMID: 21606943
- Galluzzi L, Vacchelli E, Eggermont A, Fridman WH, Galon J, Sautès-Fridman C, Tartour E, Zitvogel L, Kroemer G. Trial Watch: Experimental Toll-like receptor agonists for cancer therapy. OncoImmunology 2012; 1:699 - 739; http://dx.doi.org/10.4161/onci.20696; PMID: 23162757
- Kuhn S, Hyde EJ, Yang J, Rich FJ, Harper JL, Kirman JR, Ronchese F. Increased Numbers of Monocyte-Derived Dendritic Cells during Successful Tumor Immunotherapy with Immune-Activating Agents. J Immunol 2013; 191:1984 - 92; http://dx.doi.org/10.4049/jimmunol.1301135; PMID: 23858033
- Rich FJ, Kuhn S, Hyde EJ, Harper JL, Ronchese F, Kirman JR. Induction of T cell responses and recruitment of an inflammatory dendritic cell subset following tumor immunotherapy with Mycobacterium smegmatis. Cancer Immunol Immunother 2012; 61:2333 - 42; http://dx.doi.org/10.1007/s00262-012-1291-8; PMID: 22714285
- Goldszmid RS, Caspar P, Rivollier A, White S, Dzutsev A, Hieny S, Kelsall B, Trinchieri G, Sher A. NK cell-derived interferon-γ orchestrates cellular dynamics and the differentiation of monocytes into dendritic cells at the site of infection. Immunity 2012; 36:1047 - 59; http://dx.doi.org/10.1016/j.immuni.2012.03.026; PMID: 22749354
- Cheong C, Matos I, Choi JH, Dandamudi DB, Shrestha E, Longhi MP, Jeffrey KL, Anthony RM, Kluger C, Nchinda G, et al. Microbial stimulation fully differentiates monocytes to DC-SIGN/CD209(+) dendritic cells for immune T cell areas. Cell 2010; 143:416 - 29; http://dx.doi.org/10.1016/j.cell.2010.09.039; PMID: 21029863
- Marzo AL, Lake RA, Lo D, Sherman L, McWilliam A, Nelson D, Robinson BW, Scott B. Tumor antigens are constitutively presented in the draining lymph nodes. J Immunol 1999; 162:5838 - 45; PMID: 10229818