Abstract
Hepatocellular carcinoma (HCC) typically occurs in patients with chronic inflammatory liver diseases, such as viral hepatitis or (non-)alcoholic steatohepatitis. Inflammation appears indeed as a crucial factor in hepatocarcinogenesis. Nevertheless, sophisticated animal models and studies of human samples revealed that the HCC also elicits antitumor immune responses. Patrolling and infiltrating lymphocytes (e.g., NKT and T cells, respectively) can exert decisive functions in the transition from chronic hepatic inflammation to cancer as well as in antitumor immune responses. An improved understanding of the cellular and molecular mechanisms whereby inflammation promotes or restricts hepatocarcinogenesis will open new avenues for therapeutic approaches to liver cancer.
Introduction
Hepatocellular carcinoma (HCC) is the third leading cause of cancer-related death and the fifth most common tumor worldwide.Citation1,Citation2 A limited panel of treatment options, which nowadays include tumor resection, liver transplantation, radiofrequency (thermal) ablation (RF(T)A), transarterial chemoembolization (TACE) and percutaneous ethanol injection (PEI), and the advanced stage at which the disease is generally diagnosed account for an overall 5-y survival rate of only 14%.Citation3,Citation4 With respect to systemic treatments, only the multi-targeted tyrosine kinase inhibitor sorafenib can moderately prolong the survival of patients with advanced HCC, but is accompanied by various side effects.Citation5 Due to the central role of the immune system in the pathogenesis of HCC, it appears indispensable to thoroughly decipher the immunological mechanisms that operate during the initiation and progression of liver cancer, as this might lead to the development of novel, innovative therapeutic strategies.
Chronic inflammatory disorders appear to increase the incidence of various types of cancer.Citation6-Citation8 On one hand, malignant cells can activate an intracellular inflammatory program, such as that mediated by the transcription factor NFκB, which has a profound influence on oncogenesis and tumor progression by interfering with the control of cellular proliferation and cell death.Citation7 On the other hand, an inflammatory component is observed in the microenvironment of most neoplastic tissues independent of their relationship to an obvious inflammatory process.Citation6 The nature and localization of immune cells infiltrating the tumor or the surrounding stroma have an important impact on tumor progression.Citation2 Thus, while tumor infiltration by myeloid cells is often associated with poor prognosis,Citation9 the presence of TH1 cells or cytotoxic T lymphocytes correlates with a reduced risk of relapse in several settings.Citation10
The close association between inflammation and cancer is particularly evident in the liver, because HCC almost exclusively arises upon chronic hepatic inflammation.Citation11 Nonetheless, a pro-inflammatory tumor microenvironment correlates with prolonged survival among HCC patients.Citation12 Thus, inflammation appears to act as a double-edged sword in hepatocarcinogenesis as it exerts pro- and antitumor effects, possibly explaining why anti-inflammatory strategies have not yet entered the clinical practice for the treatment of hepatic neoplasms. A precise understanding of the molecular mechanisms linking hepatic inflammation with cancer as well as a detailed characterization of the functions of distinct immune cell subpopulations in the inflamed and neoplastic liver are urgently warranted. This review specifically focuses on the role of lymphocytes in liver cancer.
Inflammation Promotes Liver Cancer
In the large majority of the cases, HCC develops in the context of a persistently inflamed hepatic tissue, namely in patients with chronic hepatitis, fibrosis or cirrhosis.Citation3 Interestingly, virtually all the determinants of chronic liver inflammation, including hepatitis B or C virus (HBV or HCV, respectively) infections, obesity-associated non-alcoholic steatohepatitis (NASH) and alcoholism, dramatically increase the risk of developing HCC, corroborating the notion that inflammation itself rather than a specific carcinogenic factor promote a tumor-prone hepatic microenvironment.Citation11 Interestingly, independent studies from different geographical areas reported cases of HCC developing in the absence of concomitant cirrhotic alterations in the course of non-alcoholic fatty liver disease (NAFLD) and NASH. However, also in these settings local (NASH) or systemic inflammation (e.g., obesity, metabolic syndrome) was clearly related to an increased risk of HCC.Citation13-Citation15 Conversely, the successful resolution of chronic inflammation, such as in the case of a permanent blockage of HBV replication or enduring HCV clearance, significantly reduces the risk for HCC.Citation16,Citation17 Chronic hepatic injury, impinging on cellular stress responses coupled to the death of hepatocytes, provokes an inflammatory (‘wound-healing’) response. Such a continuous cycle of cell death, compensatory proliferation and inflammatory signaling is believed to facilitate genetic alterations and the expansion of transformed parenchymal liver cells.Citation7
Multiple studies of human specimens associated HCC with various genetic and epigenetic defects as well as with alterations in specific signal transduction cascades.Citation18 However, it often remains unclear whether these alterations are causative of HCC or represent a mere epiphenomenon of hepatocarcinogenesis. Animal models of HCC are a unique opportunity to analyze various aspects of the tumor biology, for example the genetics of oncogenesis and tumor progression, in vivo, as well as to investigate the role of inflammation in HCC.Citation19 Furthermore, animal models give the opportunity to evaluate therapeutic interventions for their ability to prevent HCC development and growth. Strikingly, the functional involvement of inflammation in hepatocarcinogenesis appears to be fundamentally different between the distinct mouse models of liver cancer that are commonly used to date.
The mouse models that are regularly employed in HCC research can be roughly subdivided into chemical- or oncogene-driven models, xenograft models and genetically-modified models ().Citation19 Several transgenic mouse models exist in which primary neoplastic lesions develop in the chronically inflamed liver, including the Mdr2−/−, NEMOΔLPC, Tak1ΔLPC and AlbLTαβ mouse strains. In these models, oncogenesis proceeds along a sequence of liver inflammation, hepatic fibrosis and dysplasia, culminating in the appearance of bona fide carcinomas. For instance, the ablation of the multidrug resistance 2 (Mdr2, official name Abcb4) induces cholestatic hepatitis and HCC, which is promoted by NFκB signaling.Citation20 In the NEMOΔLPC model, mice carry a hepatocyte-specific deletion of the gene coding for IκB kinase γ (IKKγ, best known as NEMO) and are therefore unable to activate NFκB in liver parenchymal cells, resulting in the development of steatohepatitis and HCC.Citation21 The liver-specific overexpression of lymphotoxin (LT) α and β induces hepatic inflammation and hepatitis. Hepatocarcinogenesis in this AlbLTαβ model shares some similarities with the development of HCC upon HBV or HCV infection, presumably because LTs and their receptor (LTβR) are upregulated in the course of viral hepatitis and consequent HCC. In this model, carcinogenesis is linked to the induction of the classical and alternative NFκB signaling pathways by the LTαβ-LTβR axis.Citation22 Altogether, these genetically modified mouse strains provided important insights into the hepatocarcinogenic functions of inflammation, notably into the tumor-promoting role of distinct lymphocyte subsets (see below). Conversely, these models might be less suitable to characterize the mechanisms of antitumor immunity triggered by HCC and how tumor-associated signals shape the immune response.
Table 1. Selection of mouse models of hepatocellular carcinoma*
Primary Liver Cancer Provokes (Pro- and Antitumor) Immune Responses
Primary malignancies trigger an intrinsic inflammatory response that establishes a (pro-) tumorigenic microenvironment.Citation6 Specific oncogenes (for example, members of the RAS and MYC family) have been shown to induce a remodeling of the tumor microenvironment that results in the recruitment of leukocytes and lymphocytes, the expression of tumor-promoting cytokines and chemokines as well as the activation of neoangiogenesis.Citation23 Depending on their size, solid malignancies become depleted of oxygen and nutrients as they grow, resulting in the necrotic death of cancer cells coupled to the release of pro-inflammatory mediators.Citation24 The consequent inflammatory response not only stimulates neo-angiogenesis, but also recruits immune cells that can provide malignant cells with growth factors.Citation25
To analyze the immune response provoked by HCC in the liver, several mouse models have been developed (). The administration of diethylnitrosamine (DEN) to mice at a very young age (12–14 d after birth) is the most widely used system to induce primary liver cancer. The carcinogenic activity of DEN relies on its ability to alkylate DNA. Several schedules for the administration of DEN to mice are known. In all cases, HCCs develop with a high incidence and in a highly reproducible fashion.Citation19 In this context, dysplastic lesions and tumors generally develop in otherwise non-inflamed liver tissue, therefore providing an excellent opportunity to study the immune response elicited by liver cancer. Several modifications of this model have been introduced, however, in which DEN-induced hepatocarcinogenesis and chronic liver injury (for instance, as induced by repetitive injections of carbon tetrachloride) are combined. This system is advantageous in that it allows for the study of the impact of hepatic inflammation on DEN-driven oncogenesis.Citation26 Alternative models of hepatocarcinogenesis proceeding in the absence of previous hepatic inflammation are provided by genetic defects resulting in the activation or overexpression of oncogenes (e.g., MYC, β catenin) or growth factors (e.g., transforming growth factor α, fibroblast growth factor 19).Citation19
Antitumor Activity of Lymphocytes in Liver Cancer
The microenvironment of liver neoplasms typically contains various cell subsets of the innate immune system, like macrophages, neutrophils and natural killer (NK) cells, as well as T and B lymphocytes, central components of adaptive immunity.Citation2,Citation27 Tumor-infiltrating immune cells not only influence neoplastic cells, for instance as they interfere with their proliferative potential, or the local microenvironment, for instance as they stimulate angiogenesis or metastasis, but also interact with each other.Citation2 Tumor-associated macrophages (TAMs) and T cells are the immune cells most frequently found within HCCs.Citation27 Although TAMs comprise functionally diverse macrophage populations with pro- and antitumor activities, the overall effect of myeloid cells is likely to be pro-carcinogenic. Indeed, a population of immature myeloid cells best known as myeloid-derived suppressor cells (MDSCs) is very efficient at suppressing local and systemic antitumor immune responses.Citation28 Lymphocytes, on the other hand, appear to exert important anticarcinogenic functions. Notably, a pro-inflammatory microenvironment seems to correlate with prolonged survival among HCC patients.Citation12 This effect has been linked to lymphocytes, since pro-inflammatory cytokines like tumor necrosis factor α (TNFα) or interferon γ (IFNγ) as well as Toll-like receptor 3 ligands stimulate the release of various chemokines, especially chemokine (C-C motif) ligand 2 (CCL2), CCL5 and chemokine (C-X-C motif) ligand 10 (CXCL10), in the tumor microenvironment. The expression levels of these cytokines correlate with the accumulation of TH1 cells, CD8+ T lymphocytes and NK cells within neoplastic lesions, as well as with increased degrees of cancer cell death.Citation29 Accordingly, the activation of T and B cell-related signaling pathways, as documented by microarray analyses, was associated with improved survival among HCC patients.Citation27 Next, we will describe the putative functions of different lymphocyte subsets in the pathogenesis of liver cancer (summarized in and ).
Figure 1. Functions of lymphocytes in liver cancer. (A) Influence of lymphocytes on hepatocellular carcinoma (HCC). Several immune effector cells including natural killer (NK), NKT, B, CD8+ and CD4+ T cells have been shown to exert antitumor effects either directly (NK cells, CD8+ T cells, B cells, NKT cells) or upon the activation of other lymphocytes. Conversely, regulatory T cells (Tregs) mediate immunosuppressive effects, hence promoting oncogenesis and tumor progression, as they inhibit NK and CD8+ T cells. (B) Inhibition of lymphocytes by HCC. HCCs can recruit Tregs by a CCR6-dependent mechanism that impinges on the local secretion of CCL20. Tregs are also activated by myeloid-derived suppressor cells (MDSCs) and inhibit NK and CD8+ T cells. HCCs also limit the ability of dendritic cells (DCs) to present antigen to CD4+ and CD8+ T cells.
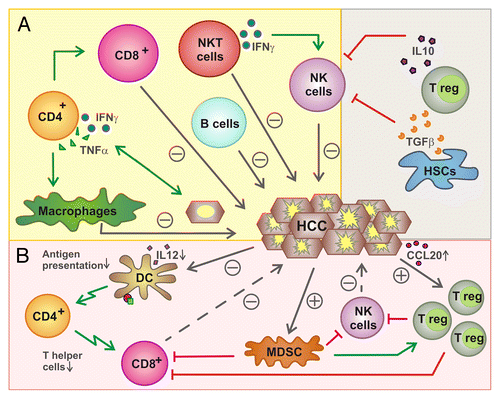
Table 2. Impact of different lymphocyte subtypes on liver cancer and proposed influence of the tumor microenvironment on tumor-infiltrating lymphocytes*
T and B Lymphocytes
The functional involvement of T and B lymphocytes, the main cellular effectors of adaptive immunity, has been investigated in mouse models of liver cancer. The administration of DEN to 14 d old mice results in the development of primary liver neoplasms after 42 weeks. In this model, T cells, especially cytotoxic CD8+ T cells, are significantly enriched in neoplastic livers.Citation27 Importantly, tumor progression, defined as both number of hepatic lesions and their size, is significantly enhanced in T and B cell-deficient Rag1−/− mice.Citation27 The distinct role of T and B cells in hepatocarcinogenesis has been dissected by administering DEN to Igh6−/− mice (so-called ‘µMT mice’, which only lack mature B cells), and MhcII−/− mice (that are characterized by the selective impairment of CD4+ T cells). In this setting, Igh6−/− mice developed larger DEN-driven tumors than their wild-type counterparts, while MhcII−/− animals developed a slightly increased number of lesions. These data demonstrate an important role for B cells in limiting the growth of DEN-driven hepatic tumors and for T cells in preventing the initial steps of DEN-mediated carcinogenesis.Citation27
In contrast to these findings, other mouse models revealed opposing functions for T and B cells in hepatocarcinogenesis. Activated T, B and NK cells are the main immune effectors expressing LTα and LTβ, which are members of the TNFα superfamily.Citation22 Upon binding to LTβR and TNFα receptor 1 (TNFR1), both of which are expressed by hepatocytes, LTs can activate the classical and alternative NFκB signaling pathway, which has an important role in HCC development.Citation30 The transgene-driven hepatocyte-specific overexpression of LTα and LTβ in mice (AlbLTαβ mice) results in chronic inflammation and liver cancer.Citation22 The role of lymphocytes in this model of hepatic carcinogenesis was investigated by crossing AlbLTαβ mice with Rag1−/− mice. Strikingly, the absence of T and B cells (which characterizes Rag1−/− mice) prevented hepatic carcinogenesis as driven by LT overexpression.Citation22
The completely contrasting results on the contribution of T and B cells to liver carcinogenesis as driven by DEN and LT overexpression perhaps reflects model-specific features that might allow for the elucidation of specific contributions of adaptive immunity to different aspects of hepatocarcinogenesis. DEN induces DNA alterations and malignant transformation very early after birth, and (pre)malignant cells appear to provoke an immune response whereby lymphocytes (at least attempt to) control oncogenesis.Citation27 Conversely, the overexpression of LTs activate NFkB signaling in hepatocytes, resulting in the secretion of inflammatory chemokines. These chemokines recruit lymphocytes that perpetuate an aggressive chronic hepatitis, constituting a prerequisite for tissue remodeling, hepatocyte proliferation and ultimately malignant transformation.Citation22
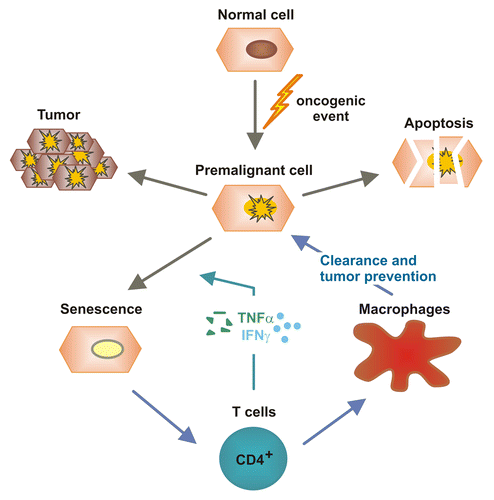
While these studies highlighted the prominent cytotoxic functions of adaptive immune effectors, either on hepatocytes (inducing chronic hepatitis) or on (pre)malignant cells (controlling oncogenesis and tumor progression), more recent works described a “sensing” function for T cells in identifying pre-malignant hepatocytes and mediating their eradication (). Upon the activation of oncogenes, cells can enter a permanent cell cycle arrest that is generally referred to as cellular senescence. Recent findings indicate that the immunosurveillance of senescent hepatocytes limits the development of hepatocellular cancer.Citation31 As a consequence of the hydrodynamic injection of plasmids coding for oncogenic NrasG12V, murine hepatocytes enter senescence in vivo, hence stimulating the development of primary liver tumors. Senescent (NrasG12V-expressing) hepatocytes were found to secrete various cytokines and chemokines that attract multiple subsets of immune cells. Among these, CD4+ TH1 cells were identified to trigger the clearance of senescent hepatocytes, a process that relied on freshly replenished macrophages .Citation31 In view of the long-standing controversy on the role of T cell-dependent immunosurveillance, these data suggest that cellular senescence might be necessary for the elicitation of a CD4+ T-cell response against pre-cancerous cells.Citation31,Citation32 A recent study lent further support to this hypothesis by showing that the combined activity of IFNγ and TNFα released by TH1 cells induces a permanent growth arrest in cancer cells.Citation33
Figure 2. Immunosurveillance of senescent hepatocytes by CD4+ T cells and TH1 cytokines. Aberrant oncogene activation in hepatocytes as well as other oncogenic events can induce a state of cellular stress from which hepatocytes can develop tumors, die via apoptosis or undergo cell senescence. CD4+ T cells can sense cellular senescence and initiate an immune response that stimulates macrophages to clear senescent cells. Moreover, TH1 cytokines such as interferon γ (IFNγ) and tumor necrosis factor α (TNFα) promote the senescence of cancer cells. These mechanisms appear important for the suppression of hepatocarcinogenesis.Citation31,Citation32,Citation33
NKT Cells
Natural killer T (NKT) cells are a subpopulation of T lymphocytes that share phenotypic and functional characteristics with NK cells. Most murine NKT cells express an invariant Vα14Jα18 T-cell receptor (TCR) and recognize glycolipids presented by the non-polymorphic MHC-like molecule CD1d. These classical NKT cells are called invariant NKT (iNKT) cells, to distinguish them from other rare NKT-cell subtypes that upregulate NK-cell markers upon stimulation.Citation34 The liver is the organ with the highest number of NKT cells, both in absolute terms and compared with other T-cell subpopulations. Indeed, NKT cells constitute about 30% of all lymphocytes in the liver.Citation35 NKT cells can rapidly produce TH1 (e.g., IFNγ and TNFα) or TH2 (e.g., IL-4 and IL-13) cytokines upon stimulation. We have recently demonstrated that iNKT cells, which are abundantly patrolling the liver in steady-state conditions, employ chemokine (C-X-C motif) receptor 6 (CXCR6) to accumulate in the liver as a very early response to experimental hepatic injury. CXCR6-dependent iNKT cells provide essential pro-inflammatory signals that activate local macrophages and perpetuate inflammation.Citation36
NKT cells may also exert important functions in antitumor immunity. For instance, an antimetastatic effect of NKT cells stimulated with IL-12 in vivo has been reported in several studies based on distinct cancer cells.Citation37,Citation38 While NKT lymphocytes appear to directly mediate antimetastatic effects upon IL-12 stimulation, they might activate other effector cells, such as NK cells, by releasing IFNγ in response to α-galactosylceramide (α-GalCer).Citation39 Even in absence of exogenous stimulation, the production of IFNγ by NKT cells appears essential for preventing the development of methylcholanthrene (MCA)-driven sarcomas in mice.Citation40 Interestingly, iNKT cells from cancer patients have been shown to produce reduced amounts of IFNγ than iNKT cells from healthy subjects, pointing to an important role for iNKT cells in oncogenesis and tumor progression.Citation41
Although NKT cells are predominant in the liver, relatively few data exist on their specific functions in hepatocarcinogenesis. In the course of β catenin-driven hepatocarcinogenesis, NKT cells constitute a key component of hepatic inflammatory responses. In line with this notion, the ablation of iNKT cells accelerates the progression of β catenin-induced liver tumors.Citation42 These findings suggest that iNKT cells may be promising targets for anticancer immunotherapy. However, especially in the context of HCC, the precise function of NKT cells remains poorly understood.
NK Cells
NK cells are also enriched among hepatic lymphocytes and seem to play an important role in liver tumorigenesis. NK cells can be activated by NKT cells, dendritic cells and Kupffer cells upon the release of IFNγ and IL-12. Conversely, regulatory T cells (Treg) and hepatic stellate cells are able to inhibit NK cell functions by secreting IL-10 and TGFβ.Citation43 The main functions of NK cells in the liver are to eliminate infected cells, malignant cells and stressed hepatocytes or to act as regulatory cells on other immune cells by producing cytokines. Thus, the cytolytic activity of NK cells seems to be important to control (pre)malignant cells in the liver.Citation43,Citation44 Accordingly, the intrahepatic levels of proliferating immune cells, predominantly T cells and CD56+ NK cells, have been found to correlate not only with the survival of HCC patients but also with the degree of cancer cell death.Citation12 Furthermore, HCC patients show significantly reduced levels of CD56dim NK cells in neoplastic lesions as compared with neighboring normal tissues, and these tumor-infiltrating NK cells produce limited amounts of IFNγ and exert minimal cytotoxicity.Citation45
The study of human specimens and mouse models of HCC provided further insights into the antineoplastic activity of NK cells in precancerous fibrotic and cirrhotic livers. Fibrosis limits the activation of NK cells, perhaps separating them from targets like (pre)malignant cells, which in part explains the link between fibrotic liver microenvironment and the increased propensity of developing hepatic tumors.Citation46,Citation47 Moreover, the phagocytosis of NK cells by hepatic stellate cells as well as alteration in the pattern of expression of NK cell-activating ligands have been observed in the context of cirrhosis and HCC.Citation48,Citation49 These data imply that NK-cell functions are important to prevent HCC, and hence often impaired as hepatic tumorigenesis proceeds.
Immunosuppression and Tumor Escape
Despite the antitumor activity of lymphocytes described above, the immune system oftentimes fails to control hepatic carcinogenesis. Indeed, some mechanisms of immunosuppression have been identified to support the development and progression of HCC. One of them consists in the selection of specific cancer cells that are able to escape immunosurveillance, a process commonly described as immunoediting. Through immunoediting, the tumor acquires the capacity to survive in spite of an intact immune system.Citation50 The downregulation of FAS and the IFNγ receptor, which are necessary for cytotoxic functions of CD8+ T cells, on hepatic cancer cells support this hypothesis.Citation51,Citation52
HCCs and their microenvironment can also influence the cytotoxic functions of CD8+ T cells. Inhibitory receptors are generally upregulated on tumor-associated antigen (TAA)-specific T cells.Citation53 Increased levels of programmed cell death 1 (PDCD1, best known as PD-1), which is frequently expressed on activated T cells, have been observed on the surface of HCC-infiltrating lymphocytes.Citation54 This is known to result in decreased expression of CD3ξ and CD28, which are critical for CD8+ T-cell activation.Citation55 In addition, the priming of T cells in the liver might be impaired in the course of HCC progression. Indeed, HCC lesions have been shown to contain reduced amounts of CD4+ T cells. This may relate to an impaired functionality of dendritic cells (DCs), which have been described to produce limited amounts of IL-12 in this context.Citation56 In any case, reduced levels of CD4+ T cells may also result in the impaired activation of cytotoxic CD8+ T cells.Citation57,Citation58
Different cells like MDSCs and Kupffer cells are known to exert immunosuppressive effects and hence may contribute to HCC progression.Citation28 For example, hepatic macrophages can express high levels of inhibitory molecules such as PD-1 ligand 1 (PD-L1), which limits the effector functions of PD-1-expressing CD8+ T cells,Citation59 or secrete immunosuppressive cytokines like IL-10.Citation60,Citation61 Among lymphocytes, Tregs have been shown to suppress antitumor immunity by inhibiting both CD8+ T and NK cells.Citation62 Tregs are enriched within HCCs, presumably owing to the local upregulation of CCL20 and the consequent recruitment of Tregs via CCR6.Citation63,Citation64 High levels of tumor-infiltrating Tregs correlate with poor prognosis in HCC patients.Citation64,Citation65
The effector functions of NK cells, which can be suppressed by Tregs and MDSC, are also dependent on the expression of specific markers on the surface of neoplastic cells, including the NK cell-activating factor MHC class I-related chain A (MICA).Citation66 Changes in MICA expression levels, for example as observed in the course of HCV infection, were found to correlate with hepatocarcinogenesis.Citation67
Impact of Immunotherapy on HCC
Considering the important role played by the immune system in the liver, notably during the development and progression of HCCs, boosting immune effector functions such as those mediated by NK or CD8+ T cells stands out as a promising treatment option for HCC patients.Citation68 One approach that has been followed to this aim was the administration of vaccine targeting the α-fetoprotein (AFP), which is expressed to high levels by many HCCs. However, short-term immune responses elicited by vaccination did not translate into a clinical benefit.Citation69,Citation70 Similarly, with the aim to stimulate CD8+ and CD4+ T cells with a broad antigenic panel, dendritic cells were pulsed with lysates from a hepatoblastoma cell line and then infused to HCC patients. In a pilot study, this approach led to disease stabilization or partial responses in 28% of patients.Citation71 The injection of dendritic cells pulsed with autologous cancer cell lysates even increased the 1-y survival rate among patients with advanced HCCs. About 13% of these patients displayed indeed a partial response and 50% stable disease.Citation72 Ongoing research aims at discovering the TAAs that may allow for the generation of optimal antitumor CD8+ and CD4+ T-cell responses.
A different immunotherapeutic strategy against HCC would be to support antitumor T-cell responses by counteracting immunosuppressive cell subsets like Tregs or MDSCs. In studies investigating the effects of Treg depletion by suitable antibodies or cyclophosphamide, an induction of AFP-specific CD4+ T-cell responses was observed.Citation73 The rather unspecific stimulation of the immune system via cytokines was also investigated as an immunotherapeutic approach to HCC by several groups. In a small trial, the injection of IL-2 and IFNγ in combination with transarterial chemotherapy was shown to lead to tumor regression in 70% of patients.Citation74
Immunotherapeutic approaches directly targeting NK and NKT cells are rare at the moment, although both cell types have been shown to exert a significant influence on tumor development and progression in murine models of HCC.Citation42,Citation43 However, the activity of these cells may very well be modulated by several (effective) chemotherapeutics, including the multi-tyrosine kinase inhibitor sorafenib, which is the systemic drug most widely used for the palliative treatment of HCC patients to date.Citation75 By blocking different tyrosine kinases, sorafenib inhibits the proliferation and survival of cancer cells and mediates antiangiogenic effects.Citation76 A recent study demonstrated that sorafenib also influences the immune system, as it might promote an NF-κB-dependent switch from immunosuppressive to immunostimulatory TAMs, eventually resulting in NK-cell activation.Citation77
Conclusions
The immune system and especially lymphocytes play a key role in the development and progression of hepatocellular carcinoma. On one hand, lymphocytes represent an important component of the inflammatory microenvironment favoring the initiation and progression of malignancies in the context of chronic liver disease. On the other hand, lymphocytes are key effectors of antitumor immune responses, for instance as they sense senescent cells (CD4+ T lymphocytes), as they release cytokines and stimulate other immune cells (NKT cells) or as they exert direct cytolytic activities against transformed cells (NK and CD8+ T lymphocytes). However, many mechanisms are in place that simultaneously suppress antitumor immunity. An improved understanding of the molecular and cellular cascades whereby inflammation promotes or restricts hepatocarcinogenesis will open novel avenues for the treatment of liver cancer.
Disclosure of Potential Conflicts of Interest
The authors disclose no competing interests.
Acknowledgments
This work was supported by the German Research Foundation (DFG Ta434/2–1 and SFB/TRR57) and by the Interdisciplinary Center for Clinical Research (IZKF) Aachen. We gratefully acknowledge the expert discussions with Prof. Tom Luedde (Aachen, Germany) and all members of the Tacke lab.
Citation: Mossanen J, Tacke F. Role of lymphocytes in liver cancer. OncoImmunology 2013; 2:e26468; 10.4161/onci.26468
References
- Schütte K, Bornschein J, Malfertheiner P. Hepatocellular carcinoma--epidemiological trends and risk factors. Dig Dis 2009; 27:80 - 92; http://dx.doi.org/10.1159/000218339; PMID: 19546545
- Hernandez-Gea V, Toffanin S, Friedman SL, Llovet JM. Role of the microenvironment in the pathogenesis and treatment of hepatocellular carcinoma. Gastroenterology 2013; 144:512 - 27; http://dx.doi.org/10.1053/j.gastro.2013.01.002; PMID: 23313965
- El-Serag HB. Hepatocellular carcinoma. N Engl J Med 2011; 365:1118 - 27; http://dx.doi.org/10.1056/NEJMra1001683; PMID: 21992124
- El-Serag HB, Marrero JA, Rudolph L, Reddy KR. Diagnosis and treatment of hepatocellular carcinoma. Gastroenterology 2008; 134:1752 - 63; http://dx.doi.org/10.1053/j.gastro.2008.02.090; PMID: 18471552
- Iavarone M, Cabibbo G, Piscaglia F, Zavaglia C, Grieco A, Villa E, Cammà C, Colombo M, SOFIA (SOraFenib Italian Assessment) study group. Field-practice study of sorafenib therapy for hepatocellular carcinoma: a prospective multicenter study in Italy. Hepatology 2011; 54:2055 - 63; http://dx.doi.org/10.1002/hep.24644; PMID: 21898496
- Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature 2008; 454:436 - 44; http://dx.doi.org/10.1038/nature07205; PMID: 18650914
- Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell 2010; 140:883 - 99; http://dx.doi.org/10.1016/j.cell.2010.01.025; PMID: 20303878
- Kuraishy A, Karin M, Grivennikov SI. Tumor promotion via injury- and death-induced inflammation. Immunity 2011; 35:467 - 77; http://dx.doi.org/10.1016/j.immuni.2011.09.006; PMID: 22035839
- Allavena P, Sica A, Solinas G, Porta C, Mantovani A. The inflammatory micro-environment in tumor progression: the role of tumor-associated macrophages. Crit Rev Oncol Hematol 2008; 66:1 - 9; http://dx.doi.org/10.1016/j.critrevonc.2007.07.004; PMID: 17913510
- Pagès F, Galon J, Dieu-Nosjean MC, Tartour E, Sautès-Fridman C, Fridman WH. Immune infiltration in human tumors: a prognostic factor that should not be ignored. Oncogene 2010; 29:1093 - 102; http://dx.doi.org/10.1038/onc.2009.416; PMID: 19946335
- El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology 2007; 132:2557 - 76; http://dx.doi.org/10.1053/j.gastro.2007.04.061; PMID: 17570226
- Chew V, Tow C, Teo M, Wong HL, Chan J, Gehring A, Loh M, Bolze A, Quek R, Lee VK, et al. Inflammatory tumour microenvironment is associated with superior survival in hepatocellular carcinoma patients. J Hepatol 2010; 52:370 - 9; http://dx.doi.org/10.1016/j.jhep.2009.07.013; PMID: 19720422
- Ertle J, Dechêne A, Sowa JP, Penndorf V, Herzer K, Kaiser G, Schlaak JF, Gerken G, Syn WK, Canbay A. Non-alcoholic fatty liver disease progresses to hepatocellular carcinoma in the absence of apparent cirrhosis. Int J Cancer 2011; 128:2436 - 43; http://dx.doi.org/10.1002/ijc.25797; PMID: 21128245
- White DL, Kanwal F, El-Serag HB. Association between nonalcoholic fatty liver disease and risk for hepatocellular cancer, based on systematic review. Clin Gastroenterol Hepatol 2012; 10:1342 - 59, e2; http://dx.doi.org/10.1016/j.cgh.2012.10.001; PMID: 23041539
- Torres DM, Harrison SA. Nonalcoholic steatohepatitis and noncirrhotic hepatocellular carcinoma: fertile soil. Semin Liver Dis 2012; 32:30 - 8; http://dx.doi.org/10.1055/s-0032-1306424; PMID: 22418886
- Wong GL, Chan HL, Mak CH, Lee SK, Ip ZM, Lam AT, Iu HW, Leung JM, Lai JW, Lo AO, et al. Entecavir treatment reduces hepatic events and deaths in chronic hepatitis B patients with liver cirrhosis. Hepatology 2013; In press PMID: 23389810
- van der Meer AJ, Veldt BJ, Feld JJ, Wedemeyer H, Dufour JF, Lammert F, Duarte-Rojo A, Heathcote EJ, Manns MP, Kuske L, et al. Association between sustained virological response and all-cause mortality among patients with chronic hepatitis C and advanced hepatic fibrosis. JAMA 2012; 308:2584 - 93; http://dx.doi.org/10.1001/jama.2012.144878; PMID: 23268517
- Zender L, Villanueva A, Tovar V, Sia D, Chiang DY, Llovet JM. Cancer gene discovery in hepatocellular carcinoma. J Hepatol 2010; 52:921 - 9; http://dx.doi.org/10.1016/j.jhep.2009.12.034; PMID: 20385424
- Vucur M, Roderburg C, Bettermann K, Tacke F, Heikenwalder M, Trautwein C, Luedde T. Mouse models of hepatocarcinogenesis: what can we learn for the prevention of human hepatocellular carcinoma?. Oncotarget 2010; 1:373 - 8; PMID: 21307402
- Pikarsky E, Porat RM, Stein I, Abramovitch R, Amit S, Kasem S, Gutkovich-Pyest E, Urieli-Shoval S, Galun E, Ben-Neriah Y. NF-kappaB functions as a tumour promoter in inflammation-associated cancer. Nature 2004; 431:461 - 6; http://dx.doi.org/10.1038/nature02924; PMID: 15329734
- Luedde T, Beraza N, Kotsikoris V, van Loo G, Nenci A, De Vos R, Roskams T, Trautwein C, Pasparakis M. Deletion of NEMO/IKKgamma in liver parenchymal cells causes steatohepatitis and hepatocellular carcinoma. Cancer Cell 2007; 11:119 - 32; http://dx.doi.org/10.1016/j.ccr.2006.12.016; PMID: 17292824
- Haybaeck J, Zeller N, Wolf MJ, Weber A, Wagner U, Kurrer MO, Bremer J, Iezzi G, Graf R, Clavien PA, et al. A lymphotoxin-driven pathway to hepatocellular carcinoma. Cancer Cell 2009; 16:295 - 308; http://dx.doi.org/10.1016/j.ccr.2009.08.021; PMID: 19800575
- Soucek L, Lawlor ER, Soto D, Shchors K, Swigart LB, Evan GI. Mast cells are required for angiogenesis and macroscopic expansion of Myc-induced pancreatic islet tumors. Nat Med 2007; 13:1211 - 8; http://dx.doi.org/10.1038/nm1649; PMID: 17906636
- Vakkila J, Lotze MT. Inflammation and necrosis promote tumour growth. Nat Rev Immunol 2004; 4:641 - 8; http://dx.doi.org/10.1038/nri1415; PMID: 15286730
- Karin M. Nuclear factor-kappaB in cancer development and progression. Nature 2006; 441:431 - 6; http://dx.doi.org/10.1038/nature04870; PMID: 16724054
- Dapito DH, Mencin A, Gwak GY, Pradere JP, Jang MK, Mederacke I, Caviglia JM, Khiabanian H, Adeyemi A, Bataller R, et al. Promotion of hepatocellular carcinoma by the intestinal microbiota and TLR4. Cancer Cell 2012; 21:504 - 16; http://dx.doi.org/10.1016/j.ccr.2012.02.007; PMID: 22516259
- Schneider C, Teufel A, Yevsa T, Staib F, Hohmeyer A, Walenda G, Zimmermann HW, Vucur M, Huss S, Gassler N, et al. Adaptive immunity suppresses formation and progression of diethylnitrosamine-induced liver cancer. Gut 2012; 61:1733 - 43; http://dx.doi.org/10.1136/gutjnl-2011-301116; PMID: 22267597
- Zhao F, Korangy F, Greten TF. Cellular immune suppressor mechanisms in patients with hepatocellular carcinoma. Dig Dis 2012; 30:477 - 82; http://dx.doi.org/10.1159/000341695; PMID: 23108303
- Chew V, Chen J, Lee D, Loh E, Lee J, Lim KH, Weber A, Slankamenac K, Poon RT, Yang H, et al. Chemokine-driven lymphocyte infiltration: an early intratumoural event determining long-term survival in resectable hepatocellular carcinoma. Gut 2012; 61:427 - 38; http://dx.doi.org/10.1136/gutjnl-2011-300509; PMID: 21930732
- Luedde T, Schwabe RF. NF-κB in the liver--linking injury, fibrosis and hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol 2011; 8:108 - 18; http://dx.doi.org/10.1038/nrgastro.2010.213; PMID: 21293511
- Kang TW, Yevsa T, Woller N, Hoenicke L, Wuestefeld T, Dauch D, Hohmeyer A, Gereke M, Rudalska R, Potapova A, et al. Senescence surveillance of pre-malignant hepatocytes limits liver cancer development. Nature 2011; 479:547 - 51; http://dx.doi.org/10.1038/nature10599; PMID: 22080947
- Swann JB, Smyth MJ. Immune surveillance of tumors. J Clin Invest 2007; 117:1137 - 46; http://dx.doi.org/10.1172/JCI31405; PMID: 17476343
- Braumüller H, Wieder T, Brenner E, Aßmann S, Hahn M, Alkhaled M, Schilbach K, Essmann F, Kneilling M, Griessinger C, et al. T-helper-1-cell cytokines drive cancer into senescence. Nature 2013; 494:361 - 5; http://dx.doi.org/10.1038/nature11824; PMID: 23376950
- Godfrey DI, MacDonald HR, Kronenberg M, Smyth MJ, Van Kaer L. NKT cells: what’s in a name?. Nat Rev Immunol 2004; 4:231 - 7; http://dx.doi.org/10.1038/nri1309; PMID: 15039760
- Zimmermann HW, Tacke F. Modification of chemokine pathways and immune cell infiltration as a novel therapeutic approach in liver inflammation and fibrosis. Inflamm Allergy Drug Targets 2011; 10:509 - 36; http://dx.doi.org/10.2174/187152811798104890; PMID: 22150762
- Wehr A, Baeck C, Heymann F, Niemietz PM, Hammerich L, Martin C, Zimmermann HW, Pack O, Gassler N, Hittatiya K, et al. Chemokine receptor CXCR6-dependent hepatic NK T Cell accumulation promotes inflammation and liver fibrosis. J Immunol 2013; 190:5226 - 36; http://dx.doi.org/10.4049/jimmunol.1202909; PMID: 23596313
- Takeda K, Seki S, Ogasawara K, Anzai R, Hashimoto W, Sugiura K, Takahashi M, Satoh M, Kumagai K. Liver NK1.1+ CD4+ alpha beta T cells activated by IL-12 as a major effector in inhibition of experimental tumor metastasis. J Immunol 1996; 156:3366 - 73; PMID: 8617962
- Seki S, Hashimoto W, Ogasawara K, Satoh M, Watanabe H, Habu Y, Hiraide H, Takeda K. Antimetastatic effect of NK1+ T cells on experimental haematogenous tumour metastases in the liver and lungs of mice. Immunology 1997; 92:561 - 6; http://dx.doi.org/10.1046/j.1365-2567.1997.00383.x; PMID: 9497499
- Nakagawa R, Nagafune I, Tazunoki Y, Ehara H, Tomura H, Iijima R, Motoki K, Kamishohara M, Seki S. Mechanisms of the antimetastatic effect in the liver and of the hepatocyte injury induced by alpha-galactosylceramide in mice. J Immunol 2001; 166:6578 - 84; PMID: 11359810
- Crowe NY, Smyth MJ, Godfrey DI. A critical role for natural killer T cells in immunosurveillance of methylcholanthrene-induced sarcomas. J Exp Med 2002; 196:119 - 27; http://dx.doi.org/10.1084/jem.20020092; PMID: 12093876
- Tahir SM, Cheng O, Shaulov A, Koezuka Y, Bubley GJ, Wilson SB, Balk SP, Exley MA. Loss of IFN-gamma production by invariant NK T cells in advanced cancer. J Immunol 2001; 167:4046 - 50; PMID: 11564825
- Anson M, Crain-Denoyelle AM, Baud V, Chereau F, Gougelet A, Terris B, Yamagoe S, Colnot S, Viguier M, Perret C, et al. Oncogenic β-catenin triggers an inflammatory response that determines the aggressiveness of hepatocellular carcinoma in mice. J Clin Invest 2012; 122:586 - 99; http://dx.doi.org/10.1172/JCI43937; PMID: 22251704
- Tian Z, Chen Y, Gao B. Natural killer cells in liver disease. Hepatology 2013; 57:1654 - 62; http://dx.doi.org/10.1002/hep.26115; PMID: 23111952
- Subleski JJ, Wiltrout RH, Weiss JM. Application of tissue-specific NK and NKT cell activity for tumor immunotherapy. J Autoimmun 2009; 33:275 - 81; http://dx.doi.org/10.1016/j.jaut.2009.07.010; PMID: 19682859
- Cai L, Zhang Z, Zhou L, Wang H, Fu J, Zhang S, Shi M, Zhang H, Yang Y, Wu H, et al. Functional impairment in circulating and intrahepatic NK cells and relative mechanism in hepatocellular carcinoma patients. Clin Immunol 2008; 129:428 - 37; http://dx.doi.org/10.1016/j.clim.2008.08.012; PMID: 18824414
- Zhang DY, Friedman SL. Fibrosis-dependent mechanisms of hepatocarcinogenesis. Hepatology 2012; 56:769 - 75; http://dx.doi.org/10.1002/hep.25670; PMID: 22378017
- Albertsson PA, Basse PH, Hokland M, Goldfarb RH, Nagelkerke JF, Nannmark U, Kuppen PJ. NK cells and the tumour microenvironment: implications for NK-cell function and anti-tumour activity. Trends Immunol 2003; 24:603 - 9; http://dx.doi.org/10.1016/j.it.2003.09.007; PMID: 14596885
- Muhanna N, Doron S, Wald O, Horani A, Eid A, Pappo O, Friedman SL, Safadi R. Activation of hepatic stellate cells after phagocytosis of lymphocytes: A novel pathway of fibrogenesis. Hepatology 2008; 48:963 - 77; http://dx.doi.org/10.1002/hep.22413; PMID: 18726940
- Coulouarn C, Factor VM, Conner EA, Thorgeirsson SS. Genomic modeling of tumor onset and progression in a mouse model of aggressive human liver cancer. Carcinogenesis 2011; 32:1434 - 40; http://dx.doi.org/10.1093/carcin/bgr133; PMID: 21771728
- Dunn GP, Old LJ, Schreiber RD. The immunobiology of cancer immunosurveillance and immunoediting. Immunity 2004; 21:137 - 48; http://dx.doi.org/10.1016/j.immuni.2004.07.017; PMID: 15308095
- Nagao M, Nakajima Y, Hisanaga M, Kayagaki N, Kanehiro H, Aomatsu Y, Ko S, Yagita H, Yamada T, Okumura K, et al. The alteration of Fas receptor and ligand system in hepatocellular carcinomas: how do hepatoma cells escape from the host immune surveillance in vivo?. Hepatology 1999; 30:413 - 21; http://dx.doi.org/10.1002/hep.510300237; PMID: 10421649
- Nagao M, Nakajima Y, Kanehiro H, Hisanaga M, Aomatsu Y, Ko S, Tatekawa Y, Ikeda N, Kanokogi H, Urizono Y, et al. The impact of interferon gamma receptor expression on the mechanism of escape from host immune surveillance in hepatocellular carcinoma. Hepatology 2000; 32:491 - 500; http://dx.doi.org/10.1053/jhep.2000.16470; PMID: 10960440
- Fourcade J, Sun Z, Pagliano O, Guillaume P, Luescher IF, Sander C, Kirkwood JM, Olive D, Kuchroo V, Zarour HM. CD8(+) T cells specific for tumor antigens can be rendered dysfunctional by the tumor microenvironment through upregulation of the inhibitory receptors BTLA and PD-1. Cancer Res 2012; 72:887 - 96; http://dx.doi.org/10.1158/0008-5472.CAN-11-2637; PMID: 22205715
- Mellman I, Coukos G, Dranoff G. Cancer immunotherapy comes of age. Nature 2011; 480:480 - 9; http://dx.doi.org/10.1038/nature10673; PMID: 22193102
- Maki A, Matsuda M, Asakawa M, Kono H, Fujii H, Matsumoto Y. Decreased expression of CD28 coincides with the down-modulation of CD3zeta and augmentation of caspase-3 activity in T cells from hepatocellular carcinoma-bearing patients and hepatitis C virus-infected patients. J Gastroenterol Hepatol 2004; 19:1348 - 56; http://dx.doi.org/10.1111/j.1440-1746.2004.03455.x; PMID: 15610307
- Ormandy LA, Farber A, Cantz T, Petrykowska S, Wedemeyer H, Horning M, Lehner F, Manns MP, Korangy F, Greten TF. Direct ex vivo analysis of dendritic cells in patients with hepatocellular carcinoma. World J Gastroenterol 2006; 12:3275 - 82; PMID: 16718852
- Behboudi S, Alisa A, Boswell S, Anastassiou J, Pathan AA, Williams R. Expansion of anti-AFP Th1 and Tc1 responses in hepatocellular carcinoma occur in different stages of disease. Br J Cancer 2010; 102:748 - 53; http://dx.doi.org/10.1038/sj.bjc.6605526; PMID: 20087354
- Witkowski M, Spangenberg HC, Neumann-Haefelin C, Büttner N, Breous E, Kersting N, Drognitz O, Hopt UT, Blum HE, Semmo N, et al. Lack of ex vivo peripheral and intrahepatic α-fetoprotein-specific CD4+ responses in hepatocellular carcinoma. Int J Cancer 2011; 129:2171 - 82; http://dx.doi.org/10.1002/ijc.25866; PMID: 21170957
- Wu K, Kryczek I, Chen L, Zou W, Welling TH. Kupffer cell suppression of CD8+ T cells in human hepatocellular carcinoma is mediated by B7-H1/programmed death-1 interactions. Cancer Res 2009; 69:8067 - 75; http://dx.doi.org/10.1158/0008-5472.CAN-09-0901; PMID: 19826049
- Chan T, Wiltrout RH, Weiss JM. Immunotherapeutic modulation of the suppressive liver and tumor microenvironments. Int Immunopharmacol 2011; 11:879 - 89; http://dx.doi.org/10.1016/j.intimp.2010.12.024; PMID: 21241810
- Tacke F, Yoneyama H. From NAFLD to NASH to fibrosis to HCC: role of dendritic cell populations in the liver. Hepatology 2013; 58:494 - 6; http://dx.doi.org/10.1002/hep.26405; PMID: 23519833
- Flecken T, Schmidt N, Spangenberg HC, Thimme R. [Hepatocellular carcinoma - from immunobiology to immunotherapy]. Z Gastroenterol 2012; 50:47 - 56; PMID: 22222798
- Ormandy LA, Hillemann T, Wedemeyer H, Manns MP, Greten TF, Korangy F. Increased populations of regulatory T cells in peripheral blood of patients with hepatocellular carcinoma. Cancer Res 2005; 65:2457 - 64; http://dx.doi.org/10.1158/0008-5472.CAN-04-3232; PMID: 15781662
- Chen KJ, Lin SZ, Zhou L, Xie HY, Zhou WH, Taki-Eldin A, Zheng SS. Selective recruitment of regulatory T cell through CCR6-CCL20 in hepatocellular carcinoma fosters tumor progression and predicts poor prognosis. PLoS One 2011; 6:e24671; http://dx.doi.org/10.1371/journal.pone.0024671; PMID: 21935436
- Fu J, Xu D, Liu Z, Shi M, Zhao P, Fu B, Zhang Z, Yang H, Zhang H, Zhou C, et al. Increased regulatory T cells correlate with CD8 T-cell impairment and poor survival in hepatocellular carcinoma patients. Gastroenterology 2007; 132:2328 - 39; http://dx.doi.org/10.1053/j.gastro.2007.03.102; PMID: 17570208
- Kohga K, Takehara T, Tatsumi T, Ohkawa K, Miyagi T, Hiramatsu N, Kanto T, Kasugai T, Katayama K, Kato M, et al. Serum levels of soluble major histocompatibility complex (MHC) class I-related chain A in patients with chronic liver diseases and changes during transcatheter arterial embolization for hepatocellular carcinoma. Cancer Sci 2008; 99:1643 - 9; http://dx.doi.org/10.1111/j.1349-7006.2008.00859.x; PMID: 18754878
- Kumar V, Kato N, Urabe Y, Takahashi A, Muroyama R, Hosono N, Otsuka M, Tateishi R, Omata M, Nakagawa H, et al. Genome-wide association study identifies a susceptibility locus for HCV-induced hepatocellular carcinoma. Nat Genet 2011; 43:455 - 8; http://dx.doi.org/10.1038/ng.809; PMID: 21499248
- Schmidt N, Neumann-Haefelin C, Thimme R. Cellular immune responses to hepatocellular carcinoma: lessons for immunotherapy. Dig Dis 2012; 30:483 - 91; http://dx.doi.org/10.1159/000341697; PMID: 23108304
- Butterfield LH, Ribas A, Dissette VB, Lee Y, Yang JQ, De la Rocha P, Duran SD, Hernandez J, Seja E, Potter DM, et al. A phase I/II trial testing immunization of hepatocellular carcinoma patients with dendritic cells pulsed with four alpha-fetoprotein peptides. Clin Cancer Res 2006; 12:2817 - 25; http://dx.doi.org/10.1158/1078-0432.CCR-05-2856; PMID: 16675576
- Butterfield LH, Ribas A, Meng WS, Dissette VB, Amarnani S, Vu HT, Seja E, Todd K, Glaspy JA, McBride WH, et al. T-cell responses to HLA-A*0201 immunodominant peptides derived from alpha-fetoprotein in patients with hepatocellular cancer. Clin Cancer Res 2003; 9:5902 - 8; PMID: 14676113
- Palmer DH, Midgley RS, Mirza N, Torr EE, Ahmed F, Steele JC, Steven NM, Kerr DJ, Young LS, Adams DH. A phase II study of adoptive immunotherapy using dendritic cells pulsed with tumor lysate in patients with hepatocellular carcinoma. Hepatology 2009; 49:124 - 32; http://dx.doi.org/10.1002/hep.22626; PMID: 18980227
- Lee WC, Wang HC, Hung CF, Huang PF, Lia CR, Chen MF. Vaccination of advanced hepatocellular carcinoma patients with tumor lysate-pulsed dendritic cells: a clinical trial. J Immunother 2005; 28:496 - 504; http://dx.doi.org/10.1097/01.cji.0000171291.72039.e2; PMID: 16113606
- Greten TF, Ormandy LA, Fikuart A, Höchst B, Henschen S, Hörning M, Manns MP, Korangy F. Low-dose cyclophosphamide treatment impairs regulatory T cells and unmasks AFP-specific CD4+ T-cell responses in patients with advanced HCC. J Immunother 2010; 33:211 - 8; http://dx.doi.org/10.1097/CJI.0b013e3181bb499f; PMID: 20139774
- Lygidakis NJ, Kosmidis P, Ziras N, Parissis J, Kyparidou E. Combined transarterial targeting locoregional immunotherapy-chemotherapy for patients with unresectable hepatocellular carcinoma: a new alternative for an old problem. J Interferon Cytokine Res 1995; 15:467 - 72; http://dx.doi.org/10.1089/jir.1995.15.467; PMID: 7544232
- Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A, et al, SHARP Investigators Study Group. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 2008; 359:378 - 90; http://dx.doi.org/10.1056/NEJMoa0708857; PMID: 18650514
- Liu L, Cao Y, Chen C, Zhang X, McNabola A, Wilkie D, Wilhelm S, Lynch M, Carter C. Sorafenib blocks the RAF/MEK/ERK pathway, inhibits tumor angiogenesis, and induces tumor cell apoptosis in hepatocellular carcinoma model PLC/PRF/5. Cancer Res 2006; 66:11851 - 8; http://dx.doi.org/10.1158/0008-5472.CAN-06-1377; PMID: 17178882
- Sprinzl MF, Reisinger F, Puschnik A, Ringelhan M, Ackermann K, Hartmann D, Schiemann M, Weinmann A, Galle PR, Schuchmann M, et al. Sorafenib perpetuates cellular anticancer effector functions by modulating the crosstalk between macrophages and natural killer cells. Hepatology 2013; 57:2358 - 68; http://dx.doi.org/10.1002/hep.26328; PMID: 23424039