Abstract
Accumulating evidence suggests that high-endothelial venules (HEVs) represent major gateways for the infiltration of lymphocytes within neoplastic lesions. However, the origin of these vessels in human neoplasms remains elusive. We have recently discovered a link between lymphotoxin β-producing dendritic cells and tumor-associated HEVs.
Tumor angiogenesis is usually thought to contribute to disease progression and metastasis, and the density of tumor-associated vessels is generally associated with poor prognosis in cancer patients. Although these affirmations are globally true, we recently proposed the concept that tumor-associated blood vessels exhibit a high degree of heterogeneity and that some of them, notably, high-endothelial venules (HEVs) might be associated with favorable clinical outcomes.Citation1 HEVs are specialized vessels normally found in lymph nodes that mediate the extravasation of naïve and central memory lymphocytes from the peripheral blood to lymphoid tissues.Citation2 Recent studies suggest that, similar to their counterparts within lymph nodes, tumor-associated HEVs represent major gateways for the infiltration of lymphocytes into solid tumors. This hypothesis is supported by retrospective studies showing that high amounts of tumor-associated HEVs correlate with elevated levels of tumor-infiltrating T lymphocytes (TILs) or with favorable prognosis factors and prolonged survival in patients affected with primary melanomaCitation3 and breast carcinoma,Citation1 respectively. Tumor-associated HEVs have also been described in murine tumor models upon the administration of lymphotoxin α (LTα)Citation4 or the depletion of FOXP3+ regulatory T cells (Tregs),Citation5 in both scenarios being associated with robust T-cell infiltration and tumor regression. Taken together, these findings suggest that tumor-associated HEVs favor antitumor immune responses by supporting a constant influx of lymphocytes in the tumor stroma. Therefore, a better understanding of the signals that regulate the formation of HEVs is warranted to allow the therapeutic manipulation of the phenotype of tumor-associated blood vessels.
The endothelial cells that line up HEVs are highly plastic and rapidly lose their specialized characteristics outside their natural microenvironment. Dendritic cells (DCs)Citation6 as well as signals provided by the lymphotoxin β (LTβ) receptor (LTβR) on endothelial cellsCitation7 have recently been shown to constitute essential regulators of the HEV phenotype in mouse lymph nodes. However, despite these considerable advances in the understanding of HEV formation in lymphoid organs,Citation2 the signals required for HEV formation in peripheral tissues, in particular neoplastic lesions, remained obscure. We have recently provided novel insights into the cells and signals that regulate HEVs in human breast carcinoma.Citation8 We found that LTβ is specifically overexpressed by breast cancers characterized by a high density of HEVs and that DCs were the major producers of LTβ in the breast tumor microenvironment. In addition, we observed that clusters of mature DCs often surround tumor-associated HEVs and the abundance of DC-LAMP+ DCs strongly correlates with the density of tumor-associated HEVs. Finally, the amounts of DC-LAMP+ DCs were robustly associated with favorable clinical outcome in breast carcinoma patients. Therefore, we proposed that in human breast cancer, like in mouse lymph nodes,Citation6 the presence of LTβ-producing DCs is required for the differentiation of HEVs ().
Figure 1. Role of lymphotoxin-producing dendritic cells in the regulation of tumor-associated high-endothelial venules. CCL19, chemokine (C-C motif) ligand; CCL21, chemokine (C-C motif) ligand 21; CXCL13, chemokine (C-X-C motif) ligand 13; DC, dendritic cell; HEV, high-endothelial venule; LTα1β2, lymphotoxin α1 β2.
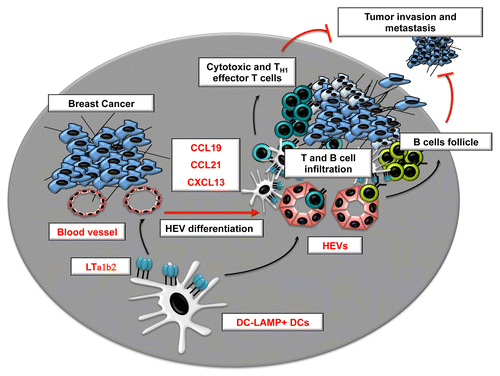
Interestingly, our results also revealed that the density of HEVs in the breast cancer stroma is associated with a reduction in the ratio between FOXP3+ Tregs and CD3+ T cells. This finding complements evidence obtained in murine models suggesting that Tregs may actually inhibit the formation of tumor-associated HEVs.Citation5 New therapeutic strategies could emerge from a better understanding of how Tregs influence HEV formation. In particular, whether this process involves DCs remains to be determined. Robust evidence indicates that Tregs constantly interact with DCs and inhibit their functions in lymph nodes, hence reducing the risk for the activation of autoreactive T cells. It is attracting to speculate that Tregs may regulate the differentiation of HEVs by directly limiting the expression of LTβR ligands on DCs. It is also possible that auto- and tumor-reactive T cells might directly promote the differentiation of HEVs upon Treg depletion, since activated T cells are known to produce LTβR ligands.
The progression of breast carcinoma is usually considered as a multistep and multifactorial process. Invasive ductal carcinoma (IDC) is thought to derive from a series of intermediate hyperplasic and neoplastic stages including ductal carcinoma in situ (DCIS). Importantly, we have documented a reduction in the density of HEVs in the IDC areas, as compared with DCIS areas, of breast carcinomas containing both a DCIS and a IDC component. Interestingly, such a reduction was associated with a decline in DC-LAMP+ DC clusters. These findings are suggestive of a causal association between DC infiltration, HEV density and tumor invasiveness. Further experiments using animal models of breast carcinogenesis are required to test this hypothesis.
HEVs are also found within the ectopic lymphoid structures that develop in chronically inflamed peripheral tissues known as tertiary lymphoid structures (TLSs). TLSs have been documented in multiple human neoplasms and their presence is correlated with improved survival in patients with lung cancer.Citation9 We found that the presence of HEVs in human breast carcinomas is associated with different levels of lymphoid organization, including (in some cases) typical TLSs characterized by large T-cell infiltrates, mature DCs, and B-cell follicles. The levels of lymphoid chemokines including chemokine (C-X-C motif) ligand 13 (CXCL13), chemokine (C-C motif) ligand 21 (CCL21) and CCL19, which are characteristic of TLSs, were also highly correlated with the presence of HEVs in the breast cancer stroma. The differentiation of HEVs most likely represents an early step leading to the development of TLSs within neoplastic lesions. Since both TLSs and HEVs are associated with high levels of DCs and TILs, it remains to be determined which of these immunological parameters is predominantly responsible for the gain in survival they are associated with in cancer patients. Whether the mere migration of effector lymphocytes into tumors is the most critical parameter, as evidenced in colon cancer patients,Citation10 or whether a high degree of organization is necessary to achieve long-term antitumor responses remains an open question in the field.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed
Citation: Martinet L, Girard J. Regulation of tumor-associated high-endothelial venules by dendritic cells: A new opportunity to promote lymphocyte infiltration into breast cancer? OncoImmunology 2013; 2:e26470; 10.4161/onci.26470
References
- Martinet L, Garrido I, Filleron T, Le Guellec S, Bellard E, Fournie JJ, Rochaix P, Girard JP. Human solid tumors contain high endothelial venules: association with T- and B-lymphocyte infiltration and favorable prognosis in breast cancer. Cancer Res 2011; 71:5678 - 87; http://dx.doi.org/10.1158/0008-5472.CAN-11-0431; PMID: 21846823
- Girard JP, Moussion C, Förster R. HEVs, lymphatics and homeostatic immune cell trafficking in lymph nodes. Nat Rev Immunol 2012; 12:762 - 73; http://dx.doi.org/10.1038/nri3298; PMID: 23018291
- Martinet L, Le Guellec S, Filleron T, Lamant L, Meyer N, Rochaix P, Garrido I, Girard JP. High endothelial venules (HEVs) in human melanoma lesions: Major gateways for tumor-infiltrating lymphocytes. Oncoimmunology 2012; 1:829 - 39; http://dx.doi.org/10.4161/onci.20492; PMID: 23162750
- Schrama D, thor Straten P, Fischer WH, McLellan AD, Bröcker EB, Reisfeld RA, Becker JC. Targeting of lymphotoxin-alpha to the tumor elicits an efficient immune response associated with induction of peripheral lymphoid-like tissue. Immunity 2001; 14:111 - 21; http://dx.doi.org/10.1016/S1074-7613(01)00094-2; PMID: 11239444
- Hindley JP, Jones E, Smart K, Bridgeman H, Lauder SN, Ondondo B, Cutting S, Ladell K, Wynn KK, Withers D, et al. T-cell trafficking facilitated by high endothelial venules is required for tumor control after regulatory T-cell depletion. Cancer Res 2012; 72:5473 - 82; http://dx.doi.org/10.1158/0008-5472.CAN-12-1912; PMID: 22962270
- Moussion C, Girard JP. Dendritic cells control lymphocyte entry to lymph nodes through high endothelial venules. Nature 2011; 479:542 - 6; http://dx.doi.org/10.1038/nature10540; PMID: 22080953
- Onder L, Danuser R, Scandella E, Firner S, Chai Q, Hehlgans T, Stein JV, Ludewig B. Endothelial cell-specific lymphotoxin-β receptor signaling is critical for lymph node and high endothelial venule formation. J Exp Med 2013; 210:465 - 73; http://dx.doi.org/10.1084/jem.20121462; PMID: 23420877
- Martinet L, Filleron T, Le Guellec S, Rochaix P, Garrido I, Girard JP. High Endothelial Venule Blood Vessels for Tumor-Infiltrating Lymphocytes Are Associated with Lymphotoxin β-Producing Dendritic Cells in Human Breast Cancer. J Immunol 2013; 191:2001 - 8; http://dx.doi.org/10.4049/jimmunol.1300872; PMID: 23825314
- Dieu-Nosjean MC, Antoine M, Danel C, Heudes D, Wislez M, Poulot V, Rabbe N, Laurans L, Tartour E, de Chaisemartin L, et al. Long-term survival for patients with non-small-cell lung cancer with intratumoral lymphoid structures. J Clin Oncol 2008; 26:4410 - 7; http://dx.doi.org/10.1200/JCO.2007.15.0284; PMID: 18802153
- Pagès F, Berger A, Camus M, Sanchez-Cabo F, Costes A, Molidor R, Mlecnik B, Kirilovsky A, Nilsson M, Damotte D, et al. Effector memory T cells, early metastasis, and survival in colorectal cancer. N Engl J Med 2005; 353:2654 - 66; http://dx.doi.org/10.1056/NEJMoa051424; PMID: 16371631