Abstract
Dendritic cell (DC)-based anticancer vaccines have yielded disappointing results in a multitude of clinical trials. New data suggest that the clinical efficacy of DC-based vaccines may be dependent on the paracrine production of interleukin-12 in the course of antigen presentation and the consequent development of therapeutic Type 1 CD8+ T-cell immunity.
Dendritic cells (DCs) are widely considered as the most potent antigen-presenting cells (APCs) in mammals and hence have been extensively harnessed for the development of anticancer vaccines.Citation1 In spite of such an intense wave of investigation, DC-based anticancer vaccines, which mainly rely on monocyte-derived DCs expanded ex vivo, have yielded low response rates so far, a finding that begs the question: “What is lacking in current DC-based vaccine formulations”?
Effective therapeutic vaccines must drive the development of high-avidity antigen-specific CD8+ T cells that display strong effector functions and long-term survival. In addition to signal 1 (provided by the peptide-MHC class I complex) and signal 2 (provided by co-stimulatory ligands), a third signal appears to be essential for CD8+ T cells to differentiate and acquire effector functions. Mescher and colleagues identified the biologically active form of interleukin-12 (IL-12p70) and Type I interferon (IFNα and IFNβ) as the critical cytokines that delivers signal 3 to resting naïve T cells.Citation2 The signal delivered by these inflammatory cytokines enforces a genetic program that results in the coordinated expression of several genes involved in T-cell effector functions, self-renewal, and homeostasis.Citation3,Citation4
Since data on the capacity of clinical DC preparations to produce IL-12p70 were not available, we initiated a clinical trial to test the hypothesis that functionally mature IL-12p70-producing DCs would drive antigen-specific immune responses in patients with metastatic (stage IV) melanoma, and improve clinical outcome.Citation5 There were 2 major points to be considered in the design of this trial. First, what would be the most appropriate clinical setting for testing of this vaccination strategy and second, what would be the optimal maturation signals needed to trigger IL-12p70 synthesis by DCs. We chose to study newly diagnosed melanoma patients with minimal yet measurable tumor burden and selected 3 well-characterized gp100-derived HLA-A*0201-restricted peptides as antigens.Citation5 A combination of CD40 ligand (CD40L, also known as CD154) and IFNγ was employed to manufacture functionally mature IL-12p70-producing DCs. Since no clinical grade CD40L material was available in 2006, good manufacturing practice (GMP)-grade, cell-bound trimeric CD40L was produced in our laboratory in the form of human CD154 expressed by K562 leukemia cells.
We treated 7 stage IV melanoma patients with autologous DCs pulsed with gp100-derived peptides and matured with CD40L and IFNγ. Although these individuals were not pre-screened for IL-12p70 production, we observed that monocyte-derived DCs from 4 of the 7 patients exhibited a selective defect (less than 1 ng/106cells/24h) in the synthesis of this cytokine upon exposure to CD40L/IFNγ in vitro. Further analyses revealed defects in the transcription of the IL-12 monomer IL-12p35, resulting in impaired IL-12p70 production. This defect was subsequently confirmed in additional melanoma patients, using age- and gender-matched healthy subjects as controls. Importantly, we found that the addition of Toll-like receptor 3 (TLR3) and TLR8 agonists, i.e., polyinosinic: polycytidylic acid (polyI:C) and R848, respectively, together with CD40L/IFNγ could correct the IL-12p70 production defect in monocyte-derived DCs from melanoma patients.
Such a specific impairment in IL-12p35 transcription allowed us to study the effects of administering DCs that secrete low or high amounts of IL-12p70, providing a unique opportunity to define the role of IL-12p70 in immunological and clinical outcomes. Immunological responses to all 3 gp100-derived peptides were observed in 6 of 7 patients. Interestingly, the magnitude of these immune responses, as assessed by HLA-A2/gp100 peptide tetramer analysis of peripheral blood mononuclear cells (PBMCs) collected in the course of vaccination, did not correlate with IL-12p70 production. Conversely, a key finding of our study was that the production levels of IL-12p70 by DCs correlated with disease outcomes, as monitored in terms of time to progression (). Patients whose DCs produced IL12p70 in amounts equal or superior to those of healthy subjects had objective radiographic responses that lasted ≥ 11.5 mo. To date, 2 of these 3 patients remain alive and well. In contrast, patients bearing DCs that produced low IL-12p70 levels exhibited rapid disease progression.
Figure 1. IL-12p70-producing dendritic cell (DC)-based vaccines elicit Tc1 immunity leading to clinical responses in melanoma patients. (A and B) Autologous monocyte-derived dendritic cells (DCs) were activated with CD40 ligand (CD40L) and interferon γ (IFNγ), resulting in the generation of functionally mature IL-12p70-producing DCs for administration to cancer patients. The amounts of IL-12p70 secreted by these cells varied dramatically among patients, and we chose to discriminate between high (> 1 ng/106cells/24h) (A) and low (< 1 ng/106cells/24hrs) (B) producers. The DCs producing low levels of IL-12p70 exhibited impaired IL12p35 transcription, resulting in the preferential secretion of IL-12p40 instead of IL-12p70. The levels of IL-12p70 secreted by DCs did not correlate with the magnitude of vaccine-induced gp100-specific CD8+ T-cell responses. However, patients immunized with DCs secreting high IL-12p70 levels developed a Tc1-biased immune response characterized by robust production of IFNγ over IL-5 and IL-13. In contrast, the administration of DCs producing low amounts of IL-12p70 drove the development of Tc2-skewed responses. A correlation was observed between the amounts of IL-12p70 produced by DCs, the development of Tc1 immunity and clinical responses.
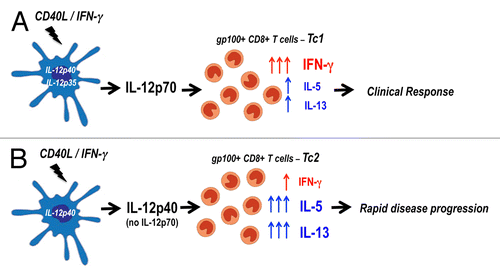
We also examined the cytokine profiles of vaccine-induced CD8+ T cells upon antigenic stimulation, in order to assess Type 1 (Tc1) or Type 2 (Tc2) polarization. A clear dichotomy in IFNγ/IL-13 and IFNγ/IL-5 ratios was observed between clinical responders and non-responders, with responders displaying a Tc1 bias (i.e., high IFNγ/IL-13 and IFNγ/IL-5 ratios) and non-responders exhibit Tc2-skewed responses (i.e., low IFNγ/IL-13 and IFNγ/IL-5 ratios). Thus, Tc1 immunity correlated with objective clinical responses while patients exhibiting a Tc2 bias exhibited rapid disease progression. This proof-of-concept clinical study indicates that the paracrine production of IL-12p70 by DCs in the course of T-cell priming plays a critical role in skewing immune responses toward a Tc1 profile, which influences disease outcome in melanoma patients with minimal disease burden.
In support of the capacity of IL-12p70 to program a therapeutic Tc1 CD8+ T-cell response, Mescher and colleagues have recently demonstrated that this cytokine, and not type I IFN, renders activated CD8+ T cells less susceptible to inhibition by the programmed cell death 1 (PDCD1, best known as PD1), leading to improved disease control in murine tumor models.Citation6 In a separate study, human circulating myeloid CD1c+ DCs were identified as robust producers of IL-12p70 upon CD40L/IFNγ maturation and as driver of IFNγ-secreting antigen-specific CD8+ T cells.Citation7 These findings suggest that myeloid CD1c+ DCs may constitute an optimal population of APCs for the development of anti-cancer vaccines based on in situ antigen targeting. In sum, accumulating evidence supports further studies of IL-12p70 as an adjuvant for anticancer vaccine in clinical trials. In our opinion, DCs may constitute the best source to provide IL-12p70 to CD4+ and CD8+ T cells in a temporally and spatially appropriate manner during antigen-presentation. The challenge is now to identify the best means to regulate the paracrine production of IL-12p70 by DCs in order to elicit therapeutic Tc1 immunity in cancer patients.
| Abbreviations: | ||
| DC | = | dendritic cell |
| IFN | = | interferon |
| mDC | = | mature DC |
Disclosure of Potential Conflicts of Interest
The authors declare no financial conflicts.
Citation: Linette GP, Carreno BM. Dendritic cell-based vaccines: Shining the spotlight on signal 3. OncoImmunology 2013; 2:e26512; 10.4161/onci.26512
References
- Kreutz M, Tacken PJ, Figdor CG. Targeting dendritic cells--why bother?. Blood 2013; 121:2836 - 44; http://dx.doi.org/10.1182/blood-2012-09-452078; PMID: 23390195
- Mescher MF, Curtsinger JM, Agarwal P, Casey KA, Gerner M, Hammerbeck CD, Popescu F, Xiao Z. Signals required for programming effector and memory development by CD8+ T cells. Immunol Rev 2006; 211:81 - 92; http://dx.doi.org/10.1111/j.0105-2896.2006.00382.x; PMID: 16824119
- Agarwal P, Raghavan A, Nandiwada SL, Curtsinger JM, Bohjanen PR, Mueller DL, Mescher MF. Gene regulation and chromatin remodeling by IL-12 and type I IFN in programming for CD8 T cell effector function and memory. J Immunol 2009; 183:1695 - 704; http://dx.doi.org/10.4049/jimmunol.0900592; PMID: 19592655
- Banerjee A, Gordon SM, Intlekofer AM, Paley MA, Mooney EC, Lindsten T, Wherry EJ, Reiner SL. Cutting edge: The transcription factor eomesodermin enables CD8+ T cells to compete for the memory cell niche. J Immunol 2010; 185:4988 - 92; http://dx.doi.org/10.4049/jimmunol.1002042; PMID: 20935204
- Carreno BM, Becker-Hapak M, Huang A, Chan M, Alyasiry A, Lie WR, Aft RL, Cornelius LA, Trinkaus KM, Linette GP. IL-12p70-producing patient DC vaccine elicits Tc1-polarized immunity. J Clin Invest 2013; 123:3383 - 94; http://dx.doi.org/10.1172/JCI68395; PMID: 23867552
- Gerner MY, Heltemes-Harris LM, Fife BT, Mescher MF. Cutting edge: IL-12 and type I IFN differentially program CD8 T cells for programmed death 1 re-expression levels and tumor control. J Immunol 2013; 191:1011 - 5; http://dx.doi.org/10.4049/jimmunol.1300652; PMID: 23804712
- Nizzoli G, Krietsch J, Weick A, Steinfelder S, Facciotti F, Gruarin P, Bianco A, Steckel B, Moro M, Crosti M, et al. Human CD1c+ dendritic cells secrete high levels of IL-12 and potently prime cytotoxic T-cell responses. Blood 2013; 122:932 - 42; http://dx.doi.org/10.1182/blood-2013-04-495424; PMID: 23794066