Abstract
In spite of significant advances in our understanding of the metastatic process, the relationship between the dissemination of primary neoplasms to the bones and antitumor immunity remains poorly understood. We have recently identified follistatin-like 1 (FSTL1), a soluble protein secreted by snail family zinc finger 1 (SNAI1)-expressing cancer cells, as a key determinant of bone metastasis that operates by inducing a systemic state of immune dysfunction.
The metastatic dissemination of cancer cells to the bone marrow (BM) is a frequent clinical finding, in particular among patients with breast and prostate carcinoma. On the long-term, bone metastases generally cause immobility and aggravate cancer-related morbidity, mainly due to skeletal complications such as hypercalcemia, pathological fractures and cord compression, overall resulting in a significant deterioration of quality of life as well as in an increase in mortality.Citation1 The mechanisms underlying the dissemination of cancer cells to the bone have been intensively investigated, and a variety of therapeutics that specifically target this process has been developed. Conversely, the relationship between the establishment of bone metastases and antitumor immunity has been the subject of much less investigational effort. As the BM is indispensable not only for normal hematopoiesis but also for the generation of immune cells that may exert anticancer activity, the changes in the BM microenvironment elicited by metastases are expected to affect not only the skeletal system but also anticancer immune responses. The mutual relationship between bone metastasis and antitumor immunity remains poorly understood.
We have been exploring novel anticancer therapeutics by focusing on the interplay between neoplastic lesions and antitumor immune responses. In a previous study, we have provided new insights into the metastatic process using murine and human melanoma cells that displayed typical features of the epithelial-to-mesenchymal transition (EMT) following transduction of a cDNA coding for snail family zinc finger 1 (SNAI1, best known as SNAIL). Thus, we demonstrated that SNAI1 expression accelerates metastasis not only by enhancing the motility of malignant cells but also by promoting immunosuppression.Citation2,Citation3 In particular, we found that SNAI1+ metastatic tumor cells secrete thrombospondin 1 (THBS1, also known as TSP1) and chemokine (C-C motif) ligand 2 (CCL2), hence favoring the development of immunosuppressive cells including immunoregulatory dendritic cells (DCregs) and regulatory T cells (Tregs).Citation2,Citation3 However, this was not the only immunosuppressive pathway elicited by SNAI1.
In a murine SNAI1+ melanoma model, we often observed all mice to exhibit black bones (which are indicative of metastatic dissemination) even after the subcutaneous implantation of malignant cells.Citation4 EMT has been reported to generate cancer cells with stem cell-like properties, including self-renewal activity and drug resistance, in the BM niche.Citation5 However, how such “cancer stem cells” CSCs would modulate the BM microenvironment and alter antitumor immune responses remains unclear. Blocking TSP1 or CCL2 did not reduce the amount of bone metastases, although the growth of primary lesions as well as the metastatic dissemination of cancer cells to other tissues were effectively inhibited following the enhancement of antitumor immune responses. Therefore, we attempted to elucidate the mechanism underlying bone metastasis from an immunological perspective, identifying a novel immunosuppressive pathway controlled by follistatin-like 1 (FSTL1), a glycoprotein that is secreted by SNAI1+ cancer cells.Citation4 FSTL1 is known as a regulator of embryonic organogenesis,Citation6 and has been detected in increased amounts in patients with rheumatoid arthritis and osteosarcoma.Citation7,Citation8 However, a functional role of FSTL1 in cancer metastasis and antitumor immunity had never been demonstrated.
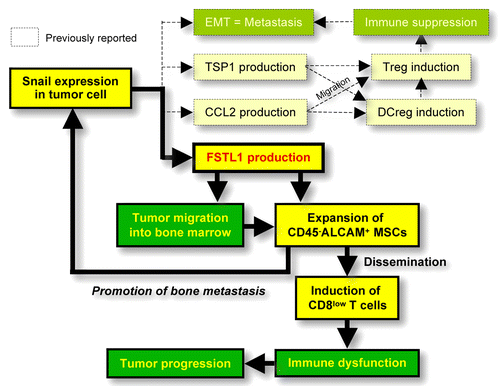
We revealed a dual role of FSTL1 in the metastatic dissemination of cancer cells to the bones.Citation4 Thus, FSTL1 promotes metastasis by accruing the invasive potential and bone tropism of malignant cells as well as by inhibiting antitumor immune responses as it favors the expansion of pluripotent and immunoregulatory activated leukocyte cell adhesion molecule (ALCAM)+CD45- mesenchymal stem cells (MSCs). Such MSCs generate functionally impaired T cells that express low levels of CD8, and further stimulate metastatic bone colonization, resulting in the amplification of the FSTL1-induced signaling cascade (). Blocking FSTL1 using specific siRNAs hampers these events and favors the elicitation of efficient antitumor immune responses in vitro and in vivo. In specimens from advanced breast carcinoma patients, the relative abundance of ALCAM+ cells significantly correlated with FSTL1 levels in malignant tissues, but not in their adjacent normal counterparts. This points to the existence of a causal connection between FSTL1 and ALCAM within the tumor microenvironment. Thus, FSTL1 stands out as a promising therapeutic target to prevent or treat bone metastasis and immune dysfunction in cancer patients.
Figure 1. Mechanisms of FSTL1-induced bone metastases. Follistatin-like 1 (FSTL1) is the third player in snail family zinc finger 1 (SNAI1)-induced immune evasion after thrombospondin 1 (THBS1, also known as TSP1) and chemokine (C-C motif) ligand 2 (CCL2), which we previously found to promote immunosuppression.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Citation: Kudo-Saito C. FSTL1 promotes bone metastasis through immune dysfunction. OncoImmunology 2013; 2:e26528; 10.4161/onci.26528
References
- Weilbaecher KN, Guise TA, McCauley LK. Cancer to bone: a fatal attraction. Nat Rev Cancer 2011; 11:411 - 25; http://dx.doi.org/10.1038/nrc3055; PMID: 21593787
- Kudo-Saito C, Shirako H, Takeuchi T, Kawakami Y. Cancer metastasis is accelerated through immunosuppression during Snail-induced EMT of cancer cells. Cancer Cell 2009; 15:195 - 206; http://dx.doi.org/10.1016/j.ccr.2009.01.023; PMID: 19249678
- Kudo-Saito C, Shirako H, Ohike M, Tsukamoto N, Kawakami Y. CCL2 is critical for immunosuppression to promote cancer metastasis. Clin Exp Metastasis 2013; 30:393 - 405; http://dx.doi.org/10.1007/s10585-012-9545-6; PMID: 23143679
- Kudo-Saito C, Fuwa T, Murakami K, Kawakami Y. Targeting FSTL1 prevents tumor bone metastasis and consequent immune dysfunction. Cancer Res 2013; Forthcoming http://dx.doi.org/10.1158/0008-5472.CAN-13-1364; PMID: 23966294
- Kong D, Li Y, Wang Z, Sarkar FH. Cancer Stem Cells and Epithelial-to-Mesenchymal Transition (EMT)-Phenotypic Cells: Are They Cousins or Twins?. Cancers (Basel) 2011; 3:716 - 29; http://dx.doi.org/10.3390/cancers30100716; PMID: 21643534
- Adams D, Larman B, Oxburgh L. Developmental expression of mouse Follistatin-like 1 (Fstl1): Dynamic regulation during organogenesis of the kidney and lung. Gene Expr Patterns 2007; 7:491 - 500; http://dx.doi.org/10.1016/j.modgep.2006.10.009; PMID: 17129766
- Li D, Wang Y, Xu N, Wei Q, Wu M, Li X, Zheng P, Sun S, Jin Y, Zhang G, et al. Follistatin-like protein 1 is elevated in systemic autoimmune diseases and correlated with disease activity in patients with rheumatoid arthritis. Arthritis Res Ther 2011; 13:R17; http://dx.doi.org/10.1186/ar3241; PMID: 21303509
- Hambrock HO, Kaufmann B, Müller S, Hanisch FG, Nose K, Paulsson M, Maurer P, Hartmann U. Structural characterization of TSC-36/Flik: analysis of two charge isoforms. J Biol Chem 2004; 279:11727 - 35; http://dx.doi.org/10.1074/jbc.M309318200; PMID: 14701841