Abstract
Adoptive or active cancer immunotherapy can fail owing to the inefficient recruitment of effector leukocytes to malignant lesions. The intratumoral injection of recombinant proteins comprising a chemokine-derived domain linked to the mucin stalk of chemokine (C-X3-C motif) ligand 1 (CX3CL1) and a glycosylphosphatidylinositol anchor can specifically enhance the recruitment of effector cell subsets to solid tumors.
Active immunotherapy and the adoptive transfer of immune effector cells are emerging approaches for the treatment of various tumors. However, the efficacy of these immunotherapeutic regimens can be significantly hampered by insufficient infiltration of neoplastic lesions by endogenous or adoptively transferred immune cells.Citation1-Citation3 At least in part, this reflects specific features of tumor vascularization, which not only provides malignant lesions with oxygen and nutrients, but also helps facilitate immune escape. Normally, in response to stress or inflammation, endothelial cells increase expression of selectins, intercellular adhesion molecules (ICAM), vascular cell adhesions molecules (VCAMs), and proteoglycans on their surface.Citation4 These events promote leukocyte rolling adhesion as leukocytes are captured from the blood stream and roll along the vessel walls. At the same time, chemokines secreted at inflammatory sites are immobilized on proteoglycan molecules at the luminal side of endothelial cells where they can engage the corresponding receptors on rolling leukocytes.Citation4 For example, chemokine (C-X-C motif) ligand 10 (CXCL10) is involved in the recruitment of activated T cells and natural killer cells, as it binds to chemokine (C-X-C motif) receptor 3 (CXCR3) found on these effector cells.
By contrast, tumor endothelium often exhibits reduced baseline expression levels as well as a limited ability to upregulate these adhesion molecules in response to pro-inflammatory stimuli. In addition, proteoglycan molecules expressed on the surface of the tumor endothelium are often subjected to cleavage (shedding) and other enzymatic modifications.Citation5 In line with this notion, a reduced interaction of leukocytes with the wall of tumor-associated vessels, resulting in defective tumor-infiltration by immune cells has been observed in many oncological settings. This phenomenon has been referred to as “endothelial anergy”, and has been suggested as a potential explanation for the limited success of adoptive T-cell therapy in some clinical studies.Citation1-Citation3
In order to overcome the problems associated with endothelial anergy, we developed a novel class of fusion proteins based on chemokine biology. These reagents were designed to enhance the migration of immune effector cells to select tissue microenvironments by creating a non-diffusible chemotactic gradient that selectively recruits specific leukocyte subsets based on their expression of a given chemokine receptor.Citation6
The backbone of these fusion proteins was derived from chemokine (C-X3-C motif) ligand 1 (CX3CL1), a membrane-anchored chemokine. CX3CL1 consists of an N-terminal 76 amino acid chemokine domain fused to a 241 amino acid mucin-like domain linked to a hydrophobic transmembrane domain and an intracellular tail. The mucin domain forms an extended stalk that protrudes 26 nm away from the cell membrane, displaying the chemokine domain at its end.Citation7 This exceptional architecture gives CX3CL1 unique characteristics in that it can directly induce the adhesion of CX3CR1-epressing cells in the absence of other adhesion molecules such as ICAM1 or VCAM1 in vitro, and can facilitate leukocyte capture under conditions of physiologic blood flow.Citation8 Importantly, the specificity of CX3CL1 can be modified by exchanging its chemokine domain with that of other chemokines resulting in the recruitment of leukocytes expressing chemokine receptors targeted by the new chemokine domain.Citation8
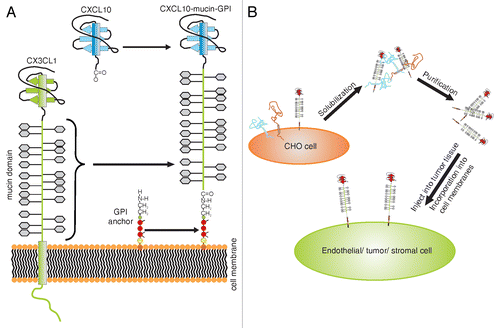
We harnessed this phenomenon by generating recombinant proteins comprising a defined N-terminal chemokine head, linked to the CX3CL1 mucin domain. In addition, the transmembrane domain of CX3CL1 was replaced by a C-terminal glycosylphosphatidylinositol (GPI) membrane anchor (). GPI anchors tether proteins to the outer leaflet of the plasma membrane.Citation9 The anchor itself hereby consists of a phosphatidylinositol group that is linked to the C-terminus of the protein via a carbohydrate core. Purified GPI-anchored proteins possess the ability to integrate spontaneously into the plasma membrane of virtually any cell. Following this incorporation, they can still exert their natural bioactivity ().Citation9 Almost any protein can be expressed as a GPI-anchored version by fusing it to an appropriate signal sequence that results in the addition of a GPI anchor.Citation9 In many settings, this concept of “cell painting” represents an efficient and safe alternative to conventional gene transfer.
Figure 1. Structure and applications of membrane-anchored chemokine fusion proteins. (A) Composition of membrane-anchored chemokine fusion proteins. The mucin domain of chemokine (C-X3-C motif) ligand 1 (CX3CL1) is combined with a new chemokine domain and stably expressed as a glycosylphosphatidylinositol (GPI)-anchored protein in Chinese hamster ovary (CHO) cells. (B) Application of membrane-anchored chemokine fusion proteins. Recombinant proteins are isolated from the plasma membrane of CHO cells and purified using fast protein liquid chromatography (FPLC). Purified recombinant proteins efficiently incorporate into plasma membranes and can hence be used to foster the recruitment of leukocyte subsets expressing the complementary chemokine receptor.
As a proof of concept for this novel class of recombinant proteins, we generated a fusion protein containing a CXCL10 chemokine head (CXCL10-mucin-GPI), along with a series of control proteins.Citation6 All proteins were expressed in a mammalian system and it was verified that the GPI anchor signal could correctly target them to the plasma membrane. The ability of the CXCL10 fusion proteins to bind and activate the CXCR3 receptor was validated in assays that measured receptor internalization, calcium mobilization, and enhanced adhesion of T cells to cell monolayers as readouts. Following the identification of a suitable detergent for solubilization, the proteins were isolated from cell extracts using affinity chromatography.
Purified fusion proteins were found to efficiently reintegrate into cell membranes in a process that critically depended upon the GPI anchor. In vitro models of leukocyte recruitment showed that primary microvascular endothelial cells incubated with low concentrations of the CXCL10-mucin-GPI chimera could efficiently recruit CXCR3-expressing NK cells under conditions of physiologic flow, in a process that relied on the presence of the mucin domain but not on inflammatory priming. When purified and injected into an experimental tumor, fusion proteins integrated into the plasma membranes of malignant and stromal cells by means of their GPI anchor. In this setting, the CXCL10-mucin-GPI chimera was found to be much more efficient in recruiting NK cells than soluble CXCL10. Thus, fusion proteins such as the CXCL10-mucin-GPI chimera represent promising candidates to act as novel adjuvants in cellular immunotherapy.
In a parallel study, a similar approach based on CXCL12 rather than CXCL10 was used to support the recruitment of CXCR4-expressing endothelial progenitor cells in an in vivo model of vessel repair, further validating the general concepts outlined here.Citation10
| Abbreviations: | ||
| ICAM | = | intercellular adhesion molecule |
| GPI | = | glycosylphosphatidylinositol |
| VCAM1 | = | vascular cell adhesion molecule 1 |
Disclosure of Potential Conflicts of Interest
PJN has patented the technology described here.
Acknowledgments
This work was funded by the Deutsche Forschungsgemeinschaft DFG TR-SFB 36.
Citation: Nelson PJ, Muenchmeier N. Membrane-anchored chemokine fusion proteins: A novel class of adjuvants for immunotherapy. OncoImmunology 2013; 2:e26619; 10.4161/onci.26619
References
- Dirkx AE, Oude Egbrink MG, Kuijpers MJ, van der Niet ST, Heijnen VV, Bouma-ter Steege JC, Wagstaff J, Griffioen AW. Tumor angiogenesis modulates leukocyte-vessel wall interactions in vivo by reducing endothelial adhesion molecule expression. Cancer Res 2003; 63:2322 - 9; PMID: 12727857
- Griffioen AW, Damen CA, Blijham GH, Groenewegen G. Tumor angiogenesis is accompanied by a decreased inflammatory response of tumor-associated endothelium. Blood 1996; 88:667 - 73; PMID: 8695814
- Griffioen AW, Damen CA, Mayo KH, Barendsz-Janson AF, Martinotti S, Blijham GH, Groenewegen G. Angiogenesis inhibitors overcome tumor induced endothelial cell anergy. Int J Cancer 1999; 80:315 - 9; http://dx.doi.org/10.1002/(SICI)1097-0215(19990118)80:2<315::AID-IJC23>3.0.CO;2-L; PMID: 9935216
- von Hundelshausen P, Weber KS, Huo Y, Proudfoot AE, Nelson PJ, Ley K, Weber C. RANTES deposition by platelets triggers monocyte arrest on inflamed and atherosclerotic endothelium. Circulation 2001; 103:1772 - 7; http://dx.doi.org/10.1161/01.CIR.103.13.1772; PMID: 11282909
- Sanderson RD, Yang Y, Kelly T, MacLeod V, Dai Y, Theus A. Enzymatic remodeling of heparan sulfate proteoglycans within the tumor microenvironment: growth regulation and the prospect of new cancer therapies. J Cell Biochem 2005; 96:897 - 905; 10.1002/jcb.20602 PMID: 16149080
- Muenchmeier N, Boecker S, Bankel L, Hinz L, Rieth N, Lapa C, Mendler AN, Noessner E, Mocikat R, Nelson PJ. A Novel CXCL10-Based GPI-Anchored Fusion Protein as Adjuvant in NK-Based Tumor Therapy. PLoS One 2013; 8:e72749; http://dx.doi.org/10.1371/journal.pone.0072749; PMID: 24023642
- Fong AM, Erickson HP, Zachariah JP, Poon S, Schamberg NJ, Imai T, Patel DD. Ultrastructure and function of the fractalkine mucin domain in CX(3)C chemokine domain presentation. J Biol Chem 2000; 275:3781 - 6; http://dx.doi.org/10.1074/jbc.275.6.3781; PMID: 10660527
- Imai T, Hieshima K, Haskell C, Baba M, Nagira M, Nishimura M, Kakizaki M, Takagi S, Nomiyama H, Schall TJ, et al. Identification and molecular characterization of fractalkine receptor CX3CR1, which mediates both leukocyte migration and adhesion. Cell 1997; 91:521 - 30; http://dx.doi.org/10.1016/S0092-8674(00)80438-9; PMID: 9390561
- Medof ME, Nagarajan S, Tykocinski ML. Cell-surface engineering with GPI-anchored proteins. FASEB J 1996; 10:574 - 86; PMID: 8621057
- Stachel G, Trenkwalder T, Götz F, El Aouni C, Muenchmeier N, Pfosser A, Nussbaum C, Sperandio M, Hatzopoulos AK, Hinkel R, et al. SDF-1 Fused to a Fractalkine Stalk and a Gpi Anchor Enables Functional Neovascularization. Stem Cells 2013; http://dx.doi.org/10.1002/stem.1439; PMID: 23744498