Abstract
Malignant cells constitutively express Natural killer group 2, member D (NKG2D) or DNAX Accessory Molecule-1 (DNAM-1) ligands, yet they are often unable to trigger a robust cytotoxic cell response. It may be therapeutically useful to implement strategies aimed at increasing the density of NKG2D and DNAM-1 ligands on the surface of cancer cells, endowing them with the capacity to activate potent antitumor natural killer-cell responses.
Natural killer (NK) cells can recognize and eliminate cells undergoing phenotypic changes in the course of neoplastic transformation. This can occur either as malignant cells express reduced levels of proteins that inhibit NK-cell cytotoxicity, such as MHC class I molecules, on their surface, or upon the upregulation of NK cell-activating ligands, including those that bind to NKG2D, and DNAM-1. Cancer immunosurveillance is indeed strongly impaired in both NKG2D- and DNAM-1-deficient mice.Citation1,Citation2 Human stress-inducible NKG2D ligands include MHC class I polypeptide-related sequence A and B (MICA and MICB) as well as UL16-binding proteins (ULBP1–6). Conversely, so far only two ligands for human DNAM-1 have been characterized: poliovirus receptor (PVR, also known as CD155) and poliovirus receptor-related 2 (herpesvirus entry mediator B) (PVRL2, also known as nectin-2 or CD112). Of note, functional studies have demonstrated that NKG2D and DNAM-1 play a key role in the killing of multiple myeloma (MM) cells,Citation1-Citation4 a malignant disorder of plasma cells.
DNA-Damaging Agents
Chemotherapeutic regimens for the treatment of MM patients typically include DNA-damaging agents, inhibiting DNA polymerases and hence affecting the replication of highly proliferating malignant cells. Unfortunately, standard-dose chemotherapy usually exerts robust immunosuppressive effects. In line with a large body of experimental and clinical evidence, we demonstrated that the exposure of MM cells to sub-lethal doses of genotoxic chemotherapeutics is able to increase the cytotoxic activity of NK cells. In particular, we have shown that, in response to doxorubicin and melphalan, MM cell lines as well as primary malignant plasma cells from MM patients, upregulate NKG2D and DNAM-1 ligands, at both the protein and mRNA levels.
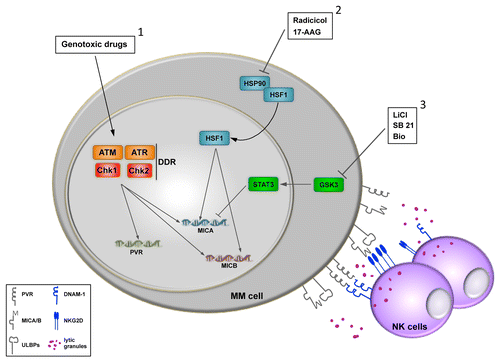
The chemotherapy-elicited expression of NKG2D and DNAM-1 ligands on the surface of MM cells depends on components of the DNA damage response (DDR), in particular ataxia telangiectasia mutated (ATM) and ataxia telangiectasia and Rad3 related (ATR). The upregulation of stress-inducible NK cell-activating ligands is preferentially associated with the onset of a senescent phenotype and an arrest in the G2 phase of the cell cycle.Citation4 In line with this notion, we have demonstrated that NK cells contacting senescent MM cells exhibit an increased propensity to degranulate, suggesting that NK-cell cytotoxicity is preferentially activated by drug-induced senescent cells.Citation4 Our studies unravel one of the mechanisms underlying the immunomodulatory effects of genotoxic chemotherapy, in particular their impact on the NK-cell effector response. Moreover, our data confirm that NK cells are major components of the immunosurveillance system that control stress-induced senescent programs such as those triggered by drugs and oncogene signaling.Citation4-Citation6
HSP90 Inhibitors
Recently, the heat shock 90 kDa protein (HSP90) family members have emerged as attractive targets for cancer therapy. Indeed, not only HSP90s are frequently overexpressed by cancer (including MM) cells but they also mediate prominent pro-survival roles, allowing for the functional expression of oncoproteins and/or for the activation of aberrant signaling pathways. Several HSP90 inhibitors have been shown to exert antineoplastic activity against human MM cells in vitro and in vivo.Citation7 We have investigated the effects of 17-allylaminogeldanamycin (17-AAG) and radicicol, 2 HSP90 inhibitors that display anti-myeloma activity, on the expression of NK cell-activating ligands by human MM cells.Citation8 We found that these drugs induce the upregulation of both MICA and MICB at the mRNA and surface-exposed protein levels, hence increasing the sensitivity of human MM cells to NK-cell mediated cytotoxicity. Mechanistically, we demonstrated that the inhibition of HSP90 activates heat shock transcription factor 1 (HSF1), a powerful enhancer of MICA/MICB transcription, stimulating the binding of HSF1 to MICA and MICB promoters in vitro and in vivo.
GSK3 Inhibitors
Glycogen synthase kinase 3 (GSK3) is a multifunctional serine/threonine kinase that participate in the regulation of multiple cellular functions including protein synthesis, motility, proliferation and survival. Moreover, GSK3 holds promise as a target for therapeutic interventions in several cancers (including MM) owing to its involvement in oncogenesis, tumor progression and chemoresistance.Citation9 We have recently described the effect of different GSK3 inhibitors (i.e., lithium chloride, SB21, BIO) on the expression of NKG2D and DNAM-1 ligands by MM cells.Citation10 We found that the inhibition of this kinase results in the upregulation of MICA on the surface of human MM cell lines as well as of primary malignant plasma cells from MM patients. Accordingly, the exposure of MM cells to GSK3 inhibitors increases their susceptibility to NKG2D-dependent NK cell-mediated killing.
We showed that MICA upregulation correlates with an increased activity of the MICA promoter. A pivotal role in this regulatory mechanism is mediated by the effect of GSK3 signal transducer and activator of transcription 3 (STAT3) activity, a transcription factor that has recently been described to specifically inhibit MICA expression in cancer cells of different origin. Thus, the pharmacological inhibition of GSK3 significantly decreases the constitutive phosphorylation of STAT3 at Y705 and its binding to the MICA promoter. Interestingly, we observed that GSK3 inhibitors can exacerbate the upregulation of MICA as induced in MM cells by standard chemotherapeutics such as melphalan and lenalidomide. GSK3 has been characterized as an important regulator of several cellular components of the immune response, including NK cells. Our data add to this knowledge by demonstrating that the pharmacological modulation of GSK3 can potentiate the antitumor activity of NK cells by stimulating the expression of stress-inducible ligands that promote their cytolytic activity.
Conclusions
In summary, our findings support the notion that the clinical benefits of chemotherapy might partly originate from the stimulation of antitumor immune responses. Of note, different classes of therapeutics agents can selectively promote the expression of NKG2D and DNAM-1 ligands on the surface of myeloma cells, suggesting that the regulation of NK cell-activating ligands is complex (). A deep comprehension of the signaling cascades involved in this process would be useful for the design of novel combination therapies to optimize antitumor NK-cell activity.
Figure 1. Upregulation of NKG2D and DNAM-1 ligands by chemotherapy increases antitumor natural killer-cell responses. Genotoxic drugs induce the expression of NKG2D or DNAM-1 ligands in the surface of cancer cells following the activation of the DNA damage responses (DDR) (1). The activation of heat shock transcription factor 1 (HSF1) resulting from the inhibition of heat shock 90 kDa protein (HSP90) family members specifically stimulates the expression of MICA and MICB (2). The inhibition of glycogen synthase kinase 3 (GSK3) correlates with that of signal transducer and activator of transcription 3 (STAT3), a negative regulator of MICA transcription.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Citation: Soriani A, Fionda C, Ricci B, Iannitto ML, Cippitelli M, Santoni A. Chemotherapy-elicited upregulation of NKG2D and DNAM1 ligands as a therapeutic target in multiple myeloma. OncoImmunology 2013; 2:e26663; 10.4161/onci.26663
References
- Guerra N, Tan YX, Joncker NT, Choy A, Gallardo F, Xiong N, Knoblaugh S, Cado D, Greenberg NM, Raulet DH. NKG2D-deficient mice are defective in tumor surveillance in models of spontaneous malignancy. Immunity 2008; 28:571 - 80; http://dx.doi.org/10.1016/j.immuni.2008.02.016; PMID: 18394936
- Iguchi-Manaka A, Kai H, Yamashita Y, Shibata K, Tahara-Hanaoka S, Honda S, Yasui T, Kikutani H, Shibuya K, Shibuya A. Accelerated tumor growth in mice deficient in DNAM-1 receptor. J Exp Med 2008; 205:2959 - 64; http://dx.doi.org/10.1084/jem.20081611; PMID: 19029379
- El-Sherbiny YM, Meade JL, Holmes TD, McGonagle D, Mackie SL, Morgan AW, Cook G, Feyler S, Richards SJ, Davies FE, et al. The requirement for DNAM-1, NKG2D, and NKp46 in the natural killer cell-mediated killing of myeloma cells. Cancer Res 2007; 67:8444 - 9; http://dx.doi.org/10.1158/0008-5472.CAN-06-4230; PMID: 17875681
- Soriani A, Zingoni A, Cerboni C, Iannitto ML, Ricciardi MR, Di Gialleonardo V, Cippitelli M, Fionda C, Petrucci MT, Guarini A, et al. ATM-ATR-dependent up-regulation of DNAM-1 and NKG2D ligands on multiple myeloma cells by therapeutic agents results in enhanced NK-cell susceptibility and is associated with a senescent phenotype. Blood 2009; 113:3503 - 11; http://dx.doi.org/10.1182/blood-2008-08-173914; PMID: 19098271
- Krizhanovsky V, Yon M, Dickins RA, Hearn S, Simon J, Miething C, Yee H, Zender L, Lowe SW. Senescence of activated stellate cells limits liver fibrosis. Cell 2008; 134:657 - 67; http://dx.doi.org/10.1016/j.cell.2008.06.049; PMID: 18724938
- Kang TW, Yevsa T, Woller N, Hoenicke L, Wuestefeld T, Dauch D, Hohmeyer A, Gereke M, Rudalska R, Potapova A, et al. Senescence surveillance of pre-malignant hepatocytes limits liver cancer development. Nature 2011; 479:547 - 51; http://dx.doi.org/10.1038/nature10599; PMID: 22080947
- Trepel J, Mollapour M, Giaccone G, Neckers L. Targeting the dynamic HSP90 complex in cancer. Nat Rev Cancer 2010; 10:537 - 49; http://dx.doi.org/10.1038/nrc2887; PMID: 20651736
- Fionda C, Soriani A, Malgarini G, Iannitto ML, Santoni A, Cippitelli M. Heat shock protein-90 inhibitors increase MHC class I-related chain A and B ligand expression on multiple myeloma cells and their ability to trigger NK cell degranulation. J Immunol 2009; 183:4385 - 94; http://dx.doi.org/10.4049/jimmunol.0901797; PMID: 19748980
- Luo J. Glycogen synthase kinase 3beta (GSK3beta) in tumorigenesis and cancer chemotherapy. Cancer Lett 2009; 273:194 - 200; http://dx.doi.org/10.1016/j.canlet.2008.05.045; PMID: 18606491
- Fionda C, Malgarini G, Soriani A, Zingoni A, Cecere F, Iannitto ML, Ricciardi MR, Federico V, Petrucci MT, Santoni A, et al. Inhibition of glycogen synthase kinase-3 increases NKG2D ligand MICA expression and sensitivity to NK cell-mediated cytotoxicity in multiple myeloma cells: role of STAT3. J Immunol 2013; 190:6662 - 72; http://dx.doi.org/10.4049/jimmunol.1201426; PMID: 23686482